Recent Developments and Applications of Food-Based Emulsifiers from Plant and Animal Sources
Abstract
1. Introduction
2. Protein-Based Emulsifiers
| Emulsifier | Food Source | Emulsion Type | Emulsion Properties | Oil Phase and Applications | Ref. |
|---|---|---|---|---|---|
| RuBisCo protein (ribulose 1,5-bisphosphate carboxylase) | Duckweed (Lemna minor) | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 0.91 μm | Oil phase: soybean oil. Application: beverage, dressing, sauce, dip | [30] |
| Soy protein | Soybean | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 0.37 μm | Oil phase: soybean oil. Application: beverage, dressing, sauce, dip | [30] |
| Soy protein | Soybean | W/O/W | Dispersed phase volume fraction: 20% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 22–25 μm | Oil phase: soybean oil. Application: double emulsion | [50] |
| Succinylated soy protein | Soybean | O/W | Dispersed phase volume fraction: 10% (mL/mL) | Oil phase: corn oil | [51] |
| Rice protein | Rice | O/W | Dispersed phase volume fraction: 3% (w/w) Emulsifier concentration: 0.6% (w/w) Dispersed phase droplet size: 1.71 μm (pH2), 1.79 μm (pH 10) | Oil phase: soybean oil | [52] |
| Rice protein | Rice | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 19.48 μm | Oil phase: linseed oil | [53] |
| Rice protein hydrolysates | Rice | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 10.15–16.69 μm | Oil phase: linseed oil | [53] |
| Rice protein fibril | Rice | O/W | Emulsifier concentration: 0.09% (w/v) | Oil phase: corn oil | [54] |
| Pea protein | Pea | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 0.5% (w/w) | Oil phase: rapeseed oil | [55] |
| Wheat germ protein (ultrasound treated) | Wheat | O/W | Dispersed phase volume fraction: 20% (v/v) Dispersed phase droplet size: 0.29 μm | Oil phase: soybean oil | [39] |
| Zanthoxylum seed protein (ultrasound) | Zanthoxylum | O/W | Dispersed phase volume fraction: 25% (v/v) Dispersed phase droplet size: 0.7896 μm | Oil phase: soybean oil | [56] |
| Whey protein | Milk | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 0.25 μm | Oil phase: soybean oil. Application: beverage, dressing, sauce, dip | [30] |
| Whey protein | Milk | W/O/W | Dispersed phase volume fraction: 20% (w/w) Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 22–25 μm | Oil phase: soybean oil. Application: double emulsion | [50] |
| Whey protein | Milk | W/O | Dispersed phase volume fraction: 15.17% (w/w) | Oil phase: milk fat. Application: butter | [57] |
| Whey protein fibril | Milk | O/W | Dispersed phase volume fraction: 20% (v/v) Emulsifier concentration: 2% (w/v) Dispersed phase droplet size: 10–100 μm | Oil phase: soybean oil | [58] |
| Whole milk powder | Milk | W/O | Dispersed phase volume fraction: 15.19% (w/w) | Oil phase: milk fat. Application: butter | [57] |
| Soy protein isolate and young apple polyphenol | Soybean and apple | O/W | Dispersed phase volume fraction: 20% (v/v) | Oil phase: rapeseed oil. Application: nano-deliver functional oils and nutrients | [59] |
| Soy protein isolate and tea polyphenol conjugates | Soybean and tea | O/W | Dispersed phase volume fraction: 25% (v/v) Emulsifier concentration: 0.3% (w/v) Dispersed phase droplet size: 496 nm | Oil phase: soybean oil | [60] |
| Casein butyrylated dextrin complex nanoparticle | Milk | O/W | Dispersed phase volume fraction: 30% (v/v) | Oil phase: corn oil | [61] |
| Sodium caseinate/phloretin complexes | Milk | O/W | Dispersed phase volume fraction: 20% (v/v) | Oil phase: sunflower oil | [62] |
| Sodium caseinate and maltodextrin | Milk | O/W | Dispersed phase volume fraction: 3% (v/v) Emulsifier concentration: 4% (w/v) Dispersed phase droplet size: 201.7–602.7 nm | Oil phase: peanut oil body | [63] |
| Cricket protein and rhamsan gum | Cricket | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 2% (w/w) Dispersed phase droplet size: 0.688 μm | Oil phase: avocado oil | [36] |
3. Polysaccharide-Based Emulsifiers
| Emulsifier | Food Source | Emulsion Type | Emulsion Properties | Oil Phase and Applications | Ref. |
|---|---|---|---|---|---|
| Mucilage | Okra | O/W | Dispersed phase volume fraction: 9.1% (v/v) | Oil phase: corn oil. Application: coconut milk | [71] |
| Pectin | Apple pomace | O/W | Emulsifier concentration: 4% (w/w) | Application: food gel | [73] |
| Pectin | Jackfruit | O/W | Emulsifier concentration: 2% (w/v) | Application: food gel | [74] |
| Gum arabic | Acacia Senegal tree | W/O/W | Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 22–25 μm | Oil phase: soybean oil. Application: double emulsion | [50] |
| Corn fiber gum | Corn | O/W | Dispersed phase volume fraction: 5% (w/w) Emulsifier concentration: 0.5–1.5% (w/w) Dispersed phase droplet size: >2.5 μm | Oil phase: soybean oil | [77] |
| Corn fiber gum (modified with octenyl succinic anhydride) | Corn | O/W | Dispersed phase volume fraction: 5% (w/w) Emulsifier concentration: 0.5–1.5% (w/w) Dispersed phase droplet size: 1.11–2.50 μm | Oil phase: soybean oil | [77] |
| Rice flour | Rice | O/W | Emulsifier concentration: 2.45% (w/w) | Application: cooked sausage | [89] |
| Tapioca starch | Tapioca | O/W | Emulsifier concentration: 2.45% (w/w) | Application: cooked sausage | [89] |
| Seaweed powder | Seaweed | O/W | Emulsifier concentration: 1% (w/w) | Application: dairy products | [90] |
| Chitosan | Exoskeleton of insects | O/W | Emulsifier concentration: 0.1–0.5% (w/w) | Application: mayonnaise | [75] |
| Quillaja saponin | Quillaja | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 0.5-2.5% (w/w) Dispersed phase droplet size: 0.15–0.5 μm | Oil phase: medium chain triglyceride oil. Application: non-dairy creamer | [91] |
| Potato starch and nanoliposomes | Potato | O/W | Dispersed phase volume fraction: 20% (v/v) Dispersed phase droplet size: 7–20 μm | Oil phase: soybean oil | [92] |
| Tapioca starch and milk protein | Cassava and milk | O/W | Emulsifier concentration: milk protein 10.5%, Modified tapioca starch 0–2% | Oil phase: anhydrous milk fat | [93] |
| Protein-polysaccharide conjugates | Sugar beet pulp | O/W | Emulsifier concentration: 1% (w/w) Dispersed phase droplet size: 0.438–0.479 μm | Oil phase: medium-chain triglycerides | [87] |
4. Phospholipid-Based Emulsifiers
| Emulsifier | Food Source | Emulsion Type | Emulsion Properties | Oil Phase and Applications | Ref. |
|---|---|---|---|---|---|
| Soy phospholipids | Soybean | O/W | Emulsifier concentration: 0.35% (w/w) | Application: gelato | [102] |
| Soy phospholipids | Soybean | O/W | Dispersed phase volume fraction: 10% (v/v) Dispersed phase droplet size: 381.27 nm | Application: infant formula | [118] |
| Milk phospholipids | Milk | O/W | Emulsifier concentration: 0.35% (w/w) | Application: gelato | [102] |
| Milk phospholipids | Milk | O/W | Dispersed phase volume fraction: 10% (v/v) Dispersed phase droplet size: 334.5 nm | Application: infant formula | [118] |
| Rice phospholipids | Rice | O/W | Emulsifier concentration: 0.35% (w/w) | Application: gelato | [102] |
| Pulp oil | Avocado | O/W | Dispersed phase volume fraction: 20–70% (v/v) Emulsifier concentration: 1, 2% (w/w) Dispersed phase droplet size: 9.47–64.49 μm | Oil phase: soybean oil | [101] |
| Soy lecithin | Soybean | O/W | Dispersed phase volume fraction: 9.65% (w/w) Emulsifier concentration: 0.5-2.0% (w/w) | Oil phase: rice bran oil | [119] |
| Sunflower phospholipids | Sunflower seed | O/W | Dispersed phase volume fraction: 10% (v/v) Dispersed phase droplet size: 378.97 nm | Application: infant formula | [118] |
| Sesame lecithin | Sesame oil | W/O | Dispersed phase volume fraction: 27% water (v/v) | Application: margarine | [120] |
| Corn lecithin | Corn oil | W/O | Dispersed phase volume fraction: 3% water (v/v) | Oil phase: corn oil. Aqueous phase: water. | [121] |
| Egg yolk phospholipids | Egg | O/W | Dispersed phase volume fraction: 10% (v/v) | Application: infant formula | [118] |
| Lecithin | Yellow mealworm | O/W | Extraction yield: 4–10% | Application: general food | [110] |
| Lecithin | Black soldier fly larvae | O/W | Extraction yield: 4–10% | Application: general food | [110] |
| Soy lecithin, rice starch, and taurine | Soybean and rice | O/W | Emulsifier concentration: 40 mg/mL soy lecithin in ethanol, added to 4% rice starch emulsion | Application: general food | [104] |
| Casein and lecithin | Milk and soybean | O/W | Dispersed phase volume fraction: 10% (w/w) Emulsifier concentration: 0.3% casein and 0.5% lecithin (w/w) Dispersed phase droplet size: 210 μm | Application: fish oil | [107] |
5. Hybrid Emulsifiers
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hadi, N.A.; Ashaari, A.; Matos, M.; Wan Rasdi, N. Exploring particle-based stabilisation of Pickering emulsions in food, aquaculture, and industrial applications. Int. J. Food Sci. Technol. 2024, 59, 6834–6855. [Google Scholar] [CrossRef]
- Ghasemi, H.; Darjani, S.; Mazloomi, H.; Mozaffari, S. Preparation of stable multiple emulsions using food-grade emulsifiers: Evaluating the effects of emulsifier concentration, W/O phase ratio, and emulsification process. SN Appl. Sci. 2020, 2, 2002. [Google Scholar] [CrossRef]
- Ishii, T.; Matsumiya, K.; Aoshima, M.; Matsumura, Y. Microgelation imparts emulsifying ability to surface-inactive polysaccharides—Bottom-up vs top-down approaches. npj Sci. Food 2018, 2, 15. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.; Xiao, Z.; Zhang, Y.; Weng, H.; Yang, Q.; Xiao, Q.; Xiao, A. A novel Pickering emulsion stabilized by rational designed agar microsphere. LWT 2023, 181, 114751. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef] [PubMed]
- Goff, H.D.; Liboff, M.; Jordan, W.K.; Kinsella, J.E. The effects of polysorbate 80 on the fat emulsion in ice cream mix: Evidence from transmission electron microscopy studies. Food Struct. 1987, 6, 11. [Google Scholar]
- Dammak, I.; Sobral, P.J.d.A.; Aquino, A.; Neves, M.A.d.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.K.; Chang, C.T.; Wada, N.; Ho, H.C.; Lo, Y.L.; Tsai, S.P.; Chen, R.A.; Huang, H.S.; Liu, P.Y.; et al. Common dietary emulsifiers promote metabolic disorders and intestinal microbiota dysbiosis in mice. Commun. Biol. 2024, 7, 749. [Google Scholar] [CrossRef]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food emulsifiers and metabolic syndrome: The role of the gut microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.C.; Lee, C.S.; Meng, Q.; Sakano, Y.; Noble, A.E.; Grant, K.A.; Esposito, A.; Gobler, C.J.; Venkatesan, A.K. Contribution of household and personal care products to 1,4-dioxane contamination of drinking water. Curr. Opin. Environ. Sci. Health 2023, 31, 100414. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.H.; Nickerson, M.T. Potential use of plant proteins in the microencapsulation of lipophilic materials in foods. Trends Food Sci. Technol. 2015, 42, 5–12. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure–function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Zeisel, S.H. Dietary choline: Biochemistry, physiology, and pharmacology. Annu. Rev. Nutr. 1981, 1, 95–121. [Google Scholar] [CrossRef]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in the food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef]
- Toker, O.; Ozonuk, S.; Güneş, R.; İçyer, N.; Rasouli Pirouzian, H.; Konar, N.; Palabiyik, I.; Altop, C. Importance of emulsifiers in the chocolate industry: Effect on structure, machinability, and quality of intermediate and final products. J. Am. Oil Chem. Soc. 2024, 101, 721–733. [Google Scholar] [CrossRef]
- Harasym, J.; Banaś, K. Lecithin’s roles in oleogelation. Gels 2024, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Chutia, H.; Mahanta, C.L. Clean label physical conjugates of protein-based bio-emulsifiers for food applications. Food Chem. Adv. 2023, 3, 100469. [Google Scholar] [CrossRef]
- Loveday, S.M. Food proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020, 105, 261–272. [Google Scholar] [CrossRef]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-based proteins and their multifaceted industrial applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Sagis, L.M.C.; Yang, J. Protein-stabilized interfaces in multiphase food: Comparing structure–function relations of plant-based and animal-based proteins. Curr. Opin. Food Sci. 2022, 43, 53–60. [Google Scholar] [CrossRef]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. Nutr. 2017, 57, 2636–2679. [Google Scholar] [CrossRef]
- McClements, D.J.; Lu, J.; Grossmann, L. Proposed methods for testing and comparing the emulsifying properties of proteins from animal, plant, and alternative sources. Colloids Interfaces 2022, 6, 19. [Google Scholar] [CrossRef]
- Tan, Y.; Lee, P.W.; Martens, T.D.; McClements, D.J. Comparison of emulsifying properties of plant and animal proteins in oil-in-water emulsions: Whey, soy, and RuBisCo proteins. Food Biophys. 2022, 17, 409–421. [Google Scholar] [CrossRef]
- Isnaini, L.; Estiasih, T.; Suseno, S.H.; Lestari, L.A. The role of vegetable proteins to stabilize emulsion: A mini review. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012036. [Google Scholar] [CrossRef]
- Santiago, L.A.; Queiroz, L.S.; Tavares, G.M.; Feyissa, A.H.; Silva, N.F.N.; Casanova, F. Edible insect proteins: How can they be a driver for food innovation? Curr. Opin. Food Sci. 2024, 58, 101195. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the development of edible insect-based foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent insight on edible insect protein: Extraction, functional properties, allergenicity, bioactivity, and applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Mudalungu, C.M.; Tchouassi, D.P.; Tanga, C.M. Techno-functional and antioxidant properties of extracted protein from edible insects. ACS Food Sci. Technol. 2024, 4, 1130–1141. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; García-Domínguez, I.; Rodríguez-Luna, A.; Hurtado-Fernández, E.; Santos, J. Cricket protein as an innovative emulsifier for avocado oil: Formulation and characterization of sustainable emulsions. Appl. Sci. 2024, 14, 1674. [Google Scholar] [CrossRef]
- Khojasteh, S.K.; Kadkhodaee, R.; Ritzoulis, C.; Emam-Djomeh, Z.; Emadzadeh, B. A comparative study on the effects of heat, ultrasound and high hydrostatic pressure on the structural and physicochemical properties of whey proteins. Appl. Food Res. 2025, 5, 101168. [Google Scholar] [CrossRef]
- Baldelli, A.; Shi, J.; Singh, A.; Guo, Y.; Fathordoobady, F.; Amiri, A.; Pratap-Singh, A. Effect of high-pressure on protein structure, refolding, and crystallization. Food Chem. Adv. 2024, 5, 100741. [Google Scholar] [CrossRef]
- Li, X.; Luo, T.; Wang, L.; Song, H.; Wang, F.; Weng, Z.; Zhou, J.; Xiang, X.; Xiong, L.; Shen, X. Emulsifying properties of wheat germ protein: Effect of different ultrasonic treatment. Ultrason. Sonochem. 2023, 98, 106479. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, L.; Gu, Y.; Zhuang, Y.; Fan, X.; Ding, Y. Exploring succinylation in proteins: A comprehensive review from functional improvements to application potential. Food Res. Int. 2025, 213, 116571. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, Y.; Lee, J.H.; Lee, S.S.; Chung, J.M.; Jung, H.S. Optimizing protein crosslinking control: Synergistic quenching effects of glycine, histidine, and lysine on glutaraldehyde reactions. Biochem. Biophys. Res. Commun. 2024, 702, 149567. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, F.; Nsor-Atindana, J.; Chen, X.; Liu, F.; Wu, J.; Zhong, F. High protein and high oil emulsions: Phase diagram, stability and interfacial adsorption. LWT 2022, 153, 112464. [Google Scholar] [CrossRef]
- Shi, A.-M.; Jiao, B.; Liu, H.-Z.; Zhu, S.; Shen, M.-J.; Feng, X.-L.; Hu, H.; Liu, L.; Faisal, S.; Wang, Q.; et al. Effects of proteolysis and transglutaminase crosslinking on physicochemical characteristics of walnut protein isolate. LWT 2018, 97, 662–667. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Selomulya, C. Exploring the relationship between physicochemical stability and interfacial properties in pea/whey protein blend-stabilised emulsions. Food Hydrocoll. 2025, 169, 111622. [Google Scholar] [CrossRef]
- Tunuhe, A.; Zheng, Z.; Rao, X.; Yu, H.; Ma, F.; Zhou, Y.; Xie, S. Protein-based materials: Applications, modification and molecular design. BioDes. Res. 2025, 7, 100004. [Google Scholar] [CrossRef]
- Xiong, D.; Xu, Q.; Tian, L.; Bai, J.; Yang, L.; Jia, J.; Liu, X.; Yang, X.; Duan, X. Mechanism of improving solubility and emulsifying properties of wheat gluten protein by pH cycling treatment and its application in powder oils. Food Hydrocoll. 2023, 135, 108132. [Google Scholar] [CrossRef]
- Pei, J.; Xiong, D.; Zhang, M.; Liu, C.; Zhang, L.; Liu, X.; Duan, X. Impact of high-soluble modified wheat gluten as an emulsifier on the structure and quality of ice cream. Food Chem. 2025, 468, 142473. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, C.; Wang, C.; Liu, C.; Yuan, Y.; Wang, B.; Wu, G.; Han, Y.; Zhao, Y.; Wu, Z.; et al. The emulsification properties of alkaline-extracted polysaccharide conjugates from Apocynum venetum L. tea residues. Food Hydrocoll. 2022, 124, 107315. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Tan, Y.; Muriel Mundo, J.L.; McClements, D.J. Comparison of plant-based emulsifier performance in water-in-oil-in-water emulsions: Soy protein isolate, pectin and gum Arabic. J. Food Eng. 2021, 307, 110625. [Google Scholar] [CrossRef]
- Lian, Z.; Yang, S.; Cheng, L.; Liao, P.; Dai, S.; Tong, X.; Tian, T.; Wang, H.; Jiang, L. Emulsifying properties and oil–water interface properties of succinylated soy protein isolate: Affected by conformational flexibility of the interfacial protein. Food Hydrocoll. 2023, 136, 108224. [Google Scholar] [CrossRef]
- Mun, S.; Shin, M.; Kim, Y.R. Emulsifying properties of proteins isolated from various rice cultivars. Food Bioprocess Technol. 2016, 9, 813–821. [Google Scholar] [CrossRef]
- Gomes, M.H.G.; Kurozawa, L.E. Performance of rice protein hydrolysates as a stabilizing agent on oil-in-water emulsions. Food Res. Int. 2023, 172, 113099. [Google Scholar] [CrossRef]
- Qi, X.; Li, Y.; Li, J.; Rong, L.; Pan, W.; Shen, M.; Xie, J. Fibrillation modification to improve the viscosity, emulsifying, and foaming properties of rice protein. Food Res. Int. 2023, 166, 112609. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. On the Emulsifying Properties of Self-Assembled Pea Protein Particles. Langmuir 2020, 36, 12221–12229. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Huang, H.; Xiong, M.; Yang, Y.; Lin, C.; Yang, F.; Xie, Y.; Yuan, Y. Improvement of the emulsifying properties of Zanthoxylum seed protein by ultrasonic modification. Ultrason. Sonochem. 2023, 100, 106638. [Google Scholar] [CrossRef]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Influence of emulsifiers and dairy ingredients on manufacturing, microstructure, and physical properties of butter. Foods 2021, 10, 1140. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Furtado, G.F.F.; Netto, F.M.; Cunha, R.L. Assessing the potential of whey protein fibril as emulsifier. J. Food Eng. 2018, 223, 99–108. [Google Scholar] [CrossRef]
- Song, Z.C.; Zhang, H.; Niu, P.F.; Shi, L.S.; Yang, X.Y.; Meng, Y.H.; Wang, X.Y.; Gong, T.; Guo, Y.R. Fabrication of a novel antioxidant emulsifier through tuning the molecular interaction between soy protein isolates and young apple polyphenols. Food Chem. 2023, 420, 136110. [Google Scholar] [CrossRef] [PubMed]
- Djuardi, A.U.P.; Yuliana, N.D.; Ogawa, M.; Akazawa, T.; Suhartono, M.T. Emulsifying properties and antioxidant activity of soy protein isolate conjugated with tea polyphenol extracts. J. Food Sci. Technol. 2020, 57, 3591–3600. [Google Scholar] [CrossRef]
- Chen, P.; Wang, R.-M.; Xu, B.-C.; Xu, F.-R.; Ye, Y.-W.; Zhang, B. Food emulsifier based on the interaction of casein and butyrylated dextrin for improving stability and emulsifying properties. J. Dairy Sci. 2023, 106, 1576–1585. [Google Scholar] [CrossRef]
- Kheynoor, N.; Jacquier, J.-C.; Khalesi, M.; Mortazavian, A.M.; Golmakani, M.-T. Formulation and characterization of sodium caseinate/phloretin complexes as antioxidant stabilizers in oil-in-water emulsions. Foods 2025, 14, 236. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, P.; Deng, Y.; Liu, G.; Li, P.; Zeng, J.; Zhang, Y.; Tang, X.; Zhao, Z.; Zhang, M. Impact of homogenization methods on the interfacial protein composition and stability of peanut oil body emulsion with sodium caseinate and maltodextrin. LWT 2025, 215, 117286. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A. Pickering emulsions in food and nutraceutical technology: From delivering hydrophobic compounds to cutting-edge food applications. Explor. Foods Foodomics 2024, 2, 408–442. [Google Scholar] [CrossRef]
- Ai, C. Recent advances on the emulsifying properties of dietary polysaccharides. eFood 2023, 4, e106. [Google Scholar] [CrossRef]
- Tan, T.B.; Nakajima, M.; Tan, C.P. Effect of polysaccharide emulsifiers on the fabrication of monodisperse oil-in-water emulsions using the microchannel emulsification method. J. Food Eng. 2018, 238, 188–194. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; Leser, M.E. Emulsification properties of hydrocolloids. Polym. Adv. Technol. 2001, 12, 123–135. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Noorlaila, A.; Aziah, S.A.; Asmeda, R.; Norizzah, A.R. Emulsifying properties of extracted okra (Abelmoschus esculentus L.) mucilage of different maturity index and its application in coconut milk emulsion. Int. Food Res. J. 2015, 22, 782–787. [Google Scholar]
- Haque, S.M.; Kabir, A.; Ratemi, E.; Elzagheid, M.; Appu, S.P.; Ghani, S.S.; Sarief, A. Greener pectin extraction techniques: Applications and challenges. Separations 2025, 12, 65. [Google Scholar] [CrossRef]
- Costa, J.M.; Wang, W.; Nakasu, P.Y.S.; Hu, C.; Forster-Carneiro, T.; Hallett, J.P. Impacts of microwaves on the pectin extraction from apple pomace: Technological properties in structuring of hydrogels. Food Hydrocoll. 2025, 160, 110766. [Google Scholar] [CrossRef]
- Chan, S.W.; Han, C.E.; Tan, C.P.; Khor, Y.P.; Lou, X. Ultrasound-assisted extraction of pectin from jackfruit (Artocarpus heterophyllus) rags: Optimization, characterization, and application in model food gel. Curr. Res. Nutr. Food Sci. 2023, 11, 991–1007. [Google Scholar] [CrossRef]
- Casariego, A.; Díaz, R. Chitosan as a natural emulsifier in mayonnaise: Stability and effects on product quality. J. Food Sci. Gastron. 2024, 2, 1–5. [Google Scholar]
- Niu, J.; Lin, Q.; Li, X.; McClements, D.J.; Ji, H.; Jin, Z.; Qiu, C. Pickering emulsions stabilized by essential oil–tannin–chitosan particles: Microstructure, stability, antibacterial activity, and antioxidant activity. Food Hydrocoll. 2024, 154, 110145. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Y.; Cai, Z.; Guo, Y.; Wu, M.; Wang, P.; Li, R.; Zhang, H. Interfacial and emulsion characterisation of chemically modified polysaccharides through a multiscale approach. J. Colloid Interface Sci. 2020, 580, 480–492. [Google Scholar] [CrossRef]
- Ma, H.; Yang, L.; Zhang, D.; Chen, H.; Kan, J. Functionalities of octenyl succinic anhydride wheat starch and its effect on the quality of model dough and noodles. Foods 2025, 14, 1688. [Google Scholar] [CrossRef]
- Nataraj, A.; Govindan, S.; Rajendran, A.; Ramani, P.; Subbaiah, K.A.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Effects of carboxymethyl modification on the acidic polysaccharides from Calocybe indica: Physicochemical properties, antioxidant, antitumor and anticoagulant activities. Antioxidants 2022, 12, 105. [Google Scholar] [CrossRef]
- Huang, B.; Hu, Q.; Zhang, G.; Zou, J.; Fei, P.; Wang, Z. Exploring the emulsification potential of chitosan modified with phenolic acids: Emulsifying properties, functional activities, and application in curcumin encapsulation. Int. J. Biol. Macromol. 2024, 263, 130450. [Google Scholar] [CrossRef] [PubMed]
- Raka, A.M.; Takada, A.; Hossain, K.S. Effect of heat treatment on conformational and structural properties of sugar beet pectin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100149. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, S.; Tan, Y.; Feng, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Biodegradation and prospect of polysaccharide from crustaceans. Mar. Drugs 2022, 20, 310. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Xin, L.; Zhang, L.; Yang, L.; Wang, P.; Liu, H. Exploring the influence of different enzymes on soy hull polysaccharide emulsion stabilization: A study on interfacial behavior and structural changes. Food Chem. 2025, 463, 141147. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical modification of polysaccharides: A review of synthetic approaches, biological activity and the structure–activity relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.S.; Brennan, C.S.; Chandrapala, J.; Gao, W.; Han, Z.; Zeng, X.A. Valorizing protein–polysaccharide conjugates from sugar beet pulp as an emulsifier. Int. J. Biol. Macromol. 2023, 226, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, E.; Mekhloufi, G.; Le Potier, I.; de Kerdaniel, T.F.; Grossiord, J.L.; Rosilio, V.; Agnely, F. Stabilization mechanism of oil-in-water emulsions by β-lactoglobulin and gum arabic. J. Colloid Interface Sci. 2011, 354, 467–477. [Google Scholar] [CrossRef]
- Pereira, J.; Hu, H.; Xing, L.; Zhang, W.; Zhou, G. Influence of rice flour, glutinous rice flour, and tapioca starch on the functional properties and quality of an emulsion-type cooked sausage. Foods 2020, 9, 9. [Google Scholar] [CrossRef]
- Rifky, M.; Abdusalomova, D.; Dissanayake, K.; Zokirov, K.; Harris, J.M.; Jesfar, M.; Esonboyev, F.; Samadiy, M. Seaweed as a functional ingredient and emulsifier in dairy processing. BIO Web Conf. 2024, 108, 18001. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Xu, T.; Pan, M.-H.; Chiou, Y.-S.; Li, Z.; Wei, S.; Yin, X.; Ding, B. Improvement of emulsifying properties of potato starch via complexation with nanoliposomes for stabilizing Pickering emulsion. Food Hydrocoll. 2023, 136, 108271. [Google Scholar] [CrossRef]
- Thaiwong, N.; Thaiudom, S. Stability of oil-in-water emulsion influenced by the interaction of modified tapioca starch and milk protein. Int. J. Dairy Technol. 2021, 74, 307–315. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Cirillo, G.; Vinci, G.; Picci, N. Determination of phospholipids in food samples. Food Rev. Int. 2012, 28, 1–46. [Google Scholar] [CrossRef]
- Mezdour, S.; Desplanques, S.; Relkin, P. Effects of residual phospholipids on surface properties of a soft-refined sunflower oil: Application to stabilization of sauce-type emulsions. Food Hydrocoll. 2011, 25, 613–619. [Google Scholar] [CrossRef]
- Xia, Z.-W.; Zhang, J.-G.; Ni, Z.-J.; Zhang, F.; Thakur, K.; Hu, F.; Wei, Z.-J. Functional and emulsification characteristics of phospholipids and derived o/w emulsions from peony seed meal. Food Chem. 2022, 389, 133112. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.M.; Masbernat, O.; Roger, K. Emulsions stabilized by phospholipids. J. Colloid Interface Sci. 2025, 678, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, H.; Lyu, X.; Chen, H.; Wei, F. Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Sci. 2023, 8, 35–44. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Zhuang, Y.; Dong, J.; He, X.; Wang, J.; Li, C.; Dong, L.; Zhang, Y.; Zhou, X.; Wang, H.; Yi, Y.; et al. Impact of heating temperature and fatty acid type on the formation of lipid oxidation products during thermal processing. Front. Nutr. 2022, 9, 913297. [Google Scholar] [CrossRef]
- Züge, L.C.B.; Maieves, H.A.; Silveira, J.L.M.; da Silva, V.R.; Scheer, A.P. Use of avocado phospholipids as emulsifier. LWT 2017, 79, 42–51. [Google Scholar] [CrossRef]
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Guizzetti, S.; Barbanti, D.; Chiavaro, E. Innovation in the Italian ice cream production: Effect of different phospholipid emulsifiers. Dairy Sci. Technol. 2014, 94, 33–49. [Google Scholar] [CrossRef]
- Pichot, R.; Watson, R.; Norton, I. Phospholipids at the interface: Current trends and challenges. Int. J. Mol. Sci. 2013, 14, 11767–11794. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Sun, L.; Dou, B.; Zhang, S.; Xin, J.; Chen, F.; Quek, S.Y.; Zhang, N. Molecular mechanism of taurine promotes the interaction between rice starch and soy lecithin. Int. J. Biol. Macromol. 2025, 310, 143240. [Google Scholar] [CrossRef]
- Darryl, D.S.; Blake, B.; Williams, I.; Mullan, B.P.; Pethick, D.W.; Dunshea, F.R. Dietary lecithin supplementation can improve the quality of the m. longissimus thoracis. Animals 2015, 5, 1180–1191. [Google Scholar] [CrossRef]
- Zhao, F.; Li, R.; Liu, Y.; Chen, H. Perspectives on lecithin from egg yolk: Extraction, physicochemical properties, modification, and applications. Front. Nutr. 2023, 9, 1082671. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Frisenfeldt Horn, A.; Jacobsen, C. Influence of casein–phospholipid combinations as emulsifier on the physical and oxidative stability of fish oil-in-water emulsions. J. Agric. Food Chem. 2014, 62, 1142–1152. [Google Scholar] [CrossRef]
- Aondoakaa, I.P.; Akoh, C.C. Microbial and insect oils: A sustainable approach to functional lipid. J. Am. Oil Chem. Soc. 2025, 102, 5–33. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Zeiri, A.; Shah, M.A. Insect lipids as novel source for future applications: Chemical composition and industry applications—A comprehensive review. Food Sci. Nutr. 2025, 13, e70553. [Google Scholar] [CrossRef]
- Li, A.; Dewettinck, K.; Verheust, Y.; Van de Walle, D.; Raes, K.; Diehl, B.; Tzompa-Sosa, D.A. Edible insects as a novel source of lecithin: Extraction and lipid characterization of black soldier fly larvae and yellow mealworm. Food Chem. 2024, 452, 139391. [Google Scholar] [CrossRef]
- Allegretti, C.; Denuccio, F.; Rossato, L.; D’Arrigo, P. Polar head modified phospholipids by phospholipase D-catalyzed transformations of natural phosphatidylcholine for targeted applications: An overview. Catalysts 2020, 10, 997. [Google Scholar] [CrossRef]
- Bravo-Alfaro, D.A.; Ochoa-Rodríguez, L.R.; Santos-Luna, D.; Villaseñor-Ortega, F.; García, H.S.; Luna-Bárcenas, G. The enzymatic modification of phospholipids improves their surface-active properties and the formation of nanoemulsions. Biocatal. Agric. Biotechnol. 2023, 48, 102652. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The phospholipase A2 superfamily: Structure, isozymes, catalysis, physiologic and pathologic roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef]
- Xu, R.; Gao, Q.; Li, J.; Su, Y.; Gu, L.; Yang, Y.; Chang, C. Characterization of liquid egg yolks hydrolyzed by phospholipase: Structure, thermal stability and emulsification properties. Food Res. Int. 2024, 198, 115325. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Li, D.; Lin, J.; Wang, W.; Yu, D.; Li, S. Effect of hydrophobic fatty acid groups on emulsifying features of rhamnolipids: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2025, 720, 137178. [Google Scholar] [CrossRef]
- Lai, Q.D.; Huynh, T.T.L.; Doan, N.T.T.; Nguyen, H.D. Particle size distribution and homogenisation efficiency in high-pressure homogenisation of wheat germ oil–water system. Int. J. Food Sci. Technol. 2022, 57, 4337–4346. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and coemulsifiers that can inhibit lipid oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Yan, Q.; Li, J.; Kouame, K.J.E.-P.; Li, X.; Liu, L.; Zong, X.; Si, K.; Liu, X.; et al. Sphingomyelin-enriched milk phospholipids offer superior benefits in improving the physicochemical properties, microstructure, and surface characteristics of infant formula. Food Chem. 2025, 463, 141549. [Google Scholar] [CrossRef]
- Tay, U.J.; Toy, J.Y.H.; He, C.; Vignuzzi, M.; Yang, X.; Subramanian, G.S.; Antipina, M.; Lorenzini, P.A.; Zhou, W.; Huang, D. Soy lecithin strengthens amaranth protein-based omelettes through protein complexation which alters protein interactions. Food Struct. 2025, 44, 100432. [Google Scholar] [CrossRef]
- Berdiansyah, S.; Arifan, F. Evaluation of the effect of sesame lecithin emulsifier generated from sesame oil water degumming process on margarine production. Food Res. 2023, 7, 236–243. [Google Scholar] [CrossRef]
- Hamad, A.; Indriani, N.; Ma’Ruf, A. Production of lecithin as an emulsifier from vegetable oil using water degumming process. Techno J. Fak. Tek. Univ. Muhammadiyah Purwok. 2022, 23, 139. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, V.K.; Du, Y.; Aghbashlo, M.; Show, P.L.; Pan, J.; Tabatabaei, M.; Rajaei, A. Potential application of polysaccharide mucilages as a substitute for emulsifiers: A review. Int. J. Biol. Macromol. 2023, 242, 124800. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Morell, P.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Protein- and polysaccharide-based particles used for Pickering emulsion stabilisation. Food Hydrocoll. 2021, 119, 106839. [Google Scholar] [CrossRef]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide–protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, J.; Zhang, T.; Karrar, E.; Chang, M.; Liu, R.; Wang, X. Impact of interactions between whey protein isolate and different phospholipids on the properties of krill oil emulsions: A consideration for functional lipids efficient delivery. Food Hydrocoll. 2022, 130, 107692. [Google Scholar] [CrossRef]
- Chen, M.; Sagis, L.M.C.; Sun, Q.J. Emulsification and dilatational surface rheology of ultrasonicated milk fat globule membrane (MFGM) materials. LWT–Food Sci. Technol. 2020, 133, 110094. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Fattori, J.; Michelon, M.; Cunha, R.L. Formation and pH-stability of whey protein fibrils in the presence of lecithin. Food Hydrocoll. 2016, 60, 288–298. [Google Scholar] [CrossRef]
- Cassani, L.; Prieto, M.A.; Gomez-Zavaglia, A. Effect of food-grade biopolymers coated Pickering emulsions on carotenoids’ stability during processing, storage, and passage through the gastrointestinal tract. Curr. Opin. Food Sci. 2023, 51, 101031. [Google Scholar] [CrossRef]
- Chai, X.; Su, Y.; Liu, Y. Emulsifier synergism in aerated emulsions: Coordinated regulation of fat crystallization and protein interfacial adsorption. Food Hydrocoll. 2026, 170, 111725. [Google Scholar] [CrossRef]
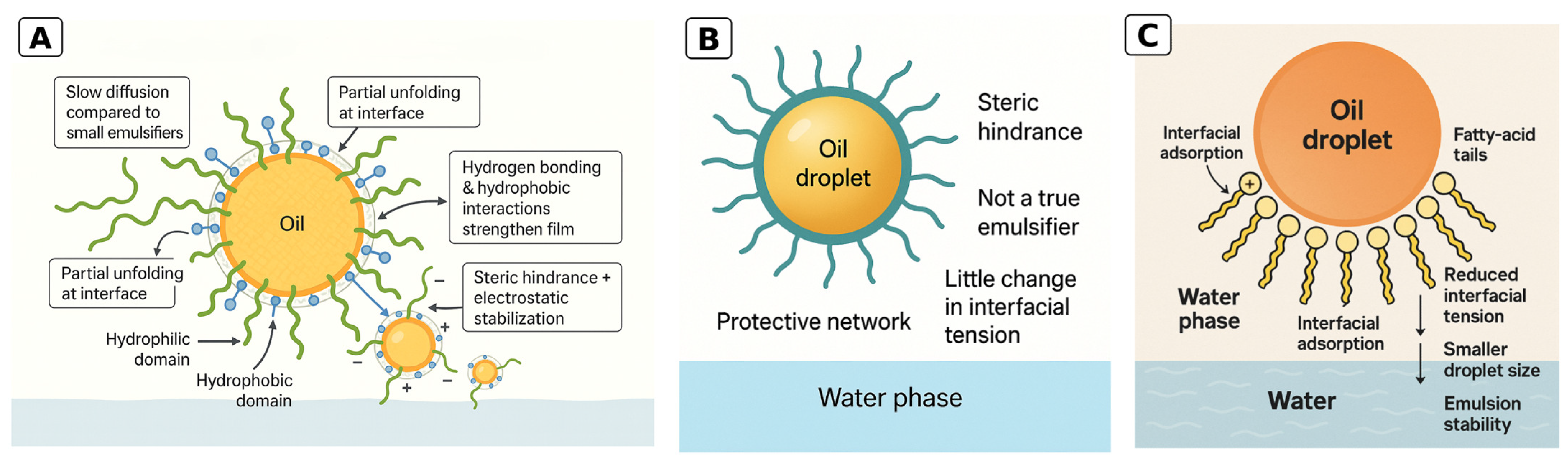
| Class | Interfacial Behavior | Stability Tendencies | Pros and Cons | Modification Methods and Examples |
|---|---|---|---|---|
| Proteins | Adsorb and partially unfold at the interface, forming a viscoelastic protein film. Provide steric hindrance and electrostatic repulsion. Produce a moderate decrease in interfacial tension compared with small-molecule surfactants. | Generally stable at moderate pH and ionic strength. Least stable near the isoelectric point. Salts can screen charges. Thermal history can denature or strengthen films. | Pros: strong interfacial films; clean label; can achieve small droplets with suitable processing. Cons: sensitive near the isoelectric point; ionic effects; slower diffusion than small surfactants; potential allergens depending on source. | Physical: heat, pH cycling, ultrasound, high pressure. Chemical: acylation or succinylation, polyphenol conjugation, glycation. Enzymatic: transglutaminase crosslinking or limited hydrolysis. |
| Polysaccharides | Do not act as classic emulsifiers. Form thick gel-like shells or networks around droplets that provide steric stabilization and reduce coalescence. Increase continuous-phase viscosity. Little direct effect on interfacial tension. | Often robust to pH and temperature (polymer dependent). Charge, substitution, molecular weight, and branching control barrier thickness and sensitivity to salts. | Pros: biocompatible and biodegradable; label friendly; improve creaming stability and mouthfeel. Cons: often require a co-emulsifier for droplet formation; higher viscosity can affect processing and sensory properties. | Physical: shear, heat, pH. Chemical: octenyl succinic anhydride modification, carboxymethylation or acetylation, protein complexation or coacervation. Enzymatic: controlled depolymerization or crosslinking. |
| Phospholipids | Amphiphilic small molecules with a polar head and hydrophobic tails that align at the interface and markedly lower interfacial tension. Facilitate droplet breakup during homogenization and produce fine droplets. | Stability is strongly influenced by fatty-acid saturation. Unsaturated phospholipids are more prone to oxidation and thermal degradation, whereas saturated fractions are more thermally and oxidatively stable. | Pros: effective at low use levels; produce fine droplets; broadly compatible. Cons: oxidation and thermal sensitivity for unsaturated fractions; possible flavor interactions; variability by source and fraction. | Blending of headgroups (PC, PE, PI, SM, lyso-PL), use of antioxidants, fractionation. Enzymatic hydrolysis to lyso-PL can increase solubility and hydrophilic–lipophilic balance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Adhikari, A. Recent Developments and Applications of Food-Based Emulsifiers from Plant and Animal Sources. Colloids Interfaces 2025, 9, 61. https://doi.org/10.3390/colloids9050061
Jin Y, Adhikari A. Recent Developments and Applications of Food-Based Emulsifiers from Plant and Animal Sources. Colloids and Interfaces. 2025; 9(5):61. https://doi.org/10.3390/colloids9050061
Chicago/Turabian StyleJin, Yuqiao, and Achyut Adhikari. 2025. "Recent Developments and Applications of Food-Based Emulsifiers from Plant and Animal Sources" Colloids and Interfaces 9, no. 5: 61. https://doi.org/10.3390/colloids9050061
APA StyleJin, Y., & Adhikari, A. (2025). Recent Developments and Applications of Food-Based Emulsifiers from Plant and Animal Sources. Colloids and Interfaces, 9(5), 61. https://doi.org/10.3390/colloids9050061










