1. Introduction
In a bid to reduce the mismatch between the energy supply and demand created by the integration of renewable energy sources, urbanisation, labour productivity and industrialisation, research and development of technologies with steadier, efficient and stable energy are gaining traction [
1,
2]. Among these, latent heat thermal energy storage systems are proving to be amongst the most compact means of storing energy for either immediate or future usage in heating and cooling in commercial and residential buildings [
3,
4]. The principle of latent heat storage revolves around the storage media themselves, phase change materials (PCMs), which store thermal energy in the form of latent heat, above their phase transition temperature, which is subsequently released via a reverse-phase transformation [
5,
6]. Since the phase change process is isothermal, they are promising materials for mitigating daily variations in solar energy, peak shifting and increasing end-user self-sufficiency of heat pump and photovoltaic systems [
7,
8,
9].
Salt hydrates are a highly promising class of PCMs due to their low cost, large volumetric storage capacity, low flammability and wide availability [
6,
9,
10]. Despite this, many salt hydrates suffer from phase separation due to incongruent melting after successive melt–freeze cycles [
11,
12] and supercooling, a phenomenon by which a material crystallises much lower than its melting point [
3,
8]. Phase separation renders salt hydrates inept after a few thermal cycles, as when segregation occurs [
13,
14], it is an irreversible process that drastically reduces the storage capacity. Developing effective methods to address this problem has remained an elusive goal. Several methods have been adopted to mitigate segregation in salt hydrates, such as mechanical agitation [
15], eutectic mixtures [
16,
17] and the addition of polymeric or gelling/thickening agents that slow down the segregation process by limiting the diffusion rate between the salt and water [
18,
19].
A salt hydrate of particular research interest is sodium sulphate decahydrate [
11,
20,
21], Glauber’s salt (Na
2SO
4·10H
2O). Not only it is low-cost and non-flammable with a high volumetric energy density (254 J/g), but it also has a melting temperature of 34 °C [
22], which is appealing for residential heating applications. However, Glauber’s salt suffers from severe phase separation and supercooling [
22,
23,
24]. Supercooling in Glauber’s salt can be reduced through the addition of sodium tetraborate in concentrations between 0.5 and 3 wt.% [
14,
15,
22,
25]. However, trying to stabilise the system, colloquially coined in the research area as “being stuck on Glauber’s salt Island”, has been a penchant of all research and researchers. Investigations into preventing phase separation have persisted since early investigations by Marks [
26] in the early 1980s, where different clays were used for thickening, and attapulgite clay showed the most promising behaviour, with 320 thermal cycles; however, a significant decrease in the enthalpy of fusion was found after the thermal cycles, from 202 J/g before cycling to 105 J/g after the 200th cycle. Gok and Paksoy [
27] encountered another issue whilst experimenting with 10% polyacrylamide gels: the addition of large amounts of gelling agents significantly reduced the enthalpy of fusion, with the most stable Glauber’s salt gel having an enthalpy of 113 J/g. This was also found in other thickening mechanisms. Liu et al. [
28] investigated the effect of 2 wt.% carboxyl methyl cellulose (CMC) and 5 wt.% octyl phenol polyoxyethylene ether (OP-10)-thickened Glauber’s salt, which had an initial enthalpy of fusion of 114 J/g. Zhang et al. [
29] showed that microencapsulated Glauber’s salt in a hydrophilic polyvinyl alcohol (PVA) shell achieved an initial enthalpy of fusion of 125.6 J/g, which decreased to 100.9 J/g after 100 cycles. From this discussion, it is evident that most of the literature on thickening agents of Glauber’s salt, and salt hydrates more broadly, is focused on stabilisation through the addition of thickeners to increase the viscosity of the salt hydrate solution, which does not prevent segregation but only slows it down. Therefore, a method to completely prevent phase separation is still required for the majority of salt hydrates.
In this work, it is hypothesised that if the characteristic distance over which sodium sulphate and water can diffuse within Glauber’s salt (the diffusion length, d) is reduced below a critical value, the segregation phenomenon can be completely prevented. Exploiting this, by trapping salt hydrates in a phase change dispersion through emulsification [
30,
31,
32], the cycling stability of salt hydrates is expected to be improved. Herein, we introduce a novel phase change dispersion (PCD), where Glauber’s salt droplets, as the dispersed phase, are suspended in a solid palmitic acid continuous phase matrix, to allow Glauber’s salt to be used reliably as a phase change material. This work represents the first proof of principle for stabilising Glauber’s salt, as a salt hydrate for thermal energy storage. This achievement is realised by minimising the diffusion length of the phase segregation pathway through the dispersion of Glauber’s salt within a continuous phase of palmitic acid, resulting in the formation of a phase change dispersion. This novel stabilisation mechanism offers unprecedented opportunities in the utilisation of phase change materials, which are currently unusable due to severe segregation issues.
2. Materials and Methods
Sodium sulphate decahydrate (Glauber’s salt; >99% purity) was purchased from Sigma Aldrich (St. Louis, MO, USA). Palmitic acid (>99% purity) was purchased from Sigma Aldrich, and sorbitan monooleate (molecular biology grade) was purchased from Sigma-Aldrich.
2.1. Characterisation
and Property Measurement
For determining the quality of the phase change dispersion, the non-destructive X-ray CT method was employed to visualise the internal structure of the whole sample. In situ static XCT tomographic experiments were performed on a Diondo d2 X-ray CT system (Luci) (Diondo, Hattingen, Germany). The measurements were conducted with the source XWT-225 TCHE+ with an acceleration voltage of 160 kV and a filament current of 188 ua. The phase change dispersion was placed on the rotatory stage inside the XCT device at ambient temperature, where it was rotated in time-step rotation mode. The projections were recorded with an X-ray detector, 4343 DX-I (Varex, Salt Lake City, UT, USA), with a pixel size of 150 μm. The projections were subsequently converted into a 3D image stack with virtual cross-sections of 352X352X346 voxels using CERA (Siemens, Healthineers, Erlangen, Germany) reconstruction software based on the filtered back projection Feldkamp–Davis–Kress algorithm. DSC measurements were performed using the DSC instrument 823e by METTLER TOLEDO (Columbus, OH, USA) between 5 and 50 °C at both 2 and 10 K min −1 heating rates. The DSC instrument had been previously calibrated with an indium standard before the measurements; the resolution of the temperature was 0.1 K, and the resolution of the melting enthalpy was 1%. Typical samples masses were between 8–10 mg ± 0.0005 mg. The melting temperatures were calculated using STARe software (Version 10.0) by METTLER TOLEDO through the tangent method, and the enthalpies of fusion were calculated through integration of the melting peak using the 0-line integration method. For analysis of the total storage capacity, melting enthalpy and sensible energy contributions, 3-layer calorimetry (3LC), by w&A Bad Saarow, Germany, was employed. The 3LC instrument was first calibrated with materials that have a known enthalpy, water and paraffin C16-99. The measurement method of 3LC is as follows: the temperature profile 5–50 °C was programmed into a standard climatic test cabinet inside which the 3LC instrument was placed, and heating rate of 0.3 K min −1 was applied to the samples, which were 100 ± 0.0005 g each. Cycling stability and degrees of supercooling were investigated with the EasyMax 102 (METTLER TOLEDO, Columbus, OH, USA). The resolution of the temperature measurements were 0.05 K, and 200 g ± 0.0005 g of the formulated material was cycled. The measuring procedure was as follows: the formulated Glauber’s salt phase change dispersion was constantly cycled for 100 cycles between 5 and 50 °C at a heating and cooling rate of 1 K min −1. The colloidal stability of the PCD under accelerated separation conditions was evaluated using a Lumisizer analytica centrifuge (LUM GmbH, Berlin, Germany). Six independent samples of the dispersion were loaded into 10 mm polycarbonate sample cells and centrifuged at 4000 rpm (equivalent to 2900× g) for 5 h at three temperatures, 20 °C, 35 °C and 60 °C. Transmission profiles were recorded over time along the sample cell length using a near-infrared light source (λ = 865 nm) and a charged couple device detector. The instability index, calculated automatically by the instrument software, expresses the degree of change in the integral transmission over the measurement period, scaled from 0 (no separation) to 1 (complete separation). These measurements allowed for the quantification of separation kinetics and temperature-dependent destabilisation phenomena.
2.2. Phase Change Dispersion Preparation
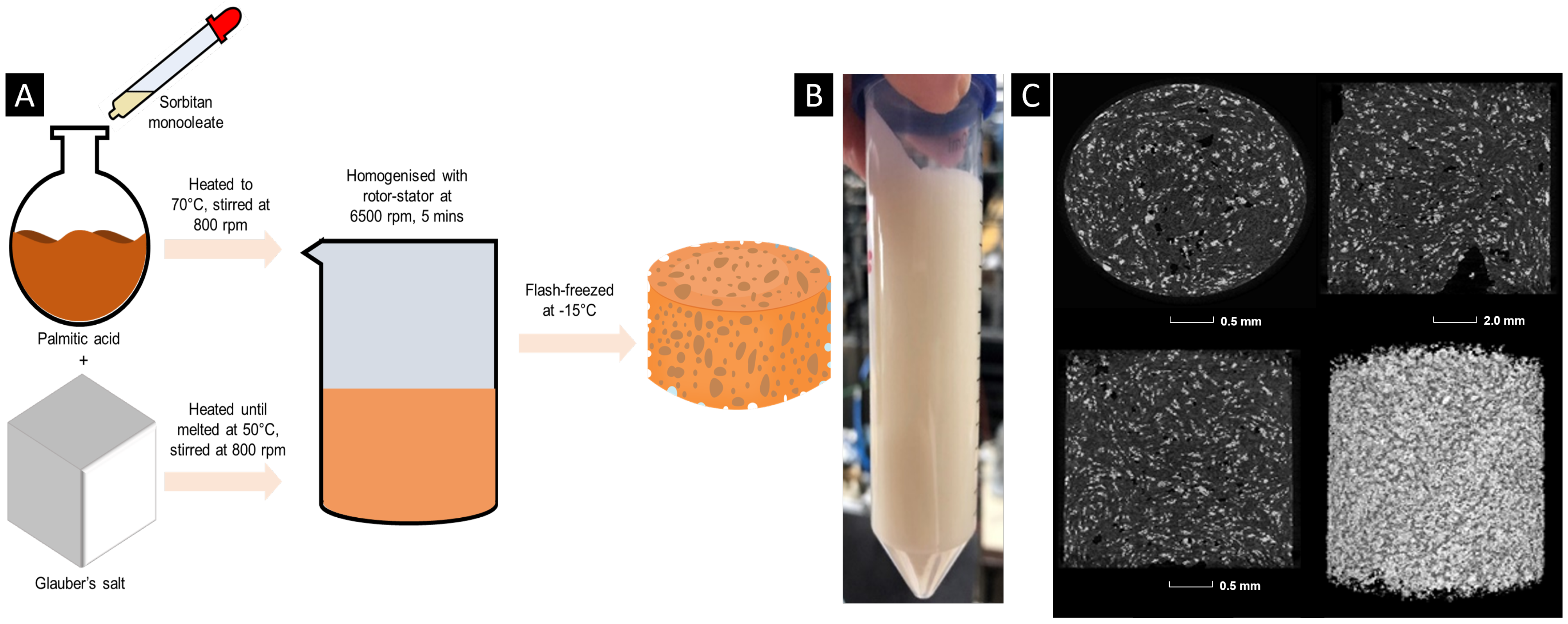
The phase change dispersion was prepared by heating Glauber’s salt and palmitic acid in a mass ratio of 85:15, respectively, in separate round-bottom flasks to 50 and 70 °C, respectively, under 800 rpm stirring. Once the palmitic acid and Glauber’s salt were fully melted, after 30 min and 1 h, respectively, and the temperature was homogeneous throughout the two samples, sorbitan monooleate (10% of the palmitic acid mass) was added to palmitic acid dropwise under stirring at 800 rpm. After 1 h, Glauber’s salt was mixed into the palmitic acid and sorbitan monooleate mixture under stirring at 500 rpm, with the heating still set to 70 °C. After all Glauber’s salt had been added in, the solution was left to stir until a homogeneously white solution appeared, for approximately 2 h. The Polytron 10-35 GT double-rimmed (outer diameter of 26 mm and inner diameter of 26.5 mm) laboratory rotor stator homogeniser, (KINEMATICA GmbH, Eschbach, Germany) was then inserted from the top of the round-bottom flask, and using a rotational speed of 6500 rpm, the phase change dispersion was dispersed for a total of 5 min whilst being kept at 70 °C. The phase change dispersion was subsequently flash-cooled in a commercial freezer overnight at −15 °C to allow the internal structure to set.
3. Results
3.1. Preparation of the Phase Change Dispersion
A phase change dispersion was prepared by dispersing droplets of Glauber’s salt, the dispersed phase, within palmitic acid, which acted as the continuous oil phase. To keep the Glauber’s salt droplets uniform and stable, a surfactant was required. For water-in-oil (W/O) emulsions, the optimal surfactant has a hydrophilic–lipophilic balance (HLB) between 3.5 and 6.0, favouring a more hydrophobic character. Sorbitan monooleate, with an HLB of 4.3, fits this requirement. Sorbitan monooleate is also non-toxic and biodegradable, making it suitable for environmentally responsible formulations.
In a mono-dispersed emulsion, the maximum achievable volume fraction of the dispersed phase is about 0.74, set by the close packing limit of spherical droplets. To maximise the mass fraction of Glauber’s salt—and therefore increase the overall phase change enthalpy—the continuous oil phase should have the lowest possible density. Palmitic acid was chosen because its relatively low density allows for a greater mass fraction of the salt hydrate within the 0.74 volume fraction limit while also being chemically compatible with the system and biodegradable.
The formation of the phase change dispersion is supported by the X-ray computer tomography (X-CT) images (
Figure 1C), whereby the lighter phase contrast demonstrates the denser Glauber’s salt and the darker phase contrast represents the less dense palmitic acid. It is deduced that the solid continuous phase of the phase change dispersion, palmitic acid, acts as a matrix providing shape stability to Glauber’s salt by preventing diffusion lengths that are larger than the critical value, which are typically in the order of magnitude of mm [
33]. Within the formulated phase change dispersion, the diffusion length is equal to the particle size of the emulsified droplets of Glauber’s salt, which, according to the X-CT images, is 0.01 mm. When diffusion lengths in salt hydrates are smaller than the critical value, phase segregation does not occur due to denser phases easily mixing back in with the bulk solution [
34].
3.2. Stability
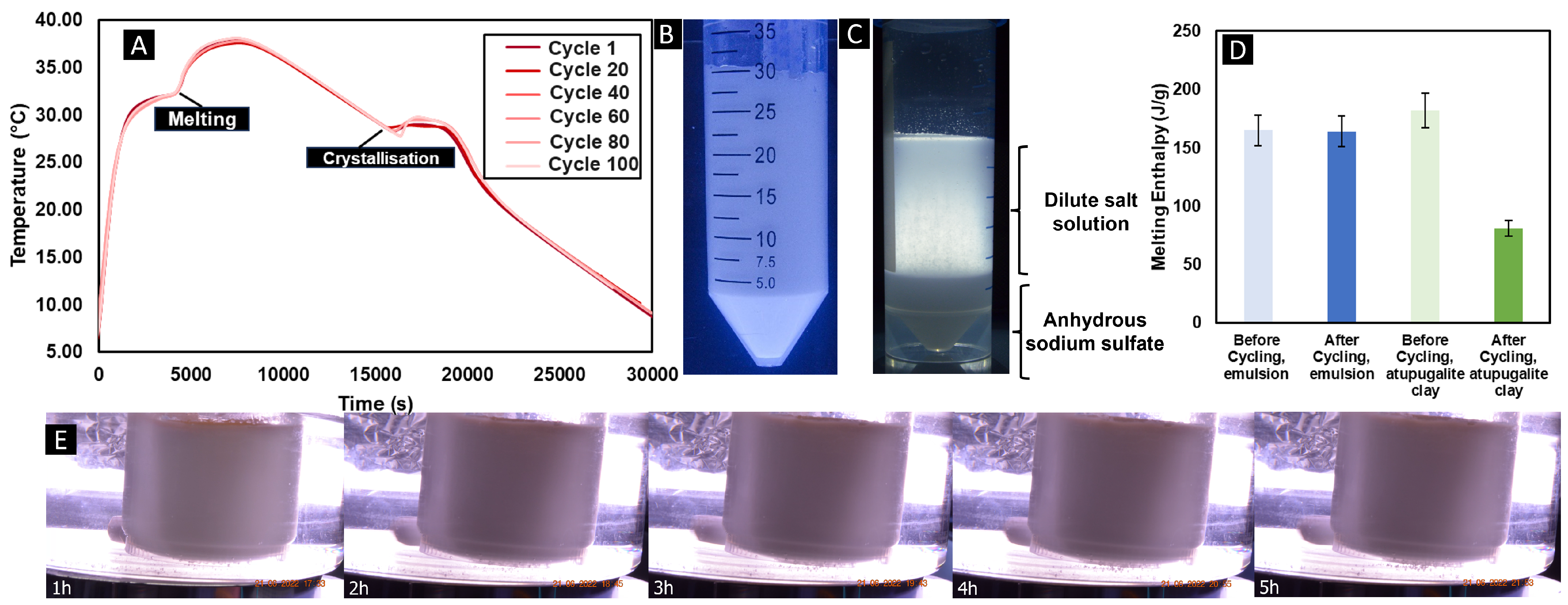
When diffusion lengths are smaller than the critical value, segregation does not occur and denser, lower-hydrate salt forms are easily mixed back in with the more dilute salt solutions through diffusion; therefore, no loss of capacity is expected with repeated thermal cycling. To verify this point, thermal cycling experiments, with optical access to monitor segregation, were performed with 50 mL of the phase change dispersion between 5 and 40 °C at a heating and cooling rate of 1 K min
−1. The phase change dispersion was subjected to 100 thermal cycles. While usually Glauber’s salt shows instability after 1 thermal cycle, the phase change dispersion showed no destabilisation or separation, as shown in
Figure 2A, where the overlay of every 20 cycles demonstrates no significant changes in the thermal profile over multiple cycles.
Figure 2B also confirms that no evident phase separation occurred, when compared with
Figure 2C, which demonstrates a reference sample of Glauber’s salt, thickened with attapulgite clay, a common method for thickening and stabilising Glauber’s salt.
Figure 2C clearly shows segregation between the anhydrous sodium sulphate and the dilute sodium sulphate decahydrate solution after 100 thermal cycles. The tests confirm the stabilisation effect of the phase change dispersion method of Glauber’s salt in a palmitic acid matrix. This conclusion could be presented more vividly when in addition, the enthalpy, as measured with a differential scanning calorimetry (DSC) instrument at 2 K min
−1, showed no reduction after those 100 cycles with the phase change dispersion (see
Figure 2D); however, the standard thickening method lost 45% of the enthalpy of fusion over the first 100 cycles.
An unexpected and interesting finding during the thermal cycling was the degree of supercooling observed in the Glauber’s salt droplets. Generally, Glauber’s salt suffers from large degrees of supercooling due to the significant and dense hydration shell in the sodium sulphate decahydrate molecules. This is particularly challenging with salts with higher hydration numbers, such as Glauber’s salt. Additionally, due to the stochastic nature of crystallisation, it was presumed that due to the smaller droplet sizes (critical volumes) of Glauber’s salt in the phase change dispersion, supercooling would be even more significant. However, the thermal cycling curves demonstrate that the supercooling is in fact reduced and therefore improved when using the phase change dispersion mechanism, demonstrating an average supercooling degree of 3 K over the 100 cycles, which can be put down to the dual action of, firstly, the templating effect of the extensive hydrogen bonding within the surfactant system. This ensures that the molecules of sodium sulphate decahydrate are well-aligned prior to crystallisation, thus reducing the Gibb’s energy barrier to nucleation. Secondly, solidified palmitic acid can act as a crystalline epitaxial nucleating agent, inducing nucleation in the typical nucleation agent theory mechanism. The addition of additional nucleating agents or surfactants that have denser hydrogen bond networks would be subject to future investigation and is further discussed in the Discussion Section of this article.
It is deduced that the phase change dispersion is stable against typical emulsion destabilisation mechanisms, such as creaming or coalescence, due to the nature of the solid continuous phase.en both phases were molten). Six independent PCD samples were analysed, and This was initially verified through a 6 h heated water bath test at 50 °C with continuous video monitoring, as shown in
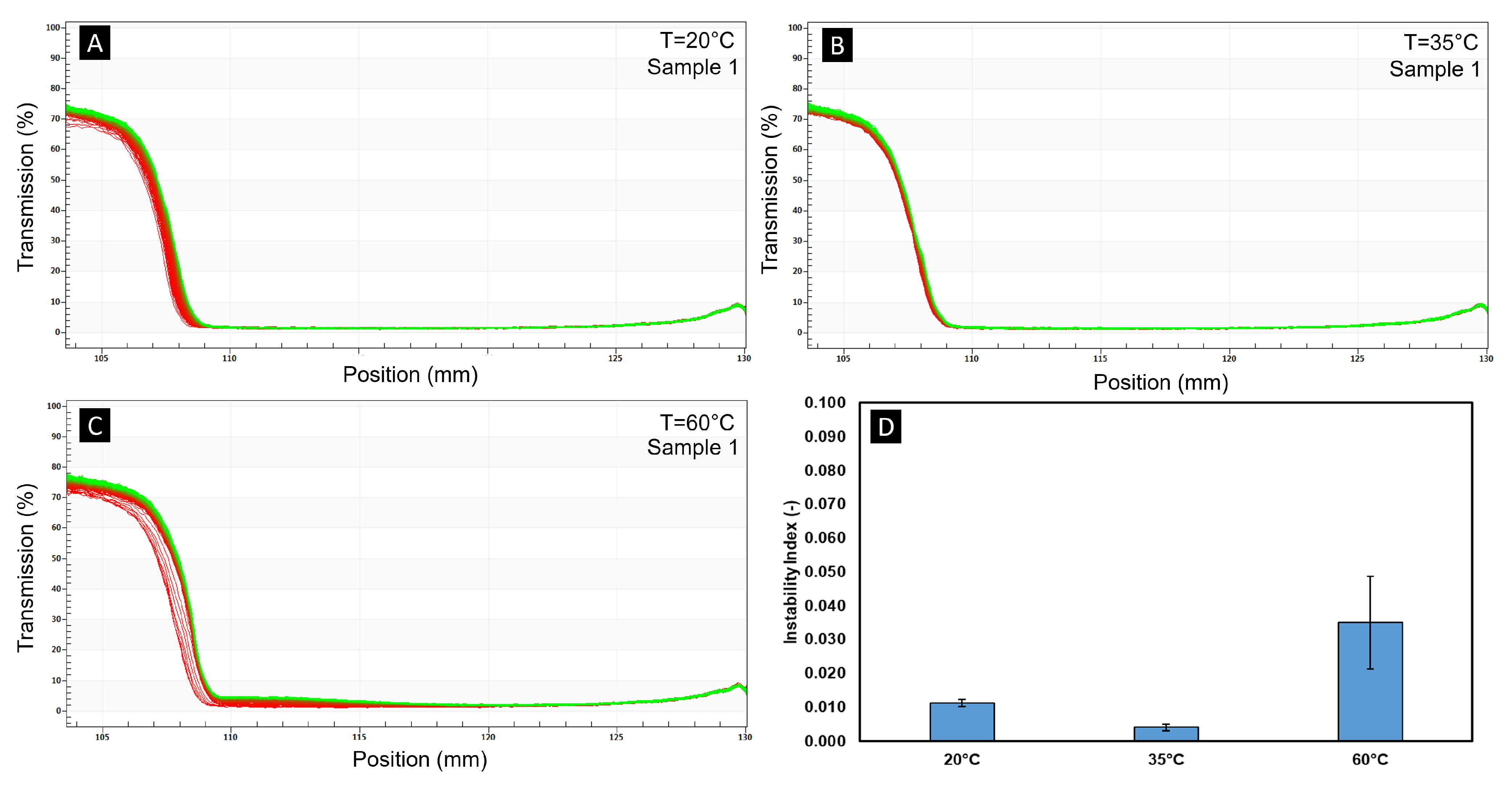
Figure 2E, which demonstrates no visual evidence of oil–water separation. To complement these macroscopic observations with a quantitative measure of stability, analytical centrifugation tests were conducted using a LumiSizer (LUM GmbH, Berlin, Germany) at 20 °C (when both phases of the PCD were fully solidified), at 35 °C (when Glauber’s salt was molten and palmitic acid solidified) and at 60 °C (
Figure 3 presents the transmission profiles for one representative sample (Sample 1), while the results for the remaining five samples are provided in the
Supplementary Information. At 20 °C and 35 °C, the transmission profiles exhibited a sharp and well-defined boundary between the supernatant (70–80% transmission) and the sediment (near-zero transmission), indicating minimal particle migration and high stability under centrifugal stress. At 60 °C, the boundary broadened slightly and the variability between scans increased, suggesting a slight acceleration of particle settling. The instability index, as shown in
Figure 3D, remained low for all temperatures; however, a slight increase was seen at 60 °C, and an increase in the standard deviation was also observed, potentially reflecting more variable separation kinetics. Despite this, the instability index remained >0.1 at all temperatures. These results confirm that the PCD remains stable under elevated shear and centrifugal acceleration in all the measured temperature ranges.
3.3. Thermal Properties
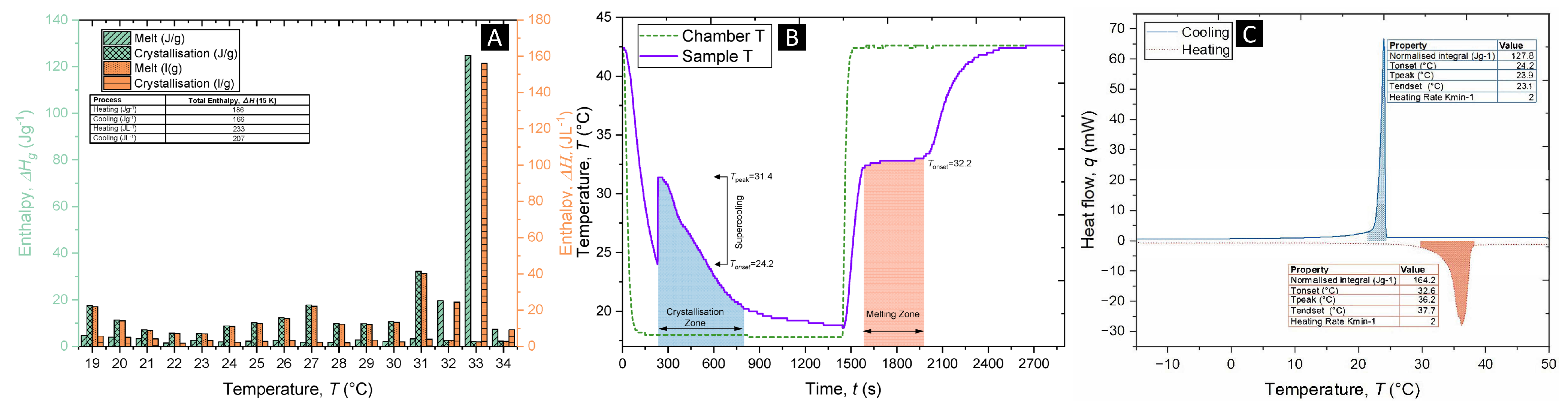
The phase change properties of the phase change dispersion were further characterised by DSC and three-layer calorimetry (3LC). The curves of the phase change dispersion from DSC, in
Figure 4C, show that the melting and crystallisation onset temperatures were 32.6 °C and 25.9 °C, respectively. The enthalpy of fusion was 164.2 J/g at a heating rate of 2 K min
−1; the reduction compared with pure Glauber’s salt (234 J/g) was attributed to the use of 20 wt.% palmitic acid as the continuous phase and 10 wt.% sorbitan monooleate as the surfactant, which do not contribute to the enthalpy of fusion.
Figure 4A shows the enthalpy collected from the 3LC, with
Figure 4B showing the corresponding thermal profile inside the 3LC instrument during measurement. The storage capacity of the phase change dispersion was measured to be 186 J/g or 233 J/L (when taking into consideration that the measured density of the phase change dispersion was 1.25 g/cm
3) in a 15 K temperature difference, from 19 to 34 °C. These values indicate a significant storage ability of the Glauber’s salt phase change dispersion and suggest promising usage of this material as a phase change material.
Figure 3.
(A) The transmission profile of Sample 1 of the PCD measured at 20 °C. The transmission profiles at (B) 35 °C and (C) 60 °C. (D) The average calculated instability index of 6 samples of the PCD at each temperature. The error bars are the associated standard deviations of each measurement.
Figure 3.
(A) The transmission profile of Sample 1 of the PCD measured at 20 °C. The transmission profiles at (B) 35 °C and (C) 60 °C. (D) The average calculated instability index of 6 samples of the PCD at each temperature. The error bars are the associated standard deviations of each measurement.
Interestingly, both the 3LC thermal profile and the DSC onset melting temperature show a melting-point depression of the Glauber’s salt phase change dispersion compared with pure Glauber’s salt, 34 °C. This is attributed to the adsorption of the surfactant in Glauber’s salt, which would initiate melting-point depression owing to defects in the crystal lattice. Furthermore, both the 3LC profile and DSC demonstrate small supercooling degrees compared with pure Glauber’s salt, which usually has supercooling between 16 and 22 K, depending on the volume size. This is further evidence of the nucleating-inducing ability of both the templating ability of the surfactant and of the crystallised palmitic acid surrounding the Glauber’s salt droplets.
Figure 4.
(A) Three-layer calorimetric data showing the enthalpy during the crystallisation and melting of the PCD between 19 and 34 °C. (B) The thermal profile measured during the 3-layer calorimeter measurement. (C) Differential scanning calorimeter curve for the PCD performed at 2 K min −1 between −10 and 50 °C.
Figure 4.
(A) Three-layer calorimetric data showing the enthalpy during the crystallisation and melting of the PCD between 19 and 34 °C. (B) The thermal profile measured during the 3-layer calorimeter measurement. (C) Differential scanning calorimeter curve for the PCD performed at 2 K min −1 between −10 and 50 °C.
4. Discussion
The goal of our work is to present a novel and unique method to stabilise Glauber’s salt and therefore enable its future application in thermal energy storage applications, as well as also showcasing a new revolutionary method for stabilisation of salt hydrates for latent heat storage applications. The design criteria of phase change dispersions for these stabilisation purposes are also explored. For applications of this method to stabilise other salt hydrates and for further improvement of the method, multiple aspects of the system need to be optimised. Firstly, we envision that by influencing the polydispersity of the emulsion, the critical volume fraction of 0.74 can be exceeded without the inversion of a water-in-oil emulsion. This, alongside careful selection of the oil phase, e.g., an organic molecule with very low density, would permit lower mass fractions of the oil phase to be used and subsequently higher mass fractions of Glauber’s salt, which would allow for the fine-tuning of the enthalpy of fusion and overall storage capacity of the phase change dispersion.
Our method has shown promising results in stabilising Glauber’s salt. However, the energy input associated with the current process is notable. Efficient strategies should be explored to reduce energy consumption during the phase change dispersion preparation stage. This could involve optimising the mixing protocol and exploring alternative dispersion techniques or even external fields (such as ultrasound) to facilitate dispersion whilst minimising energy expenditure.
By addressing the polydispersity of the suspension, reducing energy input, optimising surfactant systems, improving crystallisation behaviour and enhancing thermal conductivity through nanoparticles, significant advancements can be made in the development of highly stable and efficient phase change dispersion systems for various applications, including thermal energy storage. Continued research in these directions holds the promise for unlocking the full potential of these systems and contributing to the advancement of sustainable energy technologies.
5. Conclusions
We report a proof-of-principle system that demonstrates a novel method for stabilising Glauber’s salt, a notoriously elusive salt hydrate phase change material, by entrapping the Glauber’s salt in a phase change dispersion with solidified palmitic acid. The innovative approach has overcome the persistence challenge of phase separation, enabling Glauber’s salt to retain its impressive storage capacity over 100 thermal cycles. The phase change dispersion system, where Glauber’s salt droplets are dispersed within a solid palmitic acid matrix, effectively minimises diffusion lengths, preventing phase segregation. The thermal cycling experiments demonstrated remarkable stability, with the phase change dispersion exhibiting no signs of destabilisation or separation even after 100 thermal cycles. This stands in stark contrast to traditional thickening methods, which often show separation after 10 thermal cycles or less. Moreover, the phase change dispersion exhibited enhanced crystallisation behaviour, with reduced supercooling degrees without the addition of an external nucleating agent, an unexpected and valuable finding. This improvement is attributed to the dual action of the surfactant’s templating effect and the crystallised palmitic acid acting as a nucleating agent. Furthermore, the characterisation of the phase change dispersion’s thermal properties revealed promising results. The melting and crystallisation temperatures, as well as the enthalpy of fusion, demonstrate its suitability as a strong candidate as a phase change material to be used in domestic heating. To further optimise and extend the applicability of this method, several avenues of future research have been identified. These include fine-tuning the phase change dispersion’s polydispersity, selecting an optimal oil phase and exploring different surfactant systems. Additionally, enhancing crystallisation behaviour and introducing thermally conductive additives could lead to significant advancements in the field of thermal energy storage. In summary, this study represents a significant step forward in the development of stable and efficient phase change materials, offering unprecedented opportunities for sustainable energy technologies. Continued research and exploration of the identified optimisation strategies hold great potential for revolutionising the field of thermal energy storage.