An Innovative Approach for Assessing Foam Stability Based on Electrical Conductivity Measurements of Liquid Films
Abstract
1. Introduction
2. Materials and Methods
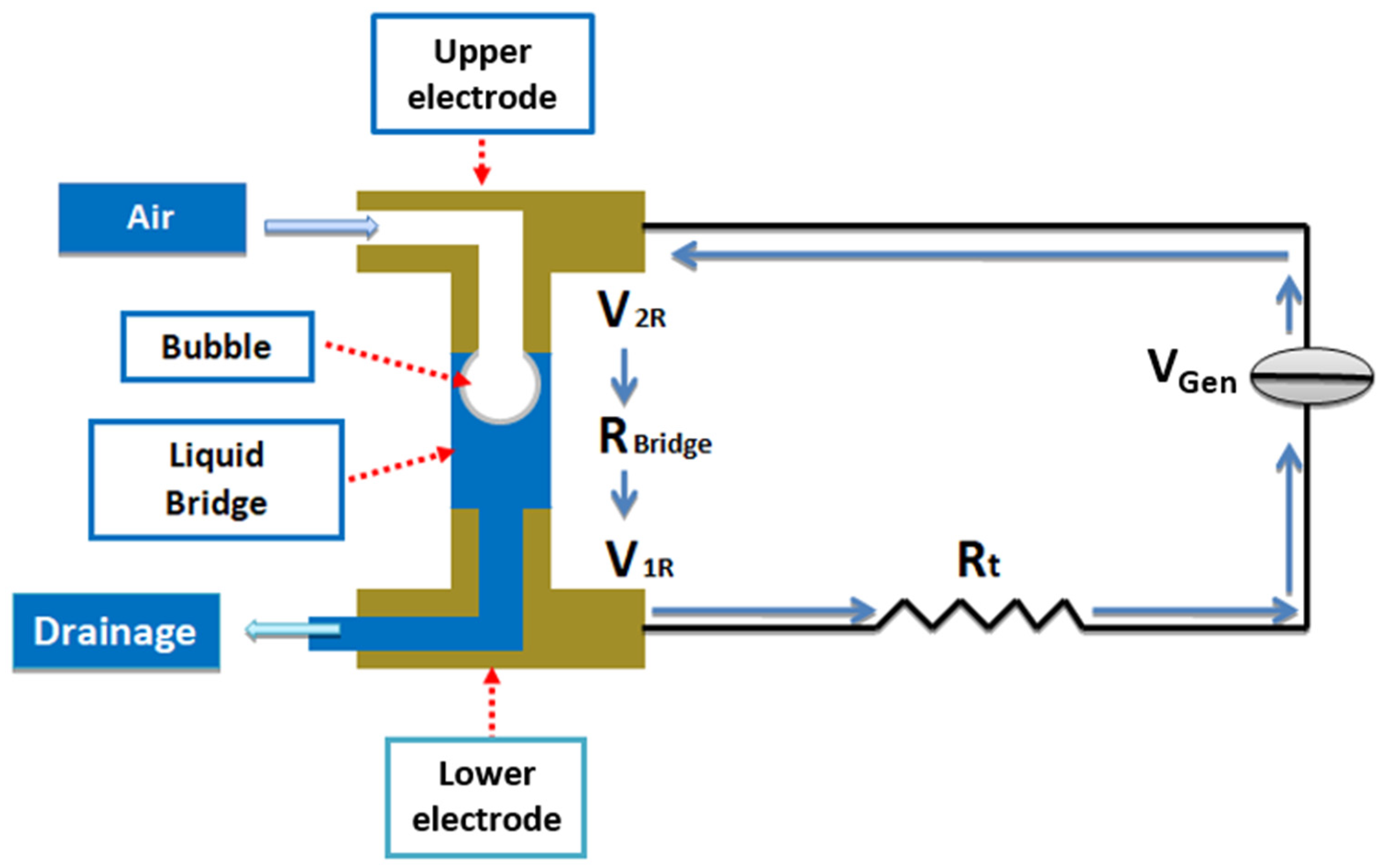
2.1. Experimental Setup and Environmental Control
2.2. Electrical Measurements and Instrumentation
2.3. Sample Preparation and Experimental Procedure
3. Results and Discussion
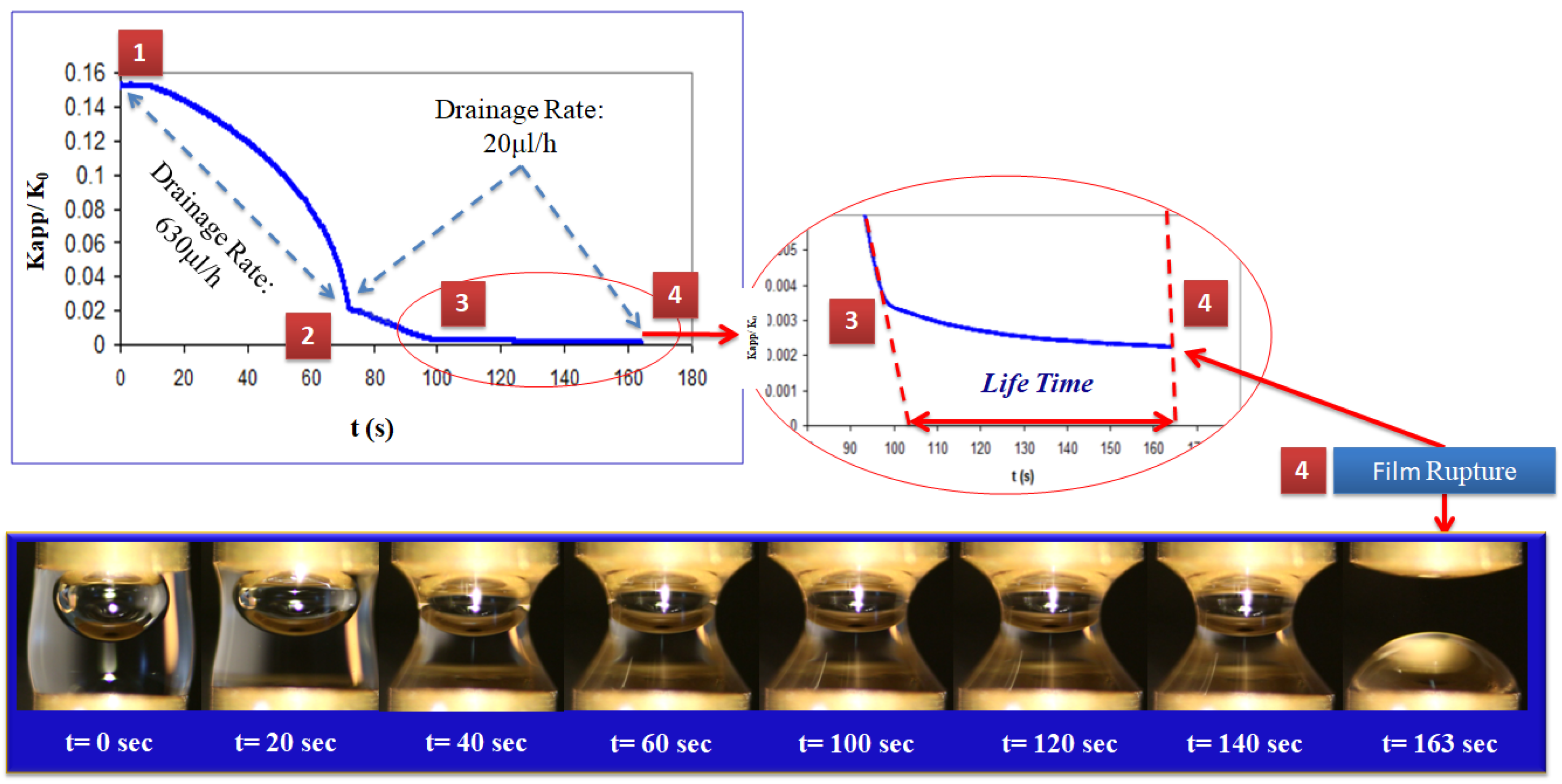
3.1. Effect of Drainage Rate on Liquid Film Lifetime
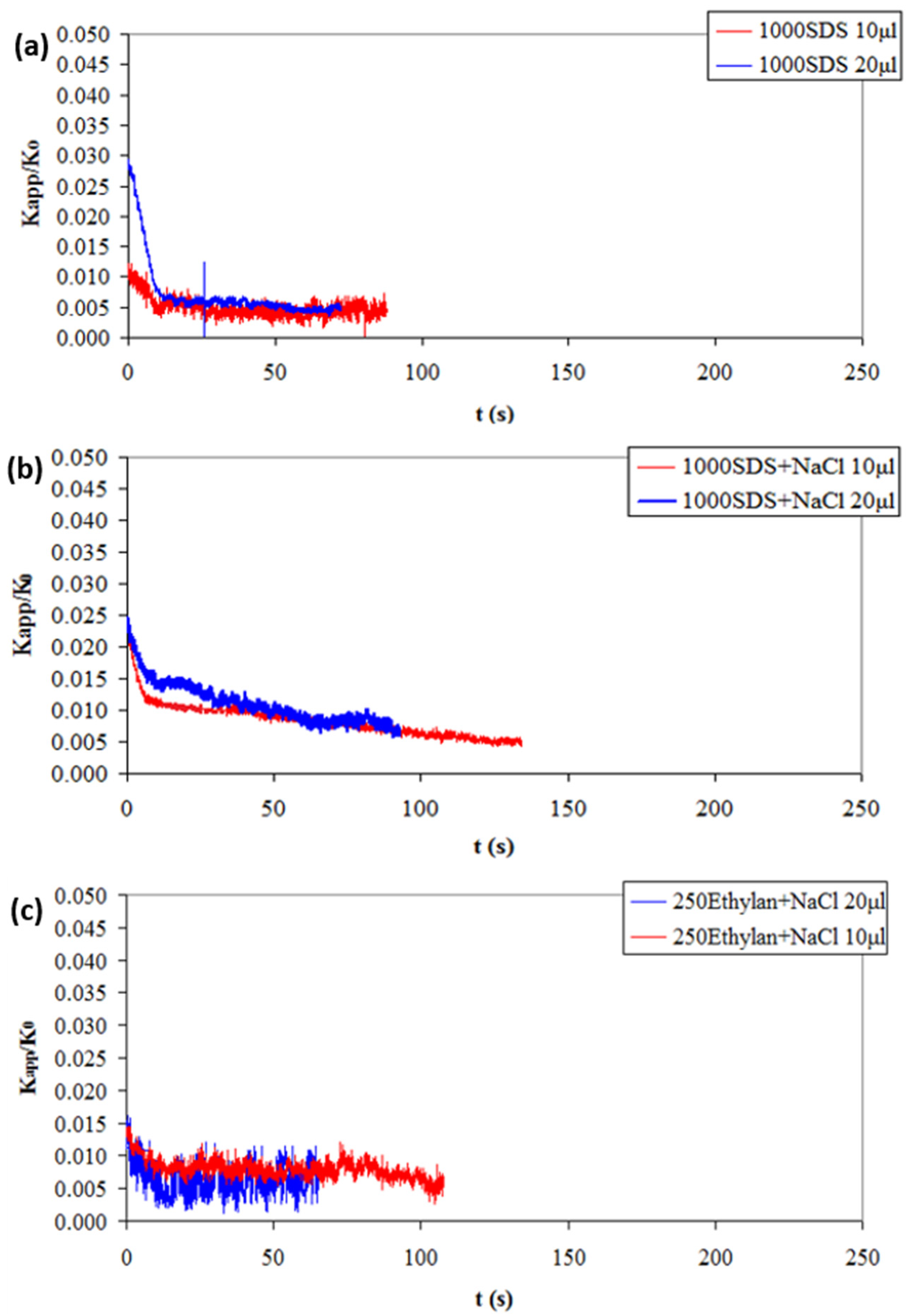
3.2. Effect of Surfactant Concentration on the Lifetime of the Liquid Film
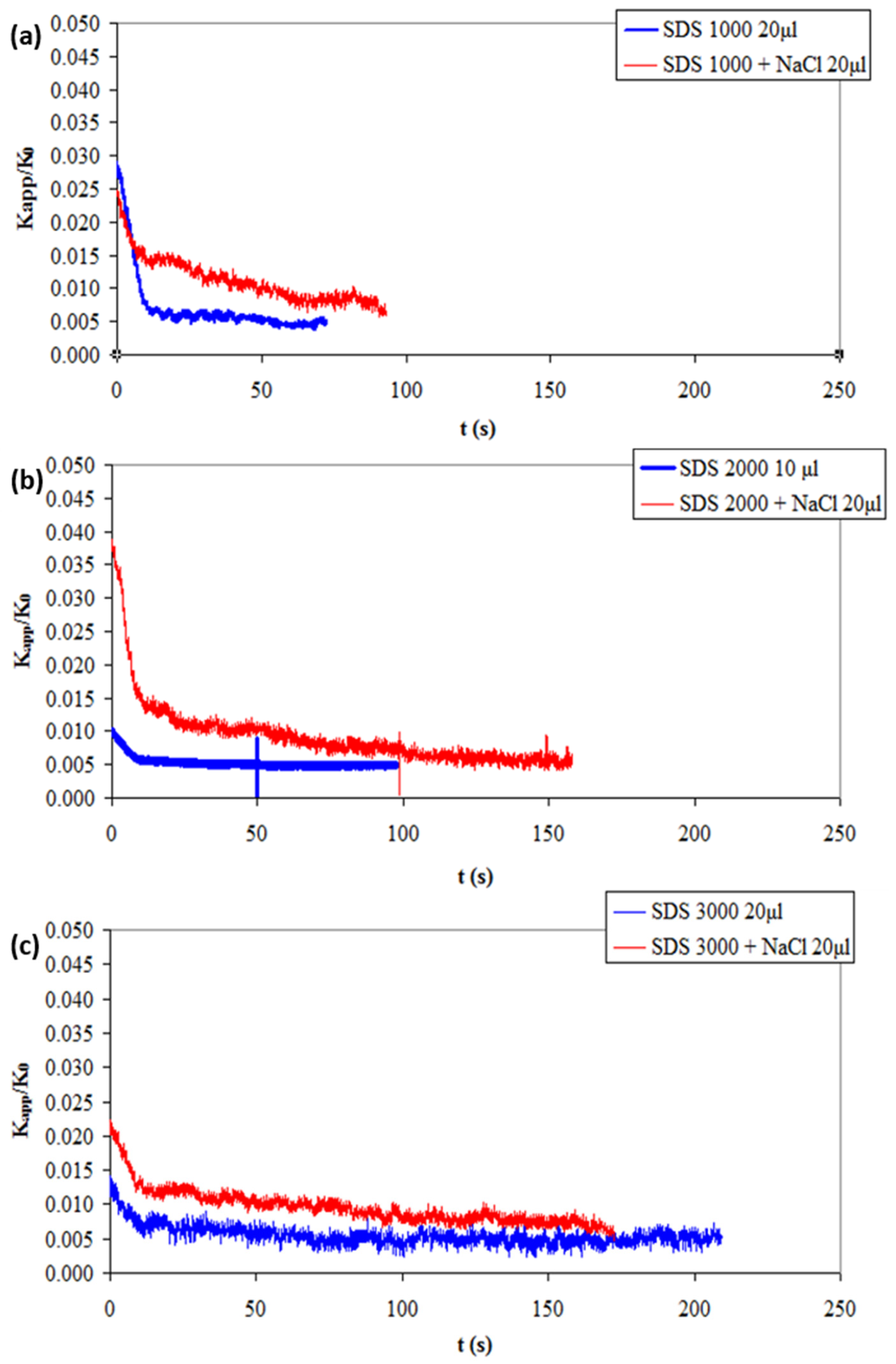
3.3. Effect of Salt Presence in Surfactant Solutions on the Lifetime of the Liquid Film
4. Conclusions
- a wider range of surfactant types (e.g., cationic, amphoteric),
- different salt species and ionic strengths, and
- complex formulations that better represent real-world systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RH | Relative Humidity |
| SDS | Sodium Dodecyl Sulfate |
| CMC | Critical Micelle Concentration |
| LD | Linear dichroism |
References
- Hutzler, S.; Weaire, D.; Saugey, A.; Cox, S.; Péron, N. The physics of foam drainage. In Proceedings of the SEPAWA Kongress, MIT European Conference, Würzburg, Germany, 12–14 October 2005. [Google Scholar]
- Exerowa, D.; Kruglyakov, P.M. Foam and Foam Films: Theory, Experiment, Application, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 5. [Google Scholar]
- Jäsberg, A.; Selenius, P.; Koponen, A.I. Experimental results on the flow rheology of fiber-laden aqueous foams. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 147–155. [Google Scholar] [CrossRef]
- Narchi, I.; Vial, C.; Djelveh, G. Effect of protein–polysaccharide mixtures on the continuous manufacturing of foamed food products. Food Hydrocoll. 2009, 23, 188–201. [Google Scholar] [CrossRef]
- Nyarko, K.; Glavaš, H.; Habschied, K.; Mastanjević, K. Determination of foam stability in lager beers using digital image analysis of images obtained using RGB and 3D cameras. Fermentation 2021, 7, 46. [Google Scholar] [CrossRef]
- Zawala, J.; Malysa, K.; Kowalczuk, P.B. On importance of external conditions and properties of the interacting phases in formation and stability of symmetrical and unsymmetrical liquid films. Adv. Colloid. Interface Sci. 2020, 276, 102085. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Zhang, Y.; Langevin, D. Quantitative description of foam drainage: Transitions with surface mobility. Eur. Phys. J. E 2004, 15, 53–60. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Zuin, V.G.; Segatto, M.L.; Ramin, L.Z. Green Chemistry in Analytical Chemistry. In Encyclopedia of Analytical Chemistry; Springer: New York, NY, USA, 2019; pp. 613–636. [Google Scholar] [CrossRef]
- Boos, J.; Drenckhan, W.; Stubenrauch, C. Protocol for studying aqueous foams stabilized by surfactant mixtures. J. Surfactants Deterg. 2013, 16, 1–12. [Google Scholar] [CrossRef]
- Safouane, M.; Saint-Jalmes, A.; Bergeron, V.; Langevin, D. Viscosity effects in foam drainage: Newtonian and non-Newtonian foaming fluids. Eur. Phys. J. E. 2006, 19, 195–202. [Google Scholar] [CrossRef]
- Cabrales, I.M.; Abrodo, P.A.; Blanco-Gomis, D. Influence of fatty acids on foaming properties of cider. J. Agric. Food Chem. 2003, 51, 6314–6316. [Google Scholar] [CrossRef]
- Zawala, J.; Miguet, J.; Rastogi, P.; Atasi, O.; Borkowski, M.; Scheid, B.; Fuller, G.G. Coalescence of surface bubbles: The crucial role of motion-induced dynamic adsorption layer. Adv. Colloid Interface Sci. 2023, 317, 102916. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, B.; Chang, C.H.; Kuo, A.T.; Siswarni, M.Z. Foam capacity and stability of Sodium Dodecyl Sulfate (SDS) on the presence of contaminant coffee and Cd ions in solution. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012042. [Google Scholar] [CrossRef]
- Zhang, L.; Mikhailovskaya, A.; Yazhgur, P.; Muller, F.; Cousin, F.; Langevin, D.; Wang, N.; Salonen, A. Precipitating sodium dodecyl sulfate to create ultrastable and stimulable foams. Angew. Chem. Int. Ed. 2015, 54, 9533–9536. [Google Scholar] [CrossRef]
- Kostoglou, M.; Lioumbas, J.; Karapantsios, T. A population balance treatment of bubble size evolution in free draining foams. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 75–84. [Google Scholar] [CrossRef]
- Subbotin, A.V.; Malkin, A.Y.; Kulichikhin, V.G. Self-organization in the flow of complex fluids (colloid and polymer systems). Part 2: Theoretical models. Adv. Colloid Interface Sci. 2011, 162, 29–38. [Google Scholar] [CrossRef]
- Suja, V.C.; Rodríguez-Hakim, M.; Tajuelo, J.; Fuller, G.G. Single bubble and drop techniques for characterizing foams and emulsions. Adv. Colloid Interface Sci. 2020, 286, 102295. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Li, M.; Shen, J.; Howes, T.; Wang, G. Rupture distance and shape of the liquid bridge with rough surface. Miner. Eng. 2021, 167, 106888. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D. A new method for the characterization of electrically conducting liquid bridges. J. Colloid Interface Sci. 2000, 227, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, M.; Karapantsios, T.D. Analysis of bubble-in-liquid bridge configuration as prototype for studying foam dynamics: Zero Bond number case. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 386–390. [Google Scholar] [CrossRef]
- Kostoglou, M.; Georgiou, E.; Karapantsios, T.D. A new device for assessing film stability in foams: Experiment and theory. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 64–73. [Google Scholar] [CrossRef]
- Evgenidis, S.; Kostoglou, M.; Karapantsios, T. Electrical conductance study of θ-liquid bridges. J. Colloid Interface Sci. 2006, 302, 597–604. [Google Scholar] [CrossRef]
- Karapantsios, T.D.; Evgenidis, S.P.; Zacharias, K.; Mesimeris, T.; European Patent Office. Method for the Detection and Characterization of Bubbles in Liquids and Device Therefor, Respectively System. EP 3005942 A1, 13 April 2016. [Google Scholar]
- Evgenidis, S.; Karapantsios, T. Increase of gas–liquid interfacial area in bubbly flows by pulsating flow conditions. Chem. Eng. J. 2024, 486, 150107. [Google Scholar] [CrossRef]
- Chondrou, A.P.; Evgenidis, S.P.; Karapantsios, T.D.; Kostoglou, M. Emulsions stability in weightlessness: Droplets size, droplets coalescence and phases spatial distribution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134943. [Google Scholar] [CrossRef]
- Oikonomidou, O.; Evgenidis, S.P.; Schwarz, C.J.; van Loon, J.W.A.; Kostoglou, M.; Karapantsios, T.D. Degassing of a decompressed flowing liquid under hypergravity conditions. Int. J. Multiph. Flow 2019, 115, 126–136. [Google Scholar] [CrossRef]
- Karakashev, S.I. Hydrodynamics of foams. Exp. Fluids 2017, 58, 91. [Google Scholar] [CrossRef]
- Xu, W.; Nikolov, A.; Wasan, D.T.; Gonsalves, A.; Borwankar, R.P. Foam film rheology and thickness stability of foam-based food products. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 13–21. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Smoukov, S.K.; Raykundaliya, N.; Grozev, N.A. Duality of foam stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126521. [Google Scholar] [CrossRef]
- Nikolov, A.D.; Kralchevsky, P.A.; Ivanov, I.B.; Wasan, D.T. Ordered micelle structuring in thin films formed from anionic surfactant solutions: II. Model development. J. Colloid Interface Sci. 1989, 133, 13–22. [Google Scholar] [CrossRef]
- Belhaij, A.; Al-Mahdy, O.A. Foamability and foam stability of several surfactants solutions: The role of screening and flooding. J. Pet. Environ. Biotechnol. 2015, 6, 227. [Google Scholar] [CrossRef]
- Patel, S.S.; Kumar, K.; Shah, D.O.; Delfino, J.J. Effect of surfactant concentration and film area on the stability of films of surfactant solutions. J. Colloid Interface Sci. 1996, 183, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xing, Y.; Xia, Y.; Guo, F.; Ding, S.; Tan, J.; Che, T.; Meng, F.; Gui, X. Synergistic adsorption mechanism of anionic and cationic surfactant mixtures on low-rank coal flotation. ACS Omega 2020, 5, 20630–20637. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhao, X.; Ye, F.; Zhao, G. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohydr. Polym. 2022, 284, 119182. [Google Scholar] [CrossRef]
- Staszak, K.; Wieczorek, D.; Michocka, K. Effect of sodium chloride on the surface and wetting properties of aqueous solutions of cocamidopropylbetaine. J. Surfactants Deterg. 2014, 17, 1221–1228. [Google Scholar] [CrossRef]
- Paulson, O.; Pugh, R.J. Flotation of inherently hydrophobic particles in aqueous solutions of inorganic electrolytes. Langmuir 1996, 12, 4808–4813. [Google Scholar] [CrossRef]
- Harvey, P.; Nguyen, A.V. Influence of electrical double-layer interaction on coal flotation. J. Colloid Interface Sci. 2002, 250, 337–343. [Google Scholar] [CrossRef]
- Kurniawan, A.U.; Ozdemir, O.; Nguyen, A.V.; Ofori, P.; Firth, B. Flotation of coal particles in MgCl2, NaCl, and NaClO3 solutions in the absence and presence of Dowfroth 250. Int. J. Miner. Process. 2011, 98, 137–144. [Google Scholar] [CrossRef]
- Bournival, G.; Pugh, R.J.; Ata, S. Examination of NaCl and MIBC as bubble coalescence inhibitor in relation to froth flotation. Miner. Eng. 2012, 25, 47–53. [Google Scholar] [CrossRef]
- Obisesan, O.; Ahmed, R.; Amani, M. The effect of salt on stability of aqueous foams. Energies 2021, 14, 279. [Google Scholar] [CrossRef]
- Firouzi, M.; Nguyen, A.V. Novel methodology for predicting the critical salt concentration of bubble coalescence inhibition. J. Phys. Chem. C 2014, 118, 1021–1026. [Google Scholar] [CrossRef]
- Katsiavria, A.; Bontozoglou, V. Stability of liquid film flow laden with the soluble surfactant sodium dodecyl sulphate: Predictions versus experimental data. J. Fluid Mech. 2020, 894, A18. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, A.V.; Farrokhpay, S. A critical review of the role of salt on froth stability. Adv. Colloid Interface Sci. 2016, 228, 38–53. [Google Scholar] [CrossRef]
- Gao, M.; Chen, G.; Bai, Y.; Zhang, R.; Zhang, J.; Zhu, S.; Zhang, Z.; Dong, S. Modification of sodium dodecyl sulfate and evaluation of foaming activity. C. R. Chim. 2020, 23, 551–561. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic effect of mixed surfactant systems on foam behavior and surface tension. J. Surfactants Deterg. 2013, 16, 621–630. [Google Scholar] [CrossRef]
- Wang, L.; Yoon, R.-H. Stability of foams and froths in the presence of ionic and non-ionic surfactants. Miner. Eng. 2020, 19, 539–547. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanova-Stancheva, D.; Grozev, N.A.; Mircheva, K.; Karakashev, S.I. Counter-ion effect on the surface potential of foam films and foams stabilized by 0.5 mmol/L sodium dodecyl sulfate. Coatings 2024, 14, 51. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, X.; Sheng, Y.; Zong, R.; Li, C.; Lu, S. Role of salts in performance of foam stabilized with sodium dodecyl sulfate. Chem. Eng. Sci. 2020, 216, 115474. [Google Scholar] [CrossRef]
- NSRDS-NBS 36; Critical Micelle Concentrations of Aqueous Surfactant Systems. National Bureau of Standards: Washington, DC, USA, 1971.
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Kinoshita, K.; Parra, E.; Needham, D. Adsorption of ionic surfactants at microscopic air–water interfaces using the micropipette interfacial area-expansion method: Measurement of the diffusion coefficient and renormalization of the mean ionic activity for SDS. J. Colloid Interface Sci. 2017, 504, 566–574. [Google Scholar] [CrossRef]

| Solution | Lifetime (20 μL/h) (s) | SD (20 μL/h) | Lifetime (10 μL/h) (s) | SD (10 μL/h) |
|---|---|---|---|---|
| SDS 3000 ppm + 4 mM NaCl | 166 | ±14 | 280 | ±8 |
| SDS 2000 ppm + 4 mM NaCl | 145 | ±17 | 195 | ±9 |
| SDS 1000 ppm + 4 mM NaCl | 85 | ±2 | 131 | ±16 |
| SDS 500 ppm + 4 mM NaCl | 0 | ±5 | 0 | ±7 |
| SDS 3000 ppm | 200 | ±5 | 296 | ±15 |
| SDS 2000 ppm | 91 | ±11 | 129 | ±13 |
| SDS 1000 ppm | 58 | ±10 | 80 | ±14 |
| SDS 500 ppm | 0 | ±4 | 0 | ±2 |
| Ethylan 500 ppm + NaCl | 131 | ±7 | 180 | ±18 |
| Ethylan 250 ppm + NaCl | 54 | ±15 | 97 | ±12 |
| Ethylan 125 ppm + NaCl | 0 | ±12 | 0 | ±17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamanis, A.T.; Evgenidis, S.P.; Karapantsios, T.D.; Kostoglou, M. An Innovative Approach for Assessing Foam Stability Based on Electrical Conductivity Measurements of Liquid Films. Colloids Interfaces 2025, 9, 52. https://doi.org/10.3390/colloids9040052
Zamanis AT, Evgenidis SP, Karapantsios TD, Kostoglou M. An Innovative Approach for Assessing Foam Stability Based on Electrical Conductivity Measurements of Liquid Films. Colloids and Interfaces. 2025; 9(4):52. https://doi.org/10.3390/colloids9040052
Chicago/Turabian StyleZamanis, Angelos T., Sotiris P. Evgenidis, Thodoris D. Karapantsios, and Margaritis Kostoglou. 2025. "An Innovative Approach for Assessing Foam Stability Based on Electrical Conductivity Measurements of Liquid Films" Colloids and Interfaces 9, no. 4: 52. https://doi.org/10.3390/colloids9040052
APA StyleZamanis, A. T., Evgenidis, S. P., Karapantsios, T. D., & Kostoglou, M. (2025). An Innovative Approach for Assessing Foam Stability Based on Electrical Conductivity Measurements of Liquid Films. Colloids and Interfaces, 9(4), 52. https://doi.org/10.3390/colloids9040052









