Evaluation of Metal–Organic Frameworks as Potential Adsorbents for Solar Cooling Applications
Abstract
1. Introduction
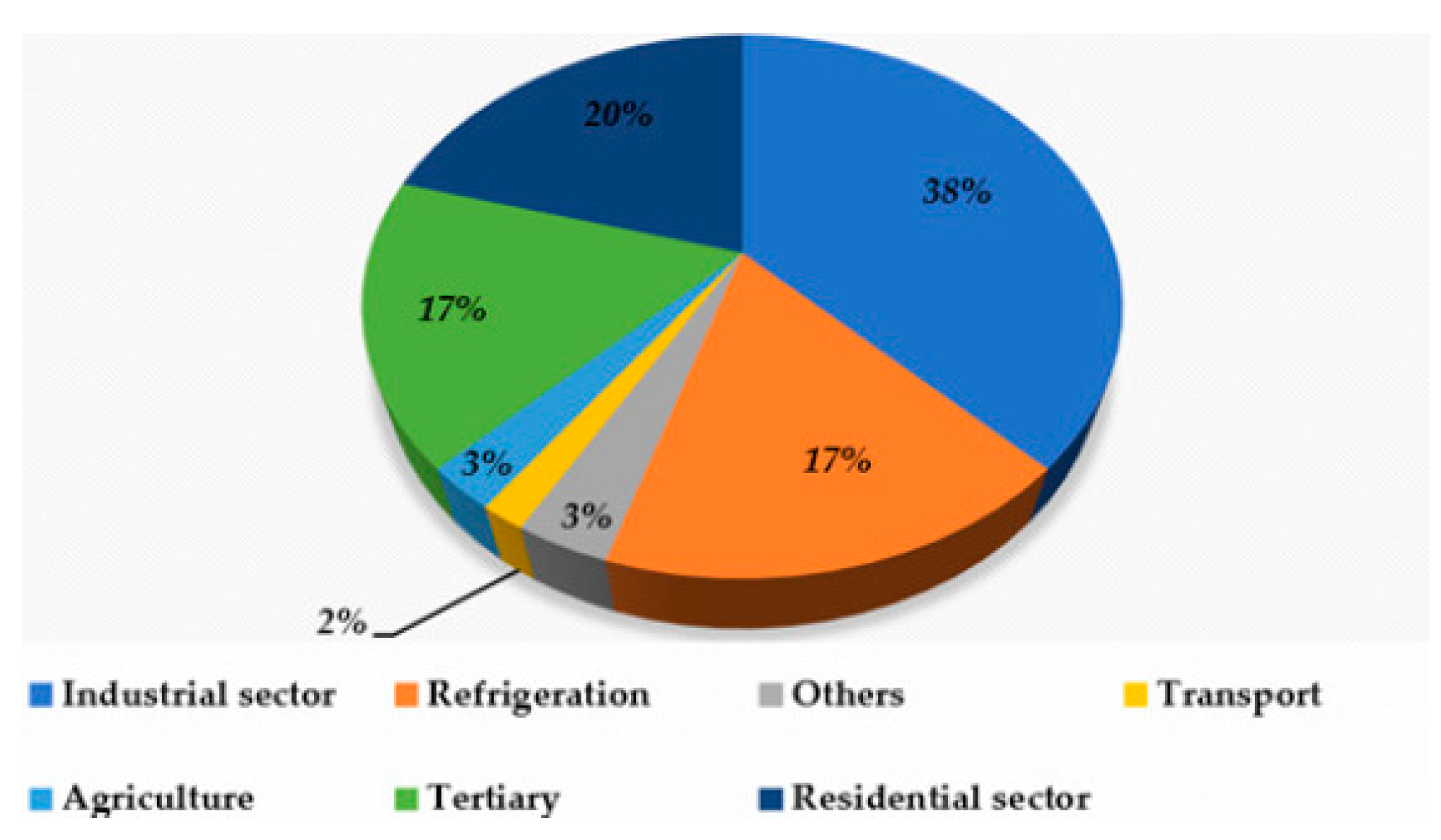
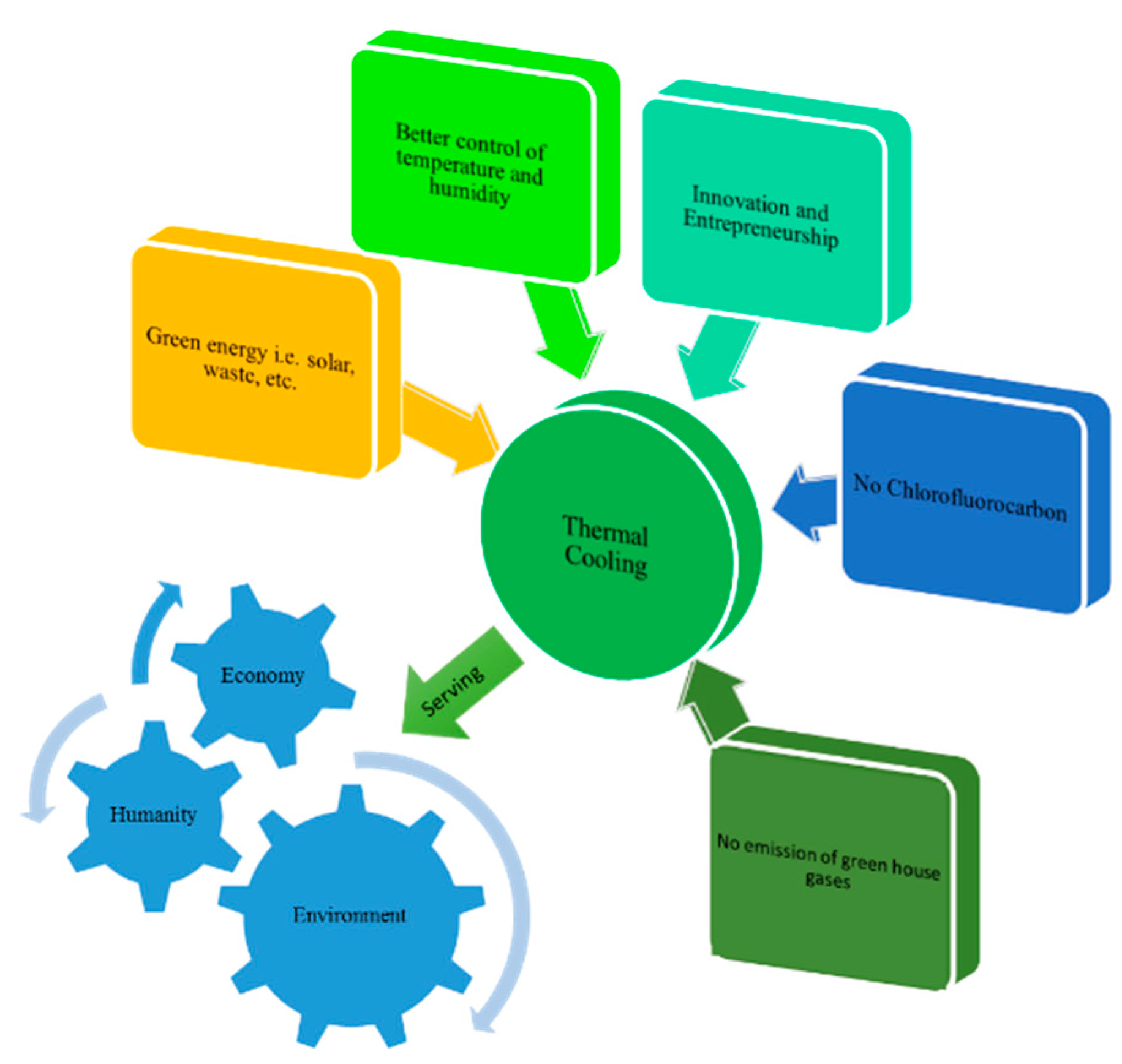
- Description of alternative cooling technologies that are both energy efficient and environmentally friendly.
- Research on thermal cooling systems for their development and promotion.
- A detailed review of available advanced adsorbent materials that can be employed for adsorption based solar cooling.
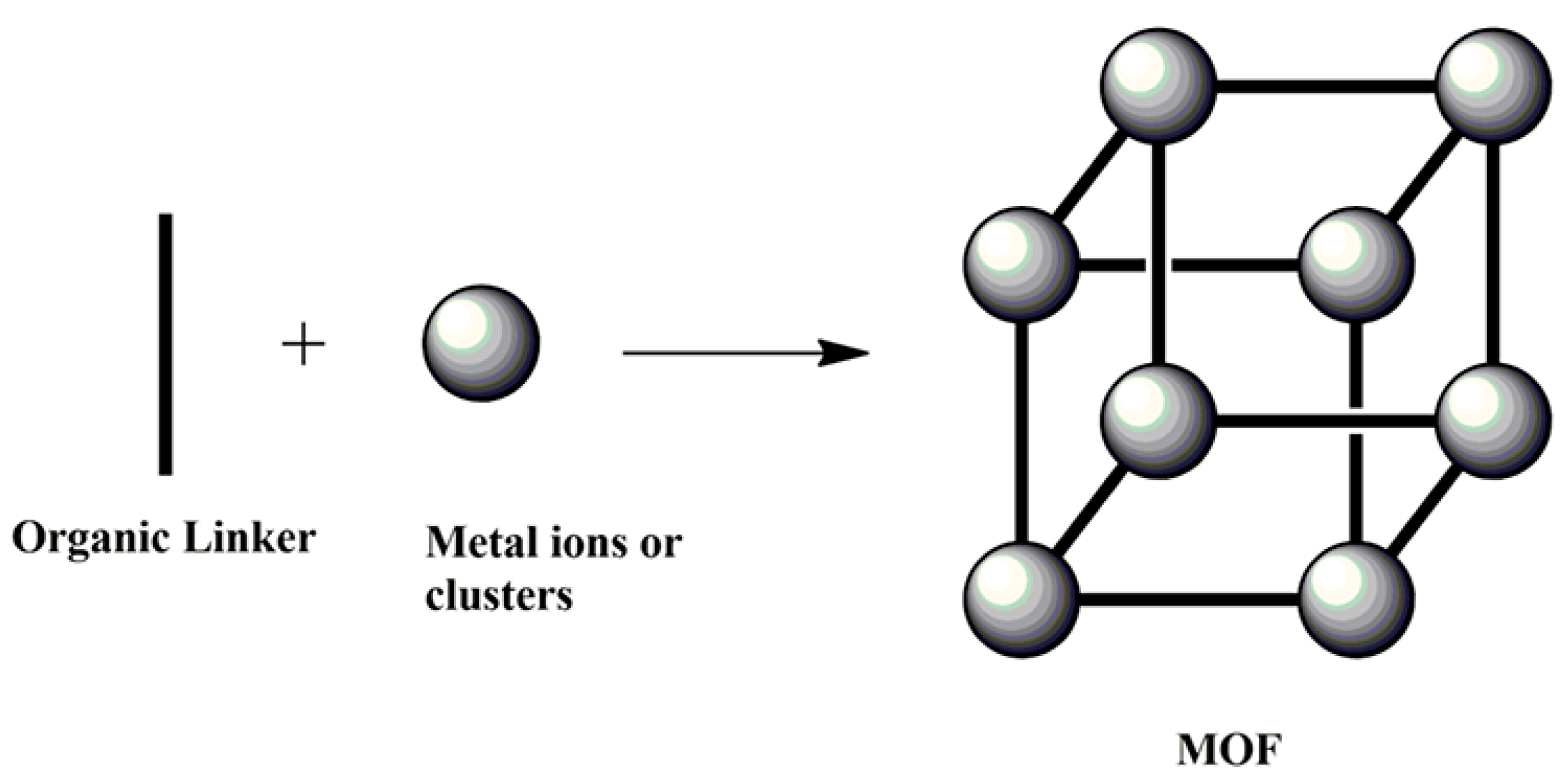
- Introduction to MOFs as an alternative to other adsorbents.
- The recent development of MOFs and their adsorption characteristics.
- Future needs for development and advancements of MOF-based cooling technology.
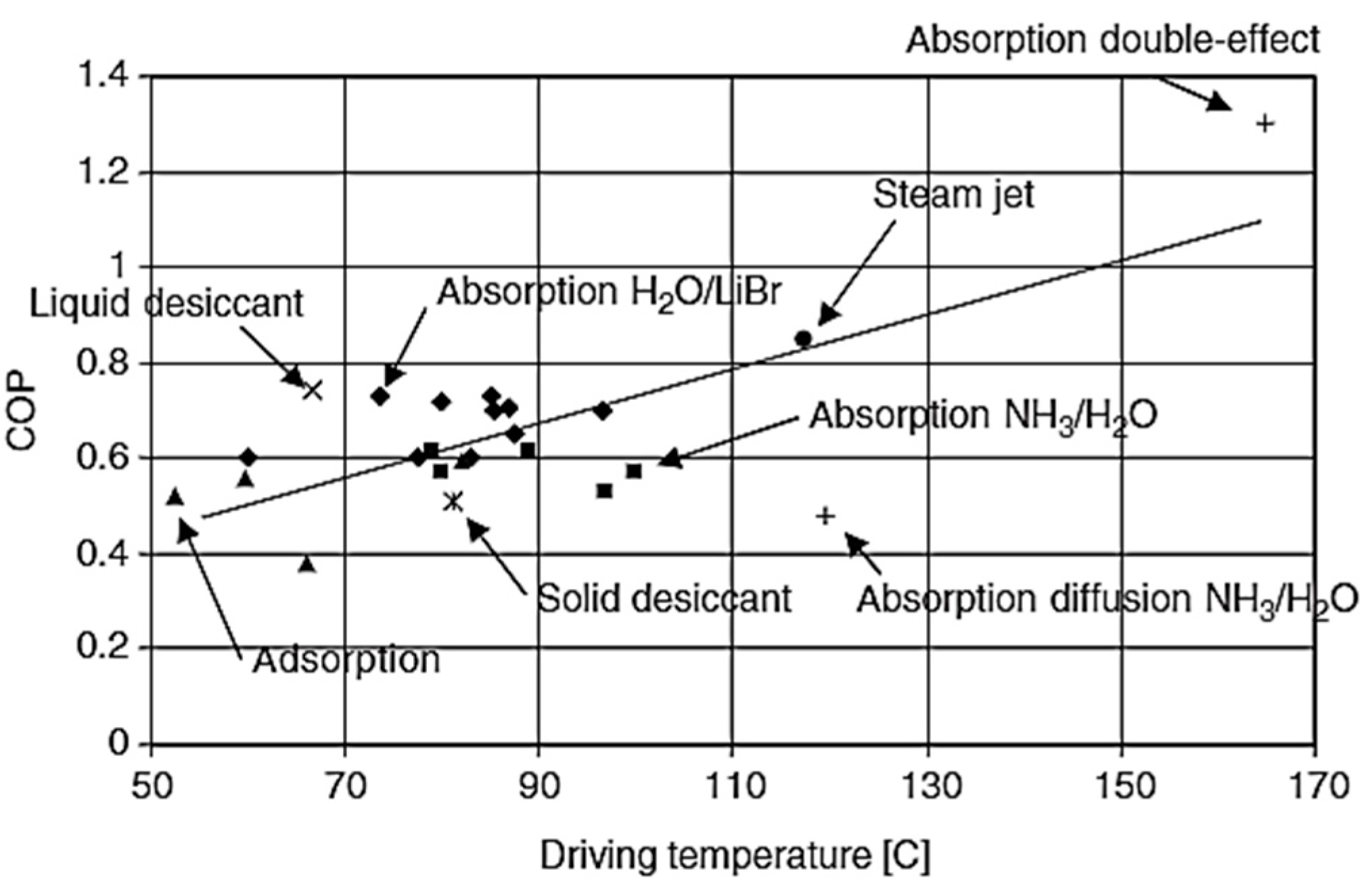
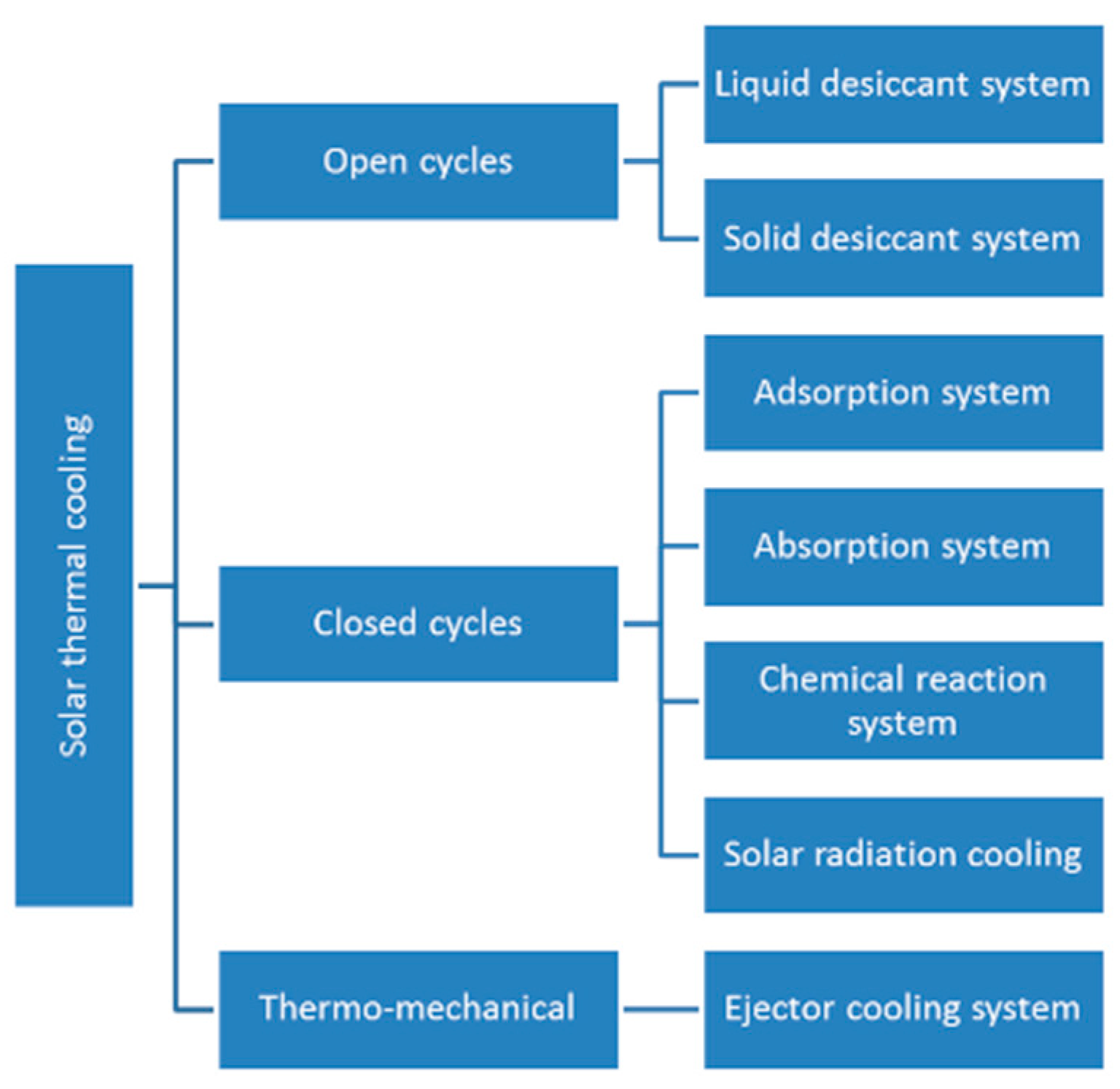
2. Thermally Activated Cooling Systems
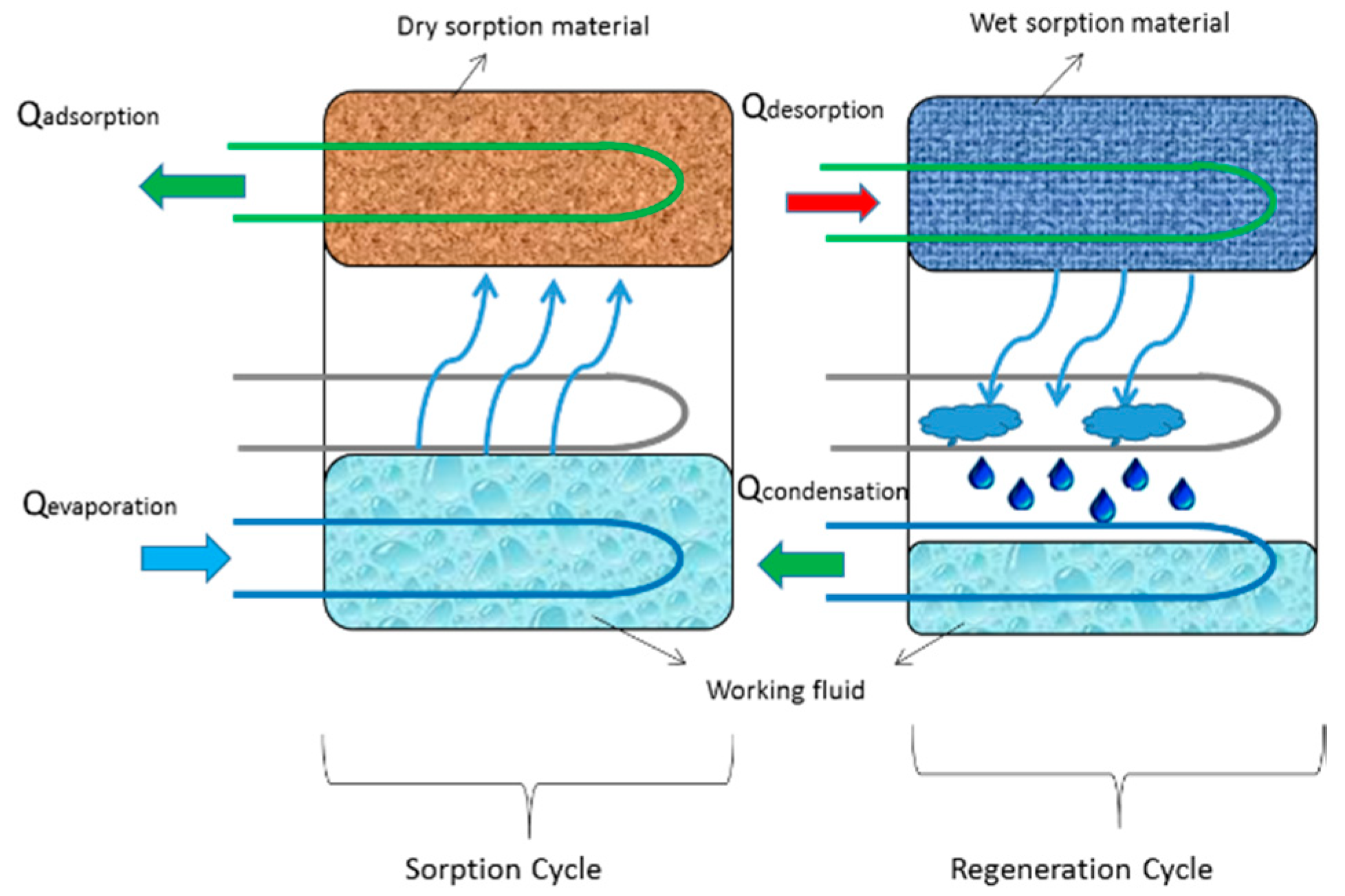
3. Physical Adsorbents and Desired Adsorption Behavior
4. MOF-Based Adsorption Cooling
5. Historical Background of MOFs as Adsorbents
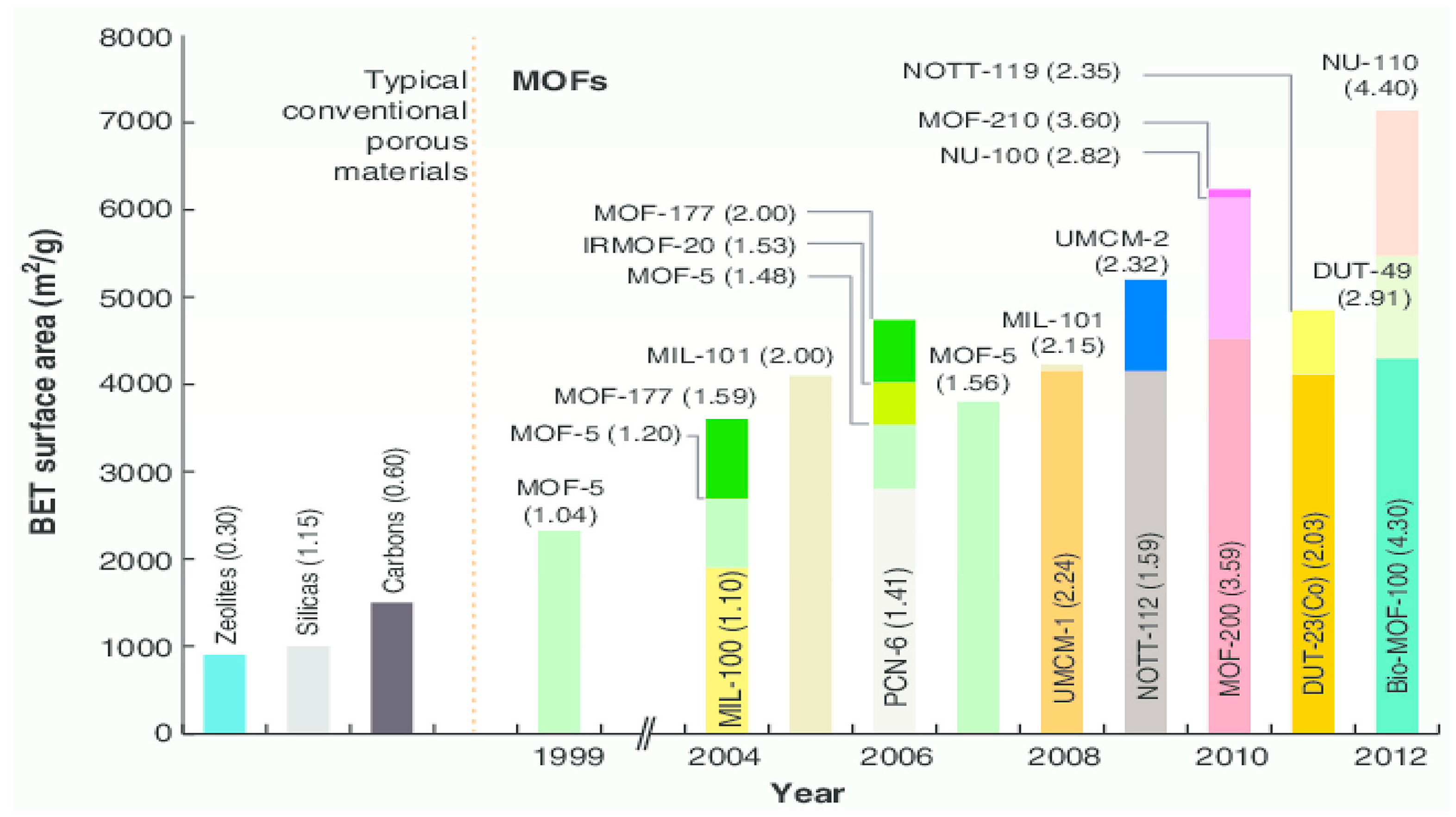
6. Comparison of MOFs with Other Adsorbents
7. The Future of MOF-Based Adsorption Cooling
8. Concluding Remarks
- Regardless of the type of adsorbent, a material with high uptake at lower partial pressure and having stepwise isotherm will be preferred for adsorption-based cooling applications. This type of adsorbent material will give better efficiency and performance.
- The adsorption-based cooling with conventional adsorbent material such as silica gel or zeolites normally have a COP value less than one but the simulation studies available in the literature showed that the use of MOF in these technologies can help to achieve a coefficient of performance greater than one.
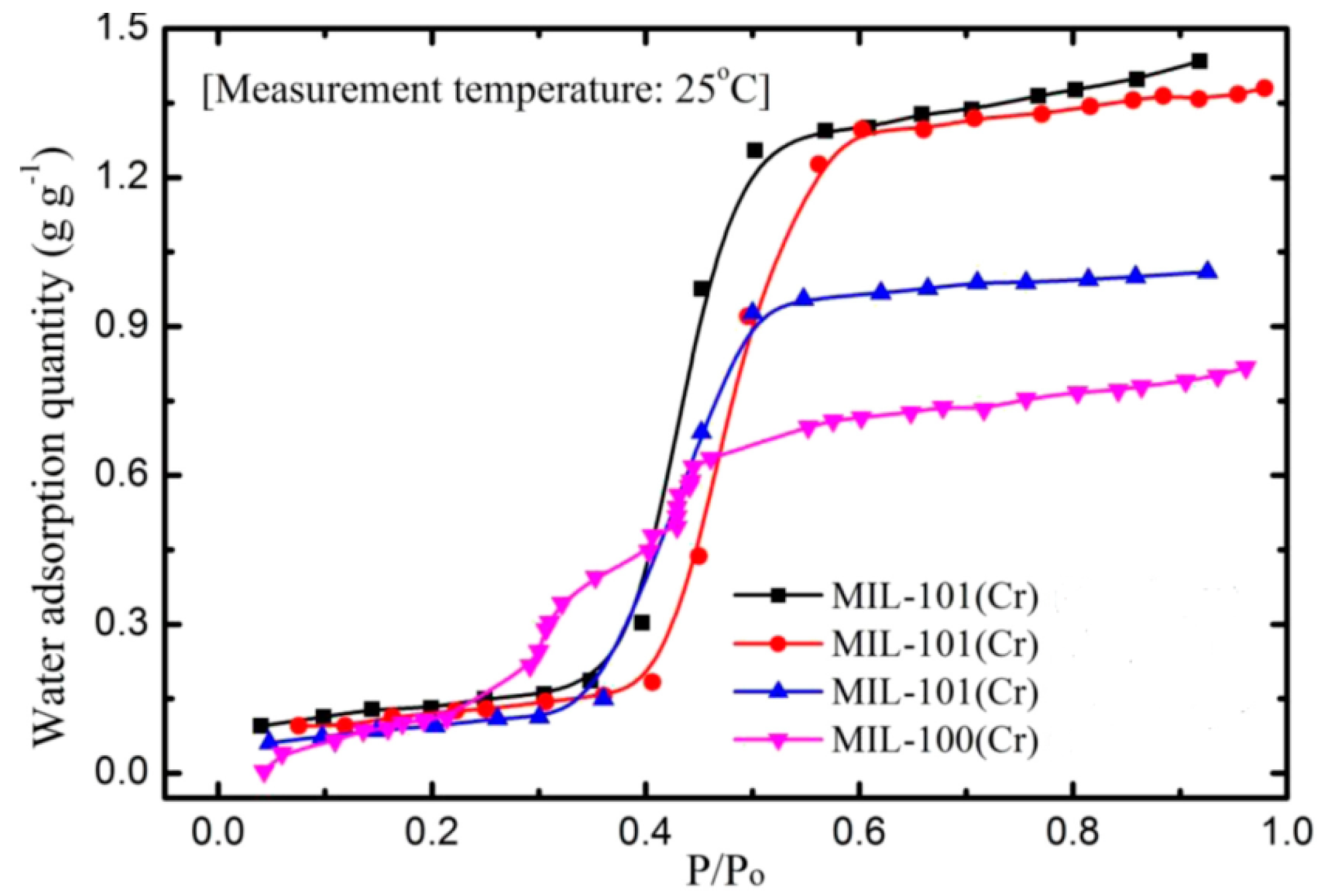
- Better adsorption characteristics, lower required heat, and stable nature has increased the interest of these materials to be used as adsorbents for cooling applications. MIL-101Cr has a high water uptake capacity at low operating pressure and there will be only a slight decrease in its adsorption capacity over time.
- The results presented in the literature showed that HKUST-1 has 93.2% better uptake as compared to silica gel RD-2060. The high-water uptake capacity of HKUST-1 shows that it could be a suitable adsorbent material for adsorption-based cooling.
- Furthermore, the literature survey showed that Cu-BTC and CPO-27 are some of the other widely researched MOFs for their potential use as adsorbent materials for these applications.
- HKUST-1/water, MIL101/water, UiO-66/water, COF-5/ethanol and Maxsorb III/ethanol working pairs have the potential to increase the performance of adsorption cooling technologies.
Funding
Conflicts of Interest
References
- Isaac, M.; Van Vuuren, D.P. Modeling global residential sector energy demand for heating and air conditioning in the context of climate change. Energy Policy 2009, 37, 507–521. [Google Scholar] [CrossRef]
- Rezk, A.R.M. Theoretical and Experimental Investigation of Silica Gel/Water Adsorption Refrigeration Systems. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2012. [Google Scholar]
- Sharafian, A.; Bahrami, M. Assessment of adsorber bed designs in waste-heat driven adsorption cooling systems for vehicle air conditioning and refrigeration. Renew. Sustain. Energy Rev. 2014, 30, 440–451. [Google Scholar] [CrossRef]
- AL-Dadah, R.; Mahmoud, S.; Elsayed, E.; Youssef, P.; Al-Mousawi, F. Metal-organic framework materials for adsorption heat pumps. Energy 2020, 190, 116356. [Google Scholar] [CrossRef]
- Eurostat Database for Energy Statistics. Available online: http://ec.europa.eu/eurostat/web/energy/publications (accessed on 24 March 2018).
- Rafique, M.M.; Gandhidasan, P.; Rehman, S.; Al-Hadhrami, L.M. A review on desiccant based evaporative cooling systems. Renew. Sustain. Energy Rev. 2015, 45, 145–159. [Google Scholar] [CrossRef]
- Rafique, M.M.; Rehman, S. Solar Electrification and Zero Energy Rural Communities. In Smart Village Technology; Springer: Cham, Switzerland, 2020; pp. 329–340. [Google Scholar]
- Rehman, S.; Rafique, M.M.; Alhems, L.M.; Alam, M. Development and Implementation of Solar Assisted Desiccant Cooling Technology in Developing Countries: A Case of Saudi Arabia. Energies 2020, 13, 524. [Google Scholar] [CrossRef]
- Rafique, M.M.; Gandhidasan, P.; Bahaidarah, H.M.; Shakir, M.A. Thermal and exergetic performance evaluation of an absorption type desiccant dehumidifier. Environ. Prog. Sustain. Energy 2018, 37, 1411–1423. [Google Scholar] [CrossRef]
- Balaras, C.A.; Grossman, G.; Henning, H.M.; Ferreira, C.A.I.; Podesser, E.; Wang, L.; Wiemken, E. Solar air conditioning in Europe—An overview. Renew. Sustain. Energy Rev. 2007, 11, 299–314. [Google Scholar] [CrossRef]
- Rafique, M.M.; Gandhidasan, P.; Bahaidarah, H.M. Liquid desiccant materials and dehumidifiers—A review. Renew. Sustain. Energy Rev. 2016, 56, 179–195. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le Pierrès, N.; Luo, L. A review of potential materials for thermal energy storage in building applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519. [Google Scholar] [CrossRef]
- Fadhel, M.I.; Sopian, K.; Daud, W.R.W.; Alghoul, M.A. Review on advanced of solar assisted chemical heat pump dryer for agriculture produce. Renew. Sustain. Energy Rev. 2011, 15, 1152–1168. [Google Scholar] [CrossRef]
- Srivastava, N.C.; Eames, I.W. A review of adsorbents and adsorbates in solid–vapour adsorption heat pump systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Water and methanol adsorption on MOFs for cycling heat transformation processes. New J. Chem. 2014, 38, 1846–1852. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Fundamentals of Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Rafique, M.M. Design and Economic Evaluation of a Solar Household Electrification System. Lamp 2018, 5, 25. [Google Scholar]
- Henning, H.M. Solar assisted air conditioning of buildings—An overview. Appl. Therm. Eng. 2007, 27, 1734–1749. [Google Scholar] [CrossRef]
- Meunier, F. Adsorption heat powered heat pumps. Appl. Therm. Eng. 2013, 61, 830–836. [Google Scholar] [CrossRef]
- Xia, Z.Z.; Chen, C.J.; Kiplagat, J.K.; Wang, R.Z.; Hu, J.Q. Adsorption equilibrium of water on silica gel. J. Chem. Eng. Data 2008, 53, 2462–2465. [Google Scholar] [CrossRef]
- Gong, L.X.; Wang, R.Z.; Xia, Z.Z.; Chen, C.J. Adsorption equilibrium of water on a composite adsorbent employing lithium chloride in silica gel. J. Chem. Eng. Data 2010, 55, 2920–2923. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, R.Z.; Xia, Z.Z.; Kiplagat, J.K. Study on a silica gel–water adsorption chiller integrated with a closed wet cooling tower. Int. J. Therm. Sci. 2010, 49, 611–620. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.Y. Theoretical research of a silica gel–water adsorption chiller in a micro combined cooling, heating and power (CCHP) system. Appl. Energy 2009, 86, 958–967. [Google Scholar] [CrossRef]
- Grisel, R.J.; Smeding, S.F.; De Boer, R. Waste heat driven silica gel/water adsorption cooling in trigeneration. Appl. Therm. Eng. 2010, 30, 1039–1046. [Google Scholar] [CrossRef]
- De Boer, R.; Smeding, S.F.; Mola, S. Silicagel-Water Adsorption Cooling Prototype System for Mobile Air Conditioning. In Proceedings of the Heat Powered Cycles Conference, Berlin, Germany, 7–9 September 2009. [Google Scholar]
- Jakob, U.; Mittelbach, W. Development and Investigation of a Compact Silica Gel/water Adsorption Chiller Integrated in Solar Cooling Systems. In Proceedings of the VII Minsk International Seminar “Heat Pipes, Heat Pumps, Refrigerators, Power Sources”, Minsk, Belarus, 8–11 September 2008; pp. 8–11. [Google Scholar]
- Chang, W.S.; Wang, C.C.; Shieh, C.C. Design and performance of a solar-powered heating and cooling system using silica gel/water adsorption chiller. Appl. Therm. Eng. 2009, 29, 2100–2105. [Google Scholar] [CrossRef]
- Chua, H.T.; Ng, K.C.; Malek, A.; Kashiwagi, T.; Akisawa, A.; Saha, B.B. Multi-bed regenerative adsorption chiller—Improving the utilization of waste heat and reducing the chilled water outlet temperature fluctuation. Int. J. Refrig. 2001, 24, 124–136. [Google Scholar] [CrossRef]
- Rafique, M.M. A statistical analysis of desiccant dehumidifier for air conditioning application. Int. J. Hybrid Inf. Technol. 2015, 8, 245–256. [Google Scholar] [CrossRef]
- Rafique, M.M.; Rehman, S.; Alhems, L.M.; Lashin, A. Parametric Analysis of a Rotary Type Liquid Desiccant Air Conditioning System. Energies 2016, 9, 305. [Google Scholar] [CrossRef]
- Rafique, M.M.; Gandhidasan, P.; Rehman, S.; Alhems, L.M. Performance analysis of a desiccant evaporative cooling system under hot and humid conditions. Environ. Prog. Sustain. Energy 2016, 35, 1476–1484. [Google Scholar] [CrossRef]
- Wang, D.C.; Li, Y.H.; Li, D.; Xia, Y.Z.; Zhang, J.P. A review on adsorption refrigeration technology and adsorption deterioration in physical adsorption systems. Renew. Sustain. Energy Rev. 2010, 14, 344–353. [Google Scholar] [CrossRef]
- Ullah, K.R.; Saidur, R.; Ping, H.W.; Akikur, R.K.; Shuvo, N.H. A review of solar thermal refrigeration and cooling methods. Renew. Sustain. Energy Rev. 2013, 24, 499–513. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Glaznev, I.S.; Ovoshchnikov, D.S.; Aristov, Y.I. Kinetics of water adsorption/desorption under isobaric stages of adsorption heat transformers: The effect of isobar shape. Int. J. Heat Mass Transf. 2009, 52, 1774–1777. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Matsushita, M.; Oku, K.; Nakano, H.; Okumura, S.I.; Yoshihara, M. Morikawa, Adsorption Refrigeration System. U.S. Patent No. 4,881,376, 21 November 1989. [Google Scholar]
- Wang, R.Z.; Oliveira, R.G. Adsorption refrigeration—An efficient way to make good use of waste heat and solar energy. Prog. Energy Combust. Sci. 2006, 32, 424–458. [Google Scholar] [CrossRef]
- Invensor. Available online: http://www.invensor.com/en/technology/cooling-system.htm (accessed on 17 November 2017).
- Dawoud, B.; Hoefle, P.; Bornmann, A.; Chmielewski, S.; Marburger, C. Vacuum Sorption Apparatus. U.S. Patent No. 8,544,293, 1 October 2013. [Google Scholar]
- Shimooka, S.; Oshima, K.; Hidaka, H.; Takewaki, T.; Kakiuchi, H.; Kodama, A.; Matsuda, H. The evaluation of direct cooling and heating desiccant device coated with FAM. J. Chem. Eng. Jpn. 2007, 40, 1330–1334. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Shimooka, S.; Iwade, M.; Oshima, K.; Yamazaki, M.; Terada, S.; Takewaki, T. Novel water vapor adsorbent FAM-Z01 and its applicability to an adsorption heat pump. Kagaku Kogaku Ronbunshu 2005, 31, 361–364. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Shimooka, S.; Iwade, M.; Oshima, K.; Yamazaki, M.; Terada, S.; Watanabe, H.; Takewaki, T. Water vapor adsorbent FAM-Z02 and its applicability to adsorption heat pump. Kagaku Kogaku Ronbunshu 2005, 31, 273–277. [Google Scholar] [CrossRef]
- MITSUBISHI PLASTICS, Zeolitic Water Vapor Adsorbent: AQSOA. Available online: http://www.aaasaveenergy.com/products/001/pdf/AQSOA_1210E.pdf (accessed on 16 March 2018).
- Wang, L.W.; Wang, R.Z.; Oliveira, R.G. A review on adsorption working pairs for refrigeration. Renew. Sustain. Energy Rev. 2009, 13, 518–534. [Google Scholar] [CrossRef]
- Saha, B.B.; Habib, K.; El-Sharkawy, I.I.; Koyama, S. Adsorption characteristics and heat of adsorption measurements of R-134a on activated carbon. Int. J. Refrig. 2009, 32, 1563–1569. [Google Scholar] [CrossRef]
- Habib, K.; Saha, B.B.; Rahman, K.A.; Chakraborty, A.; Koyama, S.; Ng, K.C. Experimental study on adsorption kinetics of activated carbon/R134a and activated carbon/R507A pairs. Int. J. Refrig. 2010, 33, 706–713. [Google Scholar] [CrossRef]
- Askalany, A.A.; Saha, B.B.; Ahmed, M.S.; Ismail, I.M. Adsorption cooling system employing granular activated carbon–R134a pair for renewable energy applications. Int. J. Refrig. 2013, 36, 1037–1044. [Google Scholar] [CrossRef]
- Zhequan, J.I.N.; Bo, T.I.A.N.; Liwei, W.A.N.G.; Ruzhu, W.A.N.G. Comparison on thermal conductivity and permeability of granular and consolidated activated carbon for refrigeration. Chin. J. Chem. Eng. 2013, 21, 676–682. [Google Scholar]
- Dawoud, B.; Sohel, M.I.; Freni, A.; Vasta, S.; Restuccia, G. On the effective thermal conductivity of wetted zeolite under the working conditions of an adsorption chiller. Appl. Therm. Eng. 2011, 31, 2241–2246. [Google Scholar] [CrossRef][Green Version]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sharmin, E.; Zafar, F. Introductory Chapter: Metal Organic Frameworks (MOFs). In Metal-Organic Frameworks; IntechOpen: London, UK, 2016. [Google Scholar]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.O.; Hupp, J.T. Metal–organic framework materials with ultrahigh surface areas: Is the sky the limit. J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yaghi, O.M. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serre, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66 (Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Wang, Z.; Cohen, S.M. Systematic functionalization of a metal− organic framework via a postsynthetic modification approach. J. Am. Chem. Soc. 2008, 130, 8508–8517. [Google Scholar] [CrossRef]
- Kim, M.; Cahill, J.F.; Su, Y.; Prather, K.A.; Cohen, S.M. Postsynthetic ligand exchange as a route to functionalization of ‘inert’metal–organic frameworks. Chem. Sci. 2012, 3, 126–130. [Google Scholar] [CrossRef]
- Goesten, M.G.; Juanalcaniz, J.; Ramosfernandez, E.V.; Gupta, K.B.S.S.; Stavitski, E.; van Bekkum, H.; Gascon, J.; Kapteijn, F. Sulfation of metal–organic frameworks: Opportunities for acid catalysis and proton conductivity. J. Catal. 2011, 281, 177–187. [Google Scholar] [CrossRef]
- Murray, L.J.; Dincă, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef]
- Choi, H.J.; Dincă, M.; Dailly, A.; Long, J.R. Hydrogen storage in water-stable metal–organic frameworks incorporating 1, 3-and 1, 4-benzenedipyrazolate. Energy Environ. Sci. 2010, 3, 117–123. [Google Scholar] [CrossRef]
- Gücüyener, C.; van den Bergh, J.; Gascon, J.; Kapteijn, F. Ethane/ethene separation turned on its head: Selective ethane adsorption on the metal− organic Framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 2010, 132, 17704–17706. [Google Scholar] [CrossRef] [PubMed]
- van den Bergh, J.; Gücüyener, C.; Pidko, E.A.; Hensen, E.J.; Gascon, J.; Kapteijn, F. Understanding the Anomalous Alkane Selectivity of ZIF-7 in the Separation of Light Alkane/Alkene Mixtures. Chem. A Eur. J. 2011, 17, 8832–8840. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Couck, S.; Denayer, J.F.; Baron, G.V.; Rémy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal− organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Hernández-Alonso, M.D.; Almeida, A.R.; van Klink, G.P.; Kapteijn, F.; Mul, G. Isoreticular MOFs as efficient photocatalysts with tunable band gap: An operando FTIR study of the photoinduced oxidation of propylene. ChemSusChem 2008, 1, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Aktay, U.; Hernandez-Alonso, M.D.; van Klink, G.P.; Kapteijn, F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal. 2009, 261, 75–87. [Google Scholar] [CrossRef]
- Hasegawa, S.; Horike, S.; Matsuda, R.; Furukawa, S.; Mochizuki, K.; Kinoshita, Y.; Kitagawa, S. Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: Selective sorption and catalysis. J. Am. Chem. Soc. 2007, 129, 2607–2614. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Jenks, J.J.; Motkuri, R.K.; TeGrotenhuis, W.; Paul, B.K.; McGrail, B.P. Simulation and experimental study of metal organic frameworks used in adsorption cooling. Heat Transf. Eng. 2017, 38, 1305–1315. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schmidt, F.P.; Henning, H.M. Water adsorption characteristics of novel materials for heat transformation applications. Appl. Therm. Eng. 2010, 30, 1692–1702. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal–organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Ul Qadir, N.; Said, S.A.; Bahaidarah, H.M. Structural stability of metal organic frameworks in aqueous media–Controlling factors and methods to improve hydrostability and hydrothermal cyclic stability. Microporous Mesoporous Mater. 2015, 201, 61–90. [Google Scholar] [CrossRef]
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual high throughput screening confirmed experimentally: Porous coordination polymer hydration. J. Am. Chem. Soc. 2009, 131, 15834–15842. [Google Scholar] [CrossRef]
- Critoph, R.E.; Zhong, Y. Review of trends in solid sorption refrigeration and heat pumping technology. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2005, 219, 285–300. [Google Scholar] [CrossRef]
- de Lange, M.F.; van Velzen, B.L.; Ottevanger, C.P.; Verouden, K.J.; Lin, L.C.; Vlugt, T.J.; Kapteijn, F. Metal–Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef] [PubMed]
- Motkuri, R.K.; Annapureddy, H.V.; Vijaykumar, M.; Schaef, H.T.; Martin, P.F.; McGrail, B.P.; Thallapally, P.K. Fluorocarbon adsorption in hierarchical porous frameworks. Nat. Commun. 2014, 5, 4368. [Google Scholar] [CrossRef]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Schossig, P.; Henning, H.M. Novel sorption materials for solar heating and cooling. Energy Procedia 2012, 30, 279–288. [Google Scholar] [CrossRef]
- Mahzoon, S.; Fatemi, S.; Hashemi, S.J. Modeling Based Investigation of Ultrafine SAPO-34 Core-shell Adsorbent in Cyclic Adsorption Process for Purification of Natural Gas from CO2. J. ISSN 2012, 1929, 1248. [Google Scholar] [CrossRef]
- Chen, H.J.; Cui, Q.; Li, Q.G.; Zheng, K.; Yao, H.Q. Adsorption-desorption characteristics of water on silico-alumino-phosphate-34 molecular sieve for cooling and air conditioning. J. Renew. Sustain. Energy 2013, 5, 053129. [Google Scholar] [CrossRef]
- Ng, E.P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Zheng, X.; Ge, T.S.; Wang, R.Z. Recent progress on desiccant materials for solid desiccant cooling systems. Energy 2014, 74, 280–294. [Google Scholar] [CrossRef]
- Aristov, Y.I. Novel materials for adsorptive heat pumping and storage: Screening and nanotailoring of sorption properties. J. Chem. Eng. Jpn. 2007, 40, 1242–1251. [Google Scholar] [CrossRef]
- Castillo, J.M.; Vlugt, T.J.; Calero, S. Understanding Water Adsorption in Cu− BTC Metal− Organic Frameworks. J. Phys. Chem. C 2008, 112, 15934–15939. [Google Scholar] [CrossRef]
- Gul-E-Noor, F.; Jee, B.; Pöppl, A.; Hartmann, M.; Himsl, D.; Bertmer, M. Effects of varying water adsorption on a Cu 3 (BTC) 2 metal–organic framework (MOF) as studied by 1 H and 13C solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 7783–7788. [Google Scholar] [CrossRef] [PubMed]
- Gul-E-Noor, F.; Michel, D.; Krautscheid, H.; Haase, J.; Bertmer, M. Time dependent water uptake in Cu 3 (btc) 2 MOF: Identification of different water adsorption states by 1 H MAS NMR. Microporous Mesoporous Mater. 2013, 180, 8–13. [Google Scholar] [CrossRef]
- Wang, Q.M.; Shen, D.; Bülow, M.; Lau, M.L.; Deng, S.; Fitch, F.R.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Ehrenmann, J.; Henninger, S.K.; Janiak, C. Water Adsorption Characteristics of MIL-101 for Heat-Transformation Applications of MOFs. Eur. J. Inorg. Chem. 2011, 2011, 471–474. [Google Scholar] [CrossRef]
- Dietzel, P.D.; Johnsen, R.E.; Blom, R.; Fjellvåg, H. Structural changes and coordinatively unsaturated metal atoms on dehydration of honeycomb analogous microporous metal–organic frameworks. Chem. A Eur. J. 2008, 14, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, P.D.; Panella, B.; Hirscher, M.; Blom, R.; Fjellvåg, H. Hydrogen adsorption in a nickel based coordination polymer with open metal sites in the cylindrical cavities of the desolvated framework. Chem. Commun. 2006, 956–961. [Google Scholar] [CrossRef]
- Glover, T.G.; Peterson, G.W.; Schindler, B.J.; Britt, D.; Yaghi, O. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Bareschino, P.; Diglio, G.; Pepe, F.; Angrisani, G.; Roselli, C.; Sasso, M. Numerical Study of a MIL101 Metal Organic Framework Based Desiccant Cooling System for Air Conditioning Applications. Appl. Therm. Eng. 2017, 124, 641–651. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of metal-organic frameworks by water adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Khutia, A.; Rammelberg, H.U.; Schmidt, T.; Henninger, S.; Janiak, C. Water sorption cycle measurements on functionalized MIL-101Cr for heat transformation application. Chem. Mater. 2013, 25, 790–798. [Google Scholar] [CrossRef]
- Yan, J.; Yu, Y.; Ma, C.; Xiao, J.; Xia, Q.; Li, Y.; Li, Z. Adsorption isotherms and kinetics of water vapor on novel adsorbents MIL-101 (Cr)@ GO with super-high capacity. Appl. Therm. Eng. 2015, 84, 118–125. [Google Scholar] [CrossRef]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as adsorbents for low temperature heating and cooling applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef] [PubMed]
- Karra, J.R.; Grabicka, B.E.; Huang, Y.G.; Walton, K.S. Adsorption study of CO2, CH4, N2, and H2O on an interwoven copper carboxylate metal–organic framework (MOF-14). J. Colloid Interface Sci. 2013, 392, 331–336. [Google Scholar] [CrossRef]
- Kondo, A.; Daimaru, T.; Noguchi, H.; Ohba, T.; Kaneko, K.; Kanoh, H. Adsorption of water on three-dimensional pillared-layer metal organic frameworks. J. Colloid Interface Sci. 2007, 314, 422–426. [Google Scholar] [CrossRef]
- Lincke, J.; Lassig, D.; Moellmer, J.; Reichenbach, C.; Puls, A.; Moeller, A.; Glaser, R.; Kalies, G.; Staudt, R.; Krautscheid, H. A novel copper-based MOF material: Synthesis, characterization and adsorption studies. Microporous Mesoporous Mater. 2011, 142, 62–69. [Google Scholar] [CrossRef]
- Furukawa, H.; Gaándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Tannert, N.; Ernst, S.J.; Jansen, C.; Bart, H.J.; Henninger, S.K.; Janiak, C. Evaluation of the highly stable metal–organic framework MIL-53 (Al)-TDC (TDC= 2, 5-thiophenedicarboxylate) as a new and promising adsorbent for heat transformation applications. J. Mater. Chem. A 2018, 6, 17706–17712. [Google Scholar] [CrossRef]
- Cui, S.; Qin, M.; Marandi, A.; Steggles, V.; Wang, S.; Feng, X.; Nouar, F.; Serre, C. Metal-Organic Frameworks as advanced moisture sorbents for energy-efficient high temperature cooling. Sci. Rep. 2018, 8, 15284. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P.; Nuhnen, A.; Lange, M.; Möllmer, J.; Weingart, O.; Janiak, C. Metal–Organic Frameworks with Potential Application for SO2 Separation and Flue Gas Desulfurization. ACS Appl. Mater. Interfaces 2019, 11, 17350–17358. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Liang, H.; Yang, W.; Liu, J.; Liu, Z.; Qiao, Z. Machine learning and in silico discovery of metal-organic frameworks: Methanol as a working fluid in adsorption-driven heat pumps and chillers. Chem. Eng. Sci. 2020, 214, 115430. [Google Scholar] [CrossRef]
- Wu, X.; Peng, L.; Xiang, S.; Cai, W. Computational design of tetrazolate-based metal–organic frameworks for CH 4 storage. Phys. Chem. Chem. Phys. 2018, 20, 30150–30158. [Google Scholar] [CrossRef]
- Li, S.; Chung, Y.G.; Snurr, R.Q. High-throughput screening of metal–organic frameworks for CO2 capture in the presence of water. Langmuir 2016, 32, 10368–10376. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Cao, M.; Li, S. Structure–property relationship of metal–organic frameworks for alcohol-based adsorption-driven heat pumps via high-throughput computational screening. J. Mater. Chem. A 2019, 7, 7470–7479. [Google Scholar] [CrossRef]
- Erdös, M.; de Lange, M.F.; Kapteijn, F.; Moultos, O.A.; Vlugt, T.J. In Silico Screening of Metal–Organic Frameworks for Adsorption-Driven Heat Pumps and Chillers. ACS Appl. Mater. Interfaces 2018, 10, 27074–27087. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Farha, O.K.; Snurr, R.Q. High Propane and Isobutane Adsorption Cooling Capacities in Zirconium-Based Metal–Organic Frameworks Predicted by Molecular Simulations. ACS Sustain. Chem. Eng. 2019, 7, 18242–18246. [Google Scholar] [CrossRef]
- Xia, X.; Liu, Z.; Li, S. Adsorption Characteristics and Cooling/Heating Performance of COF-5. Appl. Therm. Eng. 2020, 176, 115442. [Google Scholar] [CrossRef]
- Xia, X.; Cao, M.; Liu, Z.; Li, W.; Li, S. Elucidation of adsorption cooling characteristics of Zr-MOFs: Effects of structure property and working fluids. Chem. Eng. Sci. 2019, 204, 48–58. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Carboxylic-acid-functionalized UiO-66-NH2: A promising adsorbent for both aqueous-and non-aqueous-phase adsorptions. Chem. Eng. J. 2018, 331, 124–131. [Google Scholar] [CrossRef]
- Rezk, A.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A. Characterisation of metal organic frameworks for adsorption cooling. Int. J. Heat Mass Transf. 2012, 55, 7366–7374. [Google Scholar] [CrossRef]
- Elsayed, E.; Raya, A.D.; Mahmoud, S.; Elsayed, A.; Anderson, P.A. Aluminium fumarate and CPO-27 (Ni) MOFs: Characterization and thermodynamic analysis for adsorption heat pump applications. Appl. Therm. Eng. 2016, 99, 802–812. [Google Scholar] [CrossRef]
- Elsayed, A.; Elsayed, E.; Raya, A.D.; Mahmoud, S.; Elshaer, A.; Kaialy, W. Thermal energy storage using metal–organic framework materials. Appl. Energy 2017, 186, 509–519. [Google Scholar] [CrossRef]
- Youssef, P.G.; Dakkama, H.; Mahmoud, S.M.; AL-Dadah, R.K. Experimental investigation of adsorption water desalination/cooling system using CPO-27Ni MOF. Desalination 2017, 404, 192–199. [Google Scholar] [CrossRef]
- Dakkama, H.J.; Youssef, P.G.; Al-Dadah, R.K.; Mahmoud, S. Adsorption ice making and water desalination system using metal organic frameworks/water pair. Energy Convers. Manag. 2017, 142, 53–61. [Google Scholar] [CrossRef]
- Elsayed, A.; Al-Dadah, R.; Mahmoud, S.; Elshaer, A.; Bowen, J. Investigation of Efficient Adsorbents Using Transient Analysis of Adsorption Chillers with Simulink. In Proceedings of the International Conference on Heat Transfer and Fluid Flow, Prague, Czech Republic, 11−12 August 2014. [Google Scholar]
- Nunez, T.; Henning, H.M.; Mittelbach, W. Adsorption Cycle Modeling: Characterization and Comparison of Materials. In Proceedings of the International Sorption Heat Pump Conference, Munich, Germany, 24–26 March 1999; ZAE Bayern: Garching, Germany. [Google Scholar]
- Green, D.W.; Perry, R.H. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Mu, B.; Walton, K.S. Thermal analysis and heat capacity study of metal–organic frameworks. J. Phys. Chem. C 2011, 115, 22748–22754. [Google Scholar] [CrossRef]
- Song, L.F.; Jiao, C.L.; Jiang, C.H.; Zhang, J.; Sun, L.X.; Xu, F.; Cao, Z. Heat capacities and thermodynamic properties of MgNDC. J. Therm. Anal. Calorim. 2011, 103, 365–372. [Google Scholar] [CrossRef]
- Demirocak, D.E.; Kabir, M.M. Performance of a Solar Thermal Adsorption Cooling System Based on Metal Organic Frameworks in Texas. In Proceedings of the InASME 2016 10th International Conference on Energy Sustainability, American Society of Mechanical Engineers, Charlotte, NC, USA, 26–30 June 2016. [Google Scholar]
- Said, S.A.M.; Ahmed, S.; Qadir, N.U. Metal Organic Framework Adsorbent for Solar Adsorption Refrigeration. U.S. Patent No. 9,480,967, 1 November 2016. [Google Scholar]
- Markus, S.; Mueller, U.; Kiener, C. Porous Metal Organic Frameworks as Desiccants. U.S. Patent Application 12/863,339, 23 January 2009. [Google Scholar]
- Shi, B. Development of an MOF Based Adsorption Air Conditioning System for Automotive Application. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2015. [Google Scholar]

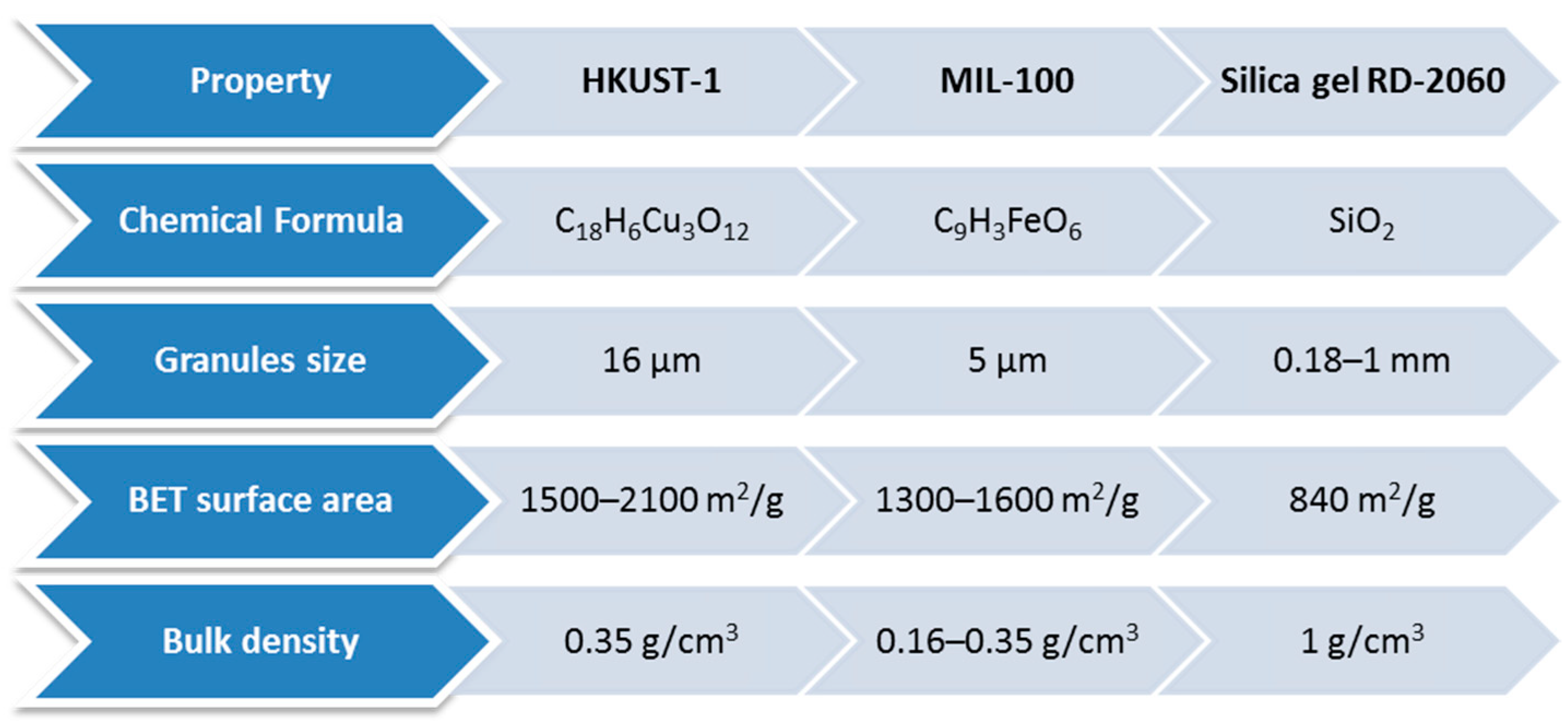
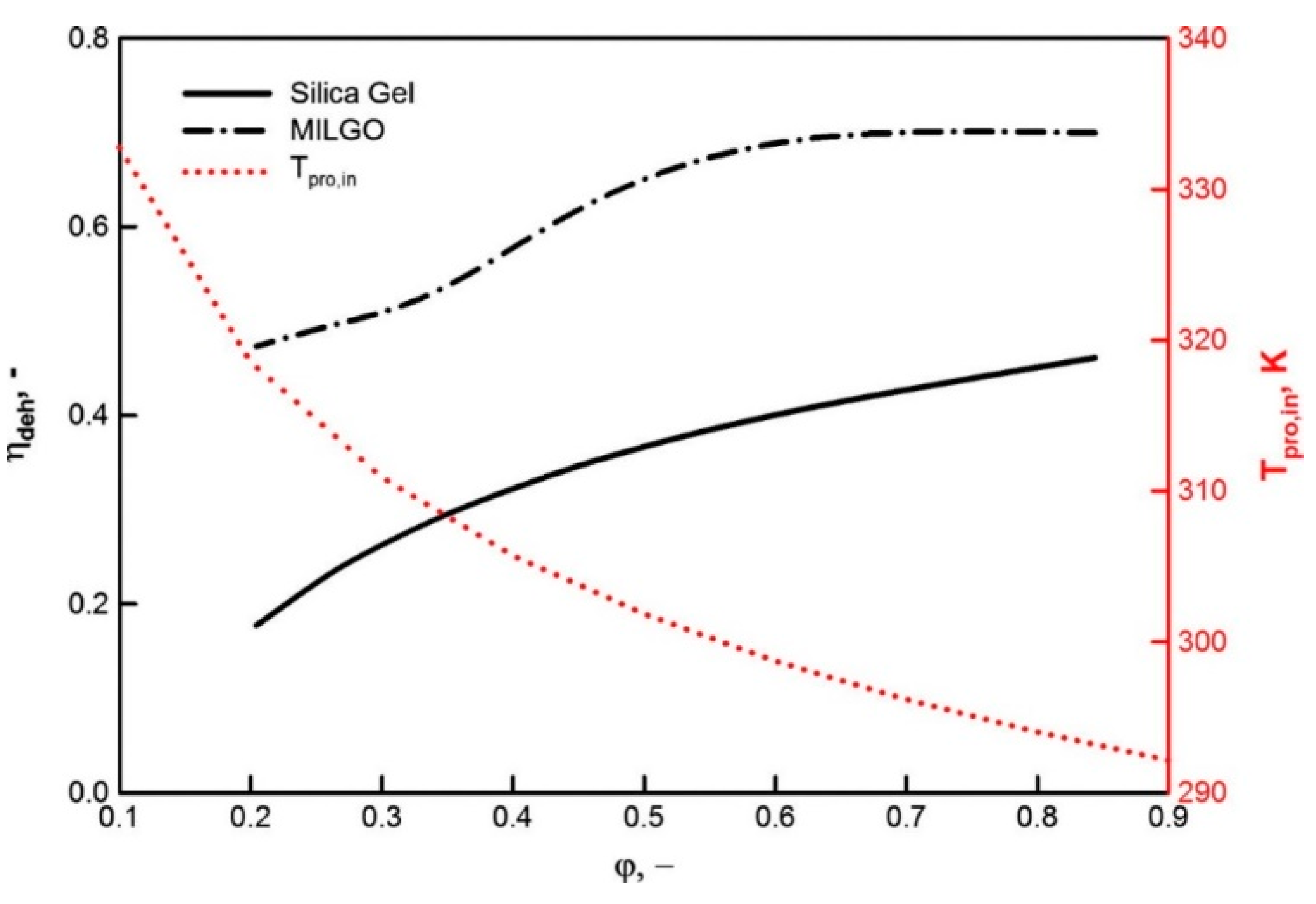
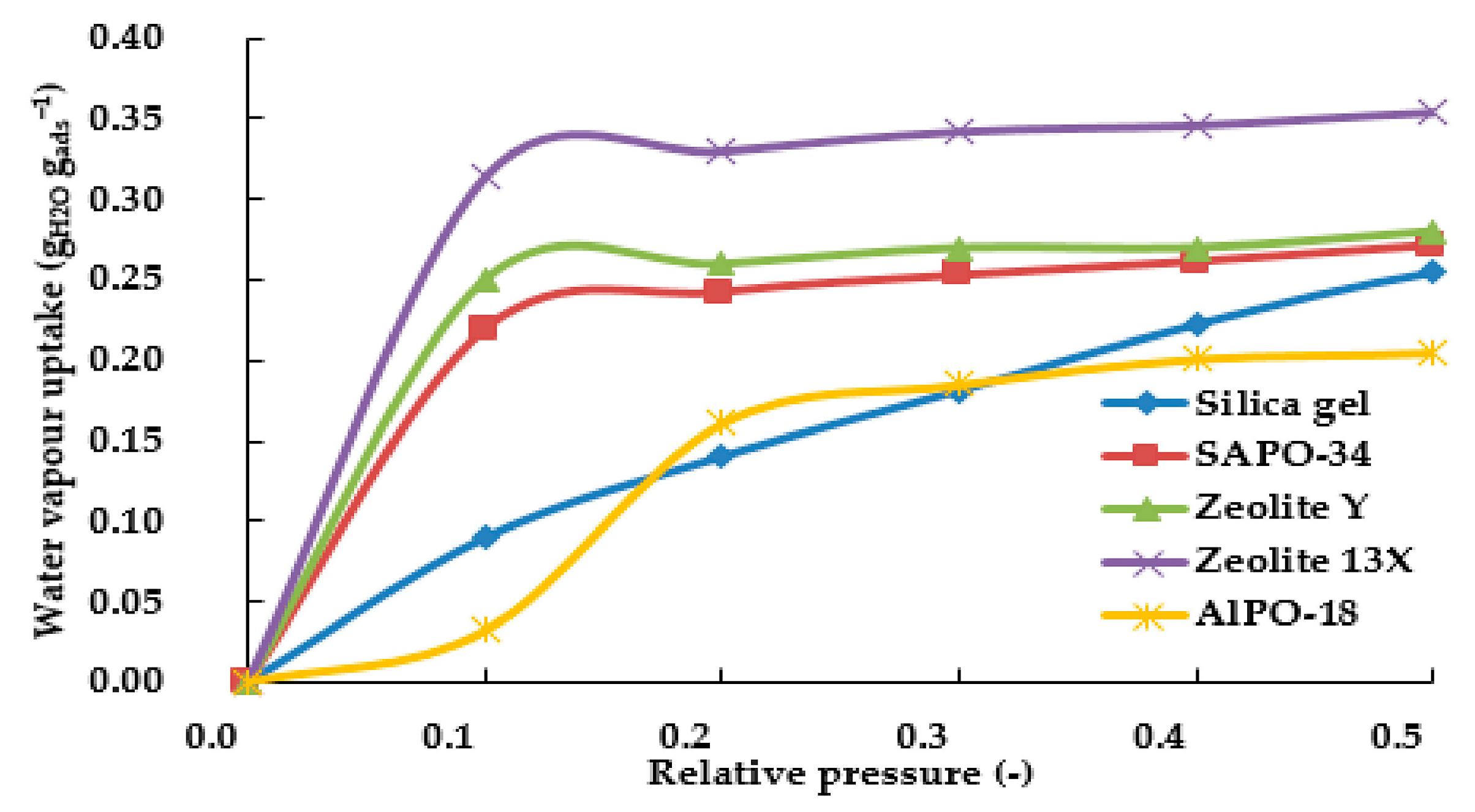
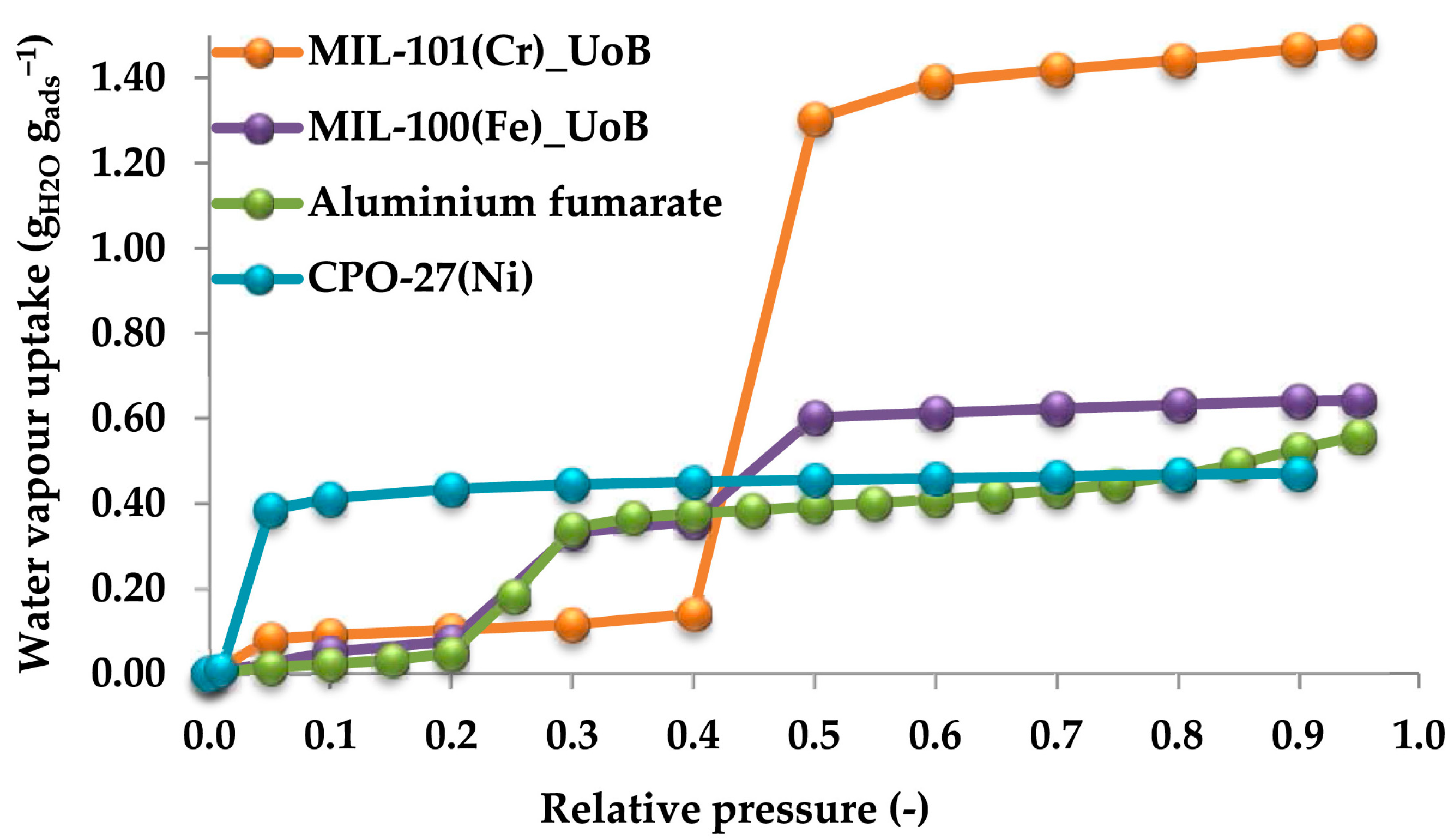
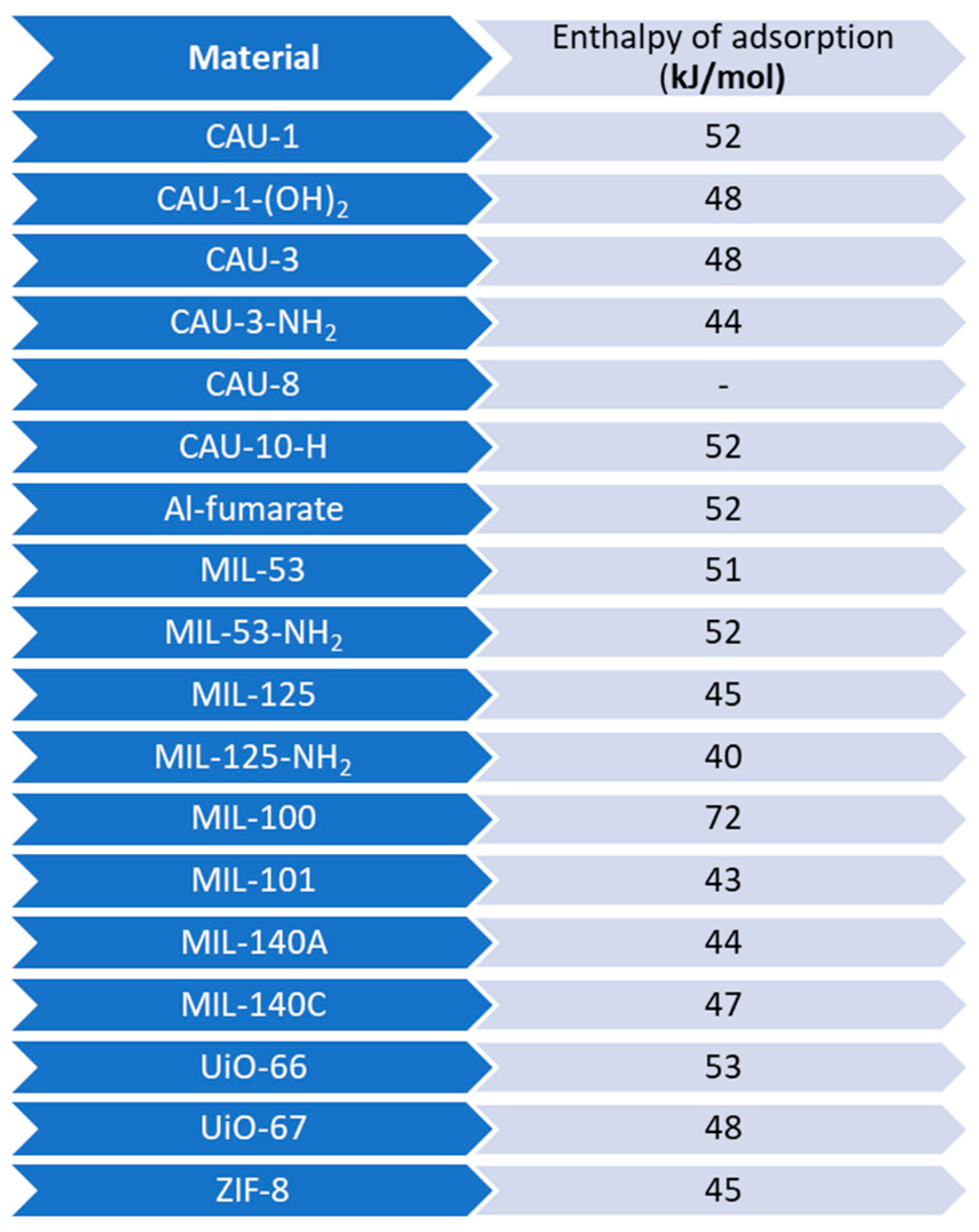
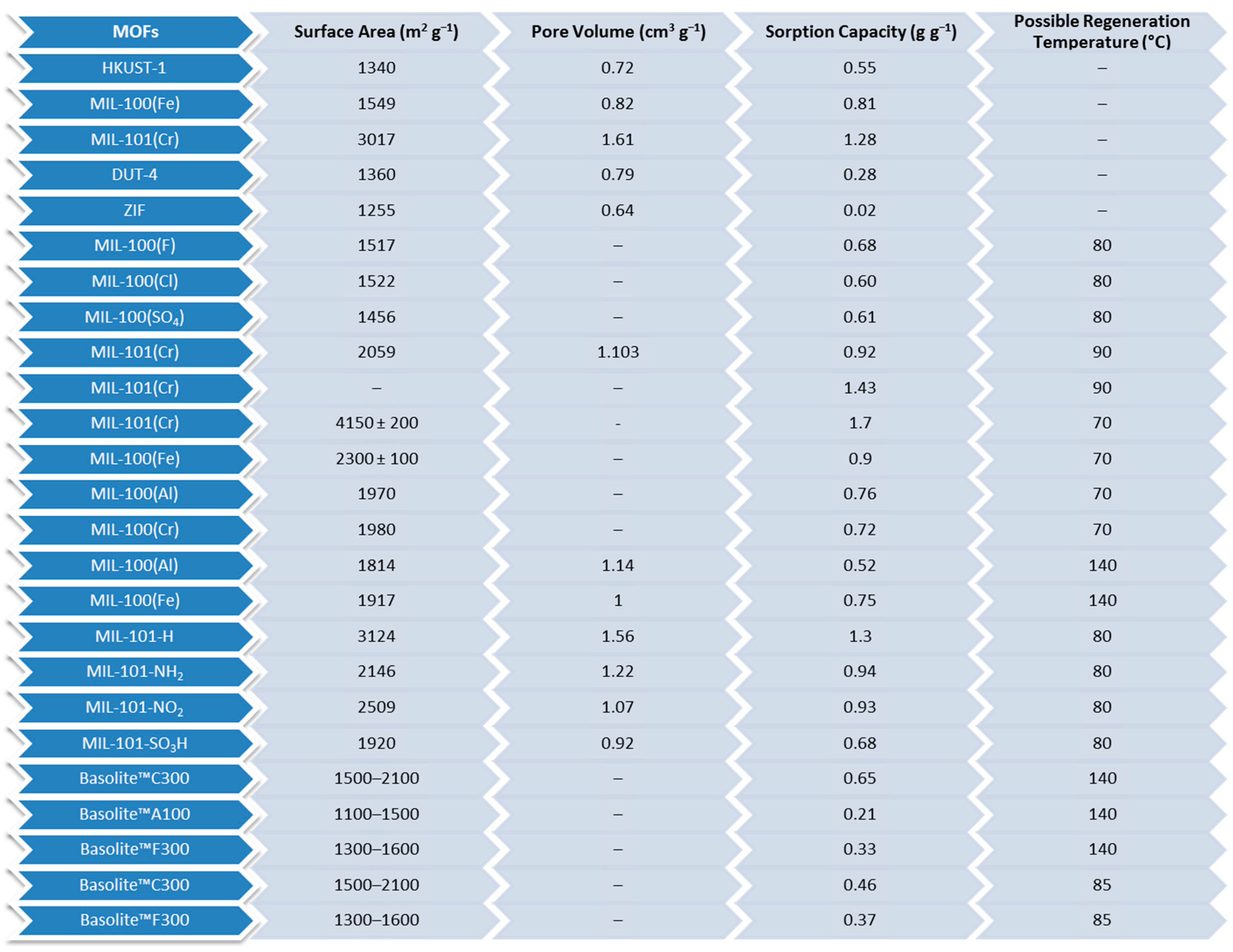
| Adsorbent | Properties | Potential Applications |
|---|---|---|
| Activated Carbon | Surface area: 500–1500 m2/g Porosity: 0.3–1.5 cm3/g | Organic removal Separation of air Purification of gases |
| Activated Alumina | Surface area: 300–400 m2/g Porosity: 0.25 cm3/g | Air separation Gas purification |
| Silica Gel | Surface area: 100–1000 m2/g Porosity: 0.4–1.2 cm3/g | Desiccant HVAC systems Humidity control of air |
| Molecular Sieves (Zeolites) | Surface area: 2800–3500 m2/g Porosity: 1.4–2 cm3/g | Deodorization of air Gas masks Wastewater purification Storage material |
| Name of MOF | Water Uptake Capacity | Remarks |
|---|---|---|
| CU-BTC | 6% by weight 0.324 kgref/kgads | Not stable |
| MIL-101Cr | 1.01 kgref/kgads 0.3 kgref/kgads | Stable |
| CPO-27 | 0.1 to 0.5 kgref/kgads | - |
| Working Pair | Working Pressure | Working Temperature (°C) | Uptake Capacity (kgref/kgads) | Is Refrigerant Thermally Stable? | Is Refrigerant Toxic? | Is Refrigerant Flammable? |
|---|---|---|---|---|---|---|
| Activated carbon-NH3 | Positive | 80 to 200 | 0.29 | Yes | Yes | Yes |
| Activated carbon-Methanol | Vacuum | 80 to 100 | 0.45 | No | Yes | Yes |
| Activated carbon-Ethanol | Vacuum | 80 to 120 | 0.19 | Yes | Yes | Yes |
| Silica gel-H2O | Vacuum | 50 to 120 | 0.30 | Yes | No | No |
| Zeolite-H2O | Vacuum | 200 to 300 | 0.17 | Yes | No | No |
| SAPO 34-H2O | Vacuum | 80 to 90 | 0.29 | Yes | No | No |
| MIL 101(Cr)-H2O | Vacuum | 90 to 140 | 1 | Yes | No | No |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafique, M.M. Evaluation of Metal–Organic Frameworks as Potential Adsorbents for Solar Cooling Applications. Appl. Syst. Innov. 2020, 3, 26. https://doi.org/10.3390/asi3020026
Rafique MM. Evaluation of Metal–Organic Frameworks as Potential Adsorbents for Solar Cooling Applications. Applied System Innovation. 2020; 3(2):26. https://doi.org/10.3390/asi3020026
Chicago/Turabian StyleRafique, Muhammad Mujahid. 2020. "Evaluation of Metal–Organic Frameworks as Potential Adsorbents for Solar Cooling Applications" Applied System Innovation 3, no. 2: 26. https://doi.org/10.3390/asi3020026
APA StyleRafique, M. M. (2020). Evaluation of Metal–Organic Frameworks as Potential Adsorbents for Solar Cooling Applications. Applied System Innovation, 3(2), 26. https://doi.org/10.3390/asi3020026





