1. Introduction
Emulsions are colloidal systems composed of two immiscible liquids, in which one phase is dispersed as droplets within a continuous phase. Despite their wide industrial and commercial relevance, emulsions are thermodynamically unstable and tend toward phase separation over time due to destabilization processes, such as coalescence, creaming, sedimentation, and Ostwald ripening [
1,
2]. Emulsion stability is intimately linked to interfacial phenomena, particularly the nature and structure of the interfacial film formed by surface-active agents. Surfactants are amphiphilic molecules that stabilize emulsions by lowering interfacial tension and forming interfacial layers that reduce droplet coalescence.
The role of surfactants in emulsions is well established, with several theoretical models describing how their molecular characteristics influence emulsion type and stability. The hydrophilic–lipophilic balance (HLB) system assigns numerical values to nonionic surfactants based on the relative strength of their hydrophilic and lipophilic segments, helping predict whether a given surfactant will favor oil-in-water (O/W) or water-in-oil (W/O) emulsion [
3]. Moreover, the hydrophilic–lipophilic deviation (HLD) framework has emerged as a comprehensive approach for predicting emulsion behavior across a wide range of surfactant systems. HLD incorporates surfactant structure, oil type, salinity, temperature, and co-surfactant effects into a unified equation that quantifies the deviation from balanced interfacial curvature. A value of HLD = 0 corresponds to optimal conditions where the surfactant exhibits equal affinity for oil and water, favoring the formation of bicontinuous or microemulsion structures. Negative HLD values favor oil-in-water (O/W) emulsions, while positive values promote water-in-oil (W/O) systems [
4].
The conversion from W/O to O/W emulsion or vice-versa is called phase inversion, which can be broadly classified into two types, catastrophic and transient inversion. Catastrophic phase inversion, first introduced by Salager [
5], is primarily driven by an increase in the volume fraction of the dispersed phase. As the internal phase volume increases and approaches the close-packing limit, droplet crowding, coalescence, and hydrodynamic instabilities lead to a sudden and often irreversible conversion of emulsion type [
6]. In contrast, transient phase inversion arises from changes in the surfactant’s physicochemical properties, such as preferential solubility and interfacial curvature [
7,
8]. The surfactant properties can be altered by varying temperature, salinity, and pH.
In surfactant-stabilized emulsions, phase inversion is governed by the dynamic redistribution of surfactant molecules at the oil–water interface coupled with droplet coalescence, interfacial curvature changes, and mixing-induced instabilities. As the internal phase volume increases during emulsification, surfactant molecules must reorient and spread over an expanding interfacial area. When surfactant adsorption becomes insufficient to maintain interfacial stability, droplet crowding and deformation lead to coalescence and a sudden reversal of phase continuity. This mechanism has been supported by conductivity and light scattering studies that track the emulsion inversion point (EIP) and show abrupt increases in droplet size and polydispersity near the inversion threshold [
6,
9]. Furthermore, studies have shown that phase inversion is accompanied by viscosity divergence and transient formation of multiple emulsions, consistent with kinetic models involving shear-induced instability and droplet–droplet interactions [
10,
11,
12]. These findings collectively support the view that catastrophic inversion is a nonequilibrium process, proceeding through metastable intermediates rather than a single thermodynamic transition. Importantly, recent studies in crude oil production systems have shown that natural surfactants, such as asphaltenes and acidic fractions, contribute significantly to the stabilization of water-in-oil emulsions and can mitigate challenges like gas hydrate agglomeration and flowline plugging. This highlights how the structural and chemical properties of interfacial films directly affect droplet stability and dispersion behavior, further underscoring the practical importance of interfacial phenomena in controlling emulsion stability and, hence, its phase inversion behavior under real operating conditions [
13].
In contrast, particle-stabilized emulsions, commonly referred to as Pickering emulsions, exhibit markedly different inversion behavior compared to surfactant-stabilized systems. In these systems, inversion and emulsion stability is closely linked to the wetting properties and surface coverage of the particles at the oil–water interface [
14,
15]. Due to the irreversible adsorption and mechanical rigidity of the particle-laden interface, phase inversion can be significantly delayed or even suppressed when particles form a densely packed or jammed interfacial layer [
16]. This resistance to inversion arises because the particle-stabilized droplets become less deformable and less prone to coalescence, effectively raising the critical internal phase volume required for structural transition. Moreover, the inversion point in Pickering systems can be further shifted by modifying particle hydrophobicity, aspect ratio, or interfacial elasticity, highlighting the importance of particle–interface interactions in controlling emulsion morphology [
17,
18].
As a result, the catastrophic phase inversion of Pickering emulsions is typically delayed to significantly higher dispersed phase volume fractions. This behavior has been demonstrated in several systems using different types of nanoparticles. For instance, Bains and Pal [
19] showed that starch nanoparticles (SNPs) stabilized W/O emulsions and shifted the inversion point to higher aqueous volume fractions, correlating the delay with increased nanoparticle concentration and interfacial packing density [
19]. Similarly, Malhotra and Pal [
20] observed that hydrophobic silica nanoparticles increased the phase inversion concentration compared to hydrophilic ones, further emphasizing the role of wettability and particle concentration in delaying inversion [
20]. Ogunlaja et al. [
21] reported that both hydrophilic and hydrophobic starch nanoparticles irreversibly adsorbed at the interface and contributed to increased emulsion stability and delayed inversion points. Furthermore, combining nanoparticles with surfactants to form hybrid stabilizers has been shown to synergistically enhance emulsion stability while extending the inversion point beyond what is achievable with either component alone [
22].
This is consistent with the findings from Binks et al. [
23], where it was demonstrated that hydrophobic silica nanoparticles delayed the inversion of W/O emulsions to O/W emulsions until water volume fractions approached
0.7, far exceeding typical inversion points seen with surfactants alone. Further, Zanini et al. [
24] reported that emulsions stabilized by rough colloidal particles exhibited delayed phase inversion due to the metastable wetting states of the particles at the interface. Their study highlighted that the surface roughness of colloids can induce contact angle hysteresis, leading to enhanced emulsion stability and resistance to inversion under mechanical agitation. Similarly, González-González et al. [
25] investigated the effects of oil phase on the inversion of Pickering emulsions stabilized by palmitic acid-decorated silica nanoparticles. Their findings indicated that the interaction between the nanoparticles and palmitic acid at the oil–water interface resulted in a more robust interfacial layer, thereby delaying phase inversion and enhancing emulsion stability [
25]. These insights highlight the broader importance of Pickering emulsions as robust, particle-stabilized systems that can maintain emulsion stability under demanding conditions. This is particularly relevant in energy applications, such as enhanced oil recovery, where stable Pickering emulsions have been shown to improve oil displacement efficiency and provide effective flow diversion and conformance control in high-temperature, high-salinity reservoirs [
26].
Such examples highlight the practical relevance of controlling phase inversion in robust particle-stabilized emulsions for flow assurance and reservoir applications. Among particle stabilizers, nanocrystalline cellulose (NCC) has emerged as a promising bio-based Pickering agent due to its unique anisotropic shape, high aspect ratio, surface charge, and renewable origin. Its amphiphilic character enables irreversible adsorption at oil–water interfaces, forming rigid, percolating interfacial networks that inhibit droplet coalescence and potentially influence phase inversion thresholds [
27,
28,
29]. In addition to its amphiphilic nature, NCC’s high aspect ratio, surface charge, and rigidity enable dense interfacial packing and strong interparticle interactions, contributing to the formation of robust, jammed interfacial films that resist coalescence and droplet deformation [
30]. Moreover, recent work has shown that modifying NCC morphology, such as producing spherical NCCs, can further enhance emulsion stability across a broad range of pH, ionic strengths, and temperatures, highlighting its versatility under diverse processing conditions [
31]. Hybrid approaches that combine NCCs with nanofibrils have also demonstrated synergistic stabilization effects through depletion interactions and network formation, offering additional routes to tailor emulsion properties [
32]. Together, these advances illustrate NCC’s unique potential as a tunable, renewable Pickering stabilizer with broad applicability in food, cosmetic, and biomedical formulations seeking to reduce reliance on synthetic surfactants.
In addition, Kinra and Pal [
33] further examined the rheology of oil-in-water (O/W) emulsions stabilized by NCC, reporting increased viscosity and improved stability with rising NCC concentration, reinforcing the role of NCC as an effective interfacial stabilizer [
33]. However, its influence on phase inversion behavior has received little attention and remains largely unexplored. Despite extensive studies on surfactant- and particle-stabilized emulsions individually, direct comparative investigations of their catastrophic phase inversion behavior under identical conditions remain scarce. Bridging this gap provides insights into the distinct mechanisms by which surfactants and particles govern catastrophic phase inversion, which is essential for designing robust, stable emulsions that perform reliably under industrial processing or harsh reservoir conditions. Beyond mechanistic insight, employing NCC provides a renewable, bio-based alternative that can enhance emulsion stability while supporting more sustainable formulation strategies.
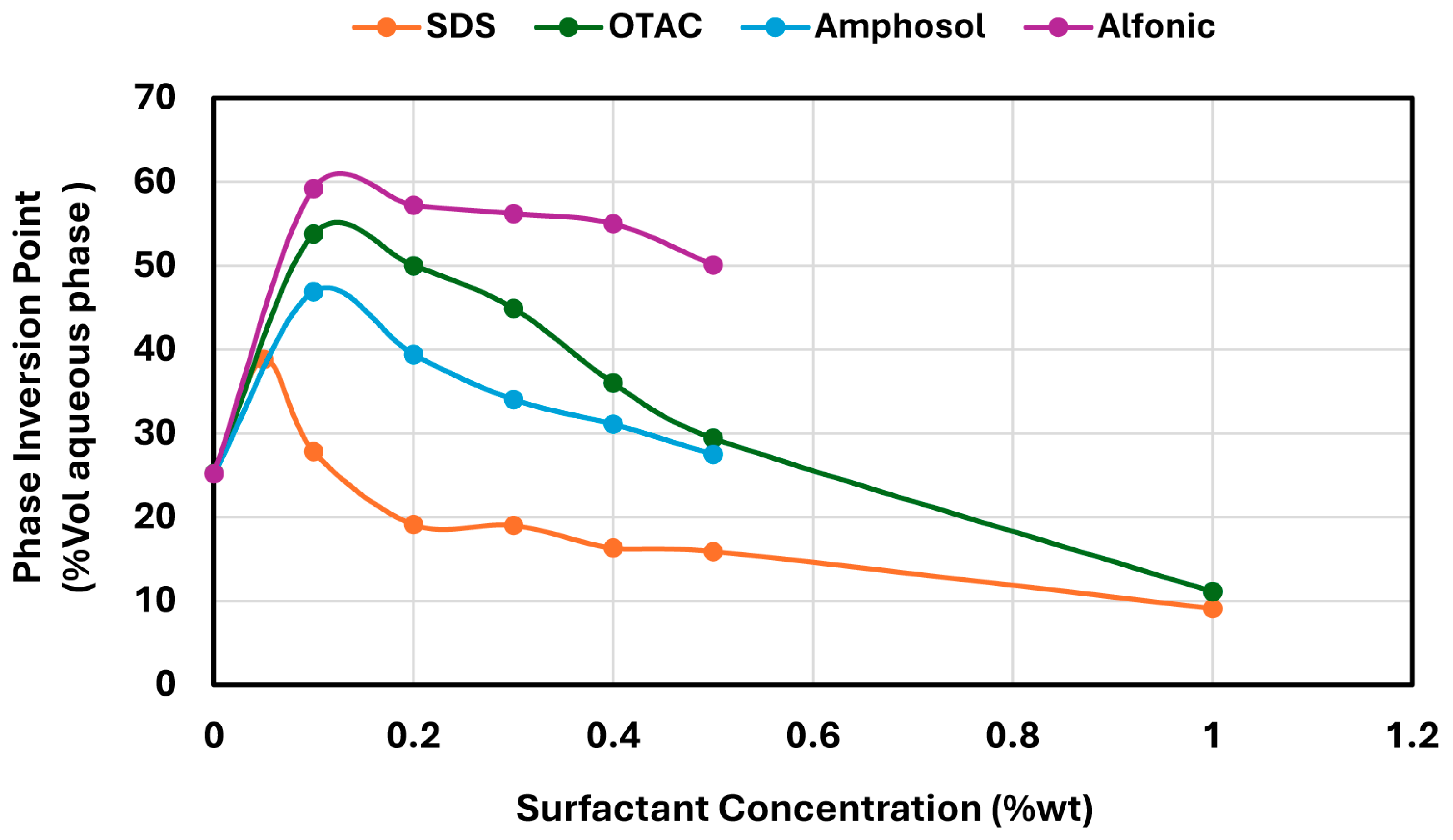
To address this gap and expand the understanding of stabilizer performance, we compare NCC-stabilized emulsions to systems stabilized by surfactants of varying chemical types: anionic (sodium dodecyl sulphate referred to as SDS), cationic (octadecyltrimethylammonium chloride referred to as OTAC), nonionic (C12–14 alcohol ethoxylate referred to as Alfonic), and zwitterionic (cetyl betaine referred to as Amphosol). These surfactants were selected to represent a range of headgroup chemistries and interfacial behaviors, enabling a systematic comparison of how surfactant charge and structure influence catastrophic phase inversion.
In this study, we show that NCC markedly delays the catastrophic inversion of water-in-oil (W/O) emulsion to oil-in-water (O/W) emulsion, whereas this phase transition in surfactant-stabilized systems occurs at much lower internal phase fractions. To the best of our knowledge, this is the first study to examine catastrophic phase inversion of W/O to O/W emulsions stabilized by nanocrystalline cellulose.
2. Materials and Methods
2.1. Chemicals
White mineral oil (petroleum), trade name Purity FG WO-15, was supplied by Petro-Canada (Mississauga, ON, Canada) and used as the continuous phase for all emulsions. The oil has a kinematic viscosity of 15.0 cSt at 40 °C and 3.4 cSt at 100 °C.
Nanocrystalline cellulose was obtained from CelluForce Inc. (Windsor, QC, Canada) under the trade name CelluForce NCC
® C100-NASD90. It is supplied as a dry white powder. The cellulose nanocrystals were rod-shaped, with a mean length of 76 nm and a mean width of 3.4 nm [
29].
Sodium dodecyl sulfate (SDS), an anionic surfactant with a molecular weight of 288.38 g/mol, was supplied as white powder (≥99% purity) by Fisher Scientific (Waltham, MA, USA). SDS is widely used as a benchmark ionic surfactant in detergents, cosmetics, and emulsion polymerization.
Octadecyltrimethylammonium chloride (OTAC), a cationic surfactant with a molecular weight of 348.05 g/mol, was provided by Molekula (Dallas, TX, USA). OTAC is a quaternary ammonium compound featuring an 18-carbon alkyl chain and is commonly used as a phase transfer agent, disinfectant, and emulsifier in industrial formulations.
C12–14 alcohol ethoxylate with 3 EO units (Alfonic 1412-3), a nonionic surfactant, was supplied by Sasol Chemicals (Sandton, South Africa). Nonionic alcohol ethoxylates are widely used in household cleaners, agrochemical formulations, and oilfield emulsions due to their mildness and broad interfacial behavior.
Cetyl betaine (Amphosol), a zwitterionic surfactant, was provided by Stepan Company (Northfield, IL, USA). Cetyl betaine contains both quaternary ammonium and carboxylate groups and is commonly used in personal care products, mild shampoos, and cosmetics as a co-surfactant with skin-friendly properties.
2.2. Preparation of NCC Dispersions
NCC was dispersed in deionized water using a variable-speed Gifford–Wood (Hudson, NY, USA) rotor–stator homogenizer (Model 1-L) equipped with an open slotted stator head for high-shear dispersion and emulsification. A batch volume of 2 L was prepared at room temperature, and NCC was added at concentrations ranging from 0 to 1.5 wt% relative to the aqueous phase. The dispersion was first premixed at 2000 rpm for 5 min to wet and break up agglomerates and then subjected to high-shear homogenization at 5000 rpm until complete dispersion was achieved, as confirmed by visual inspection for the absence of visible particles or flocs. After homogenization, sodium chloride (99.5% purity) was added to provide ionic conductivity, allowing for the detection of phase inversion by conductivity measurements during dynamic emulsification. The resulting NCC dispersions were used immediately as the aqueous phase in emulsion preparation.
2.3. Phase Inversion Experiments
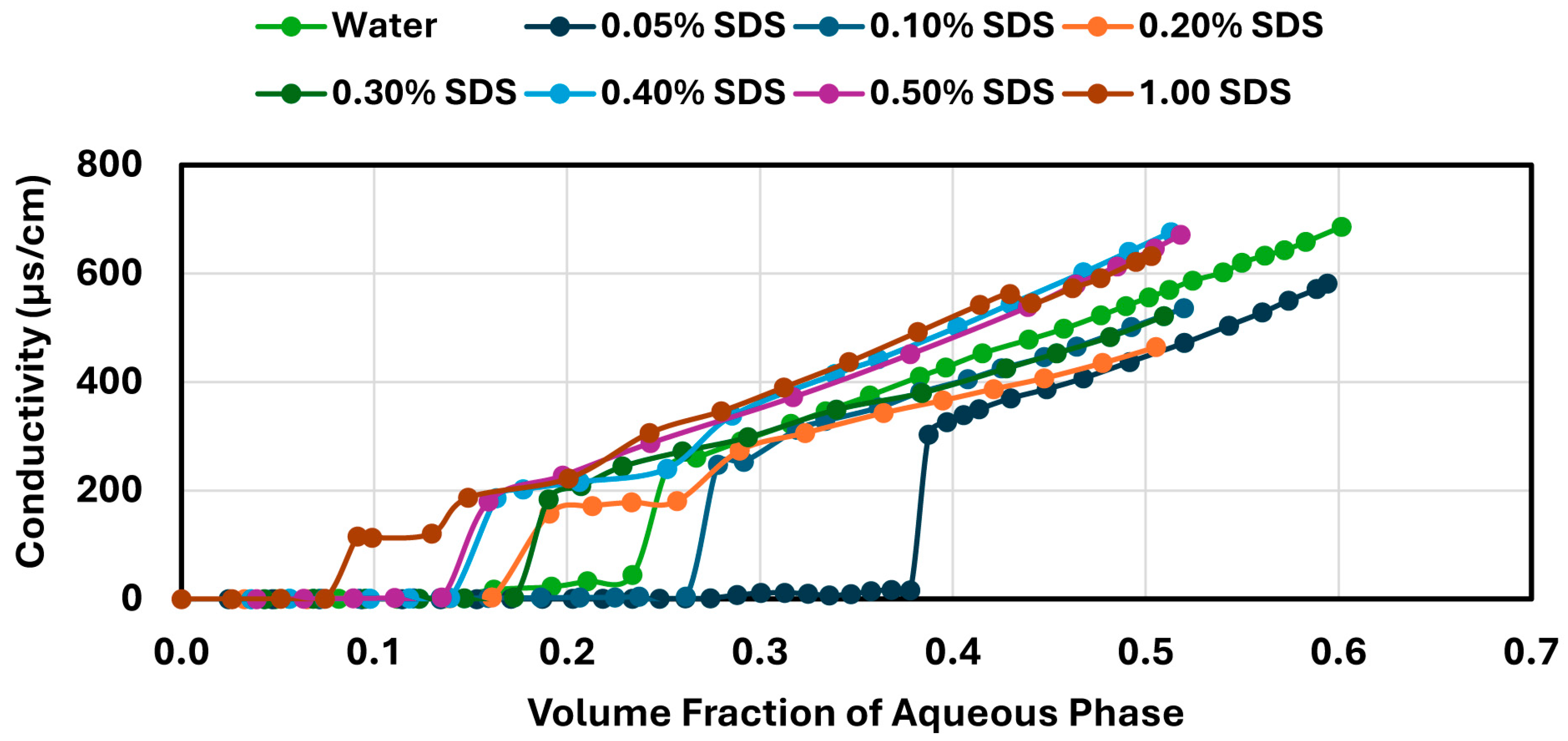
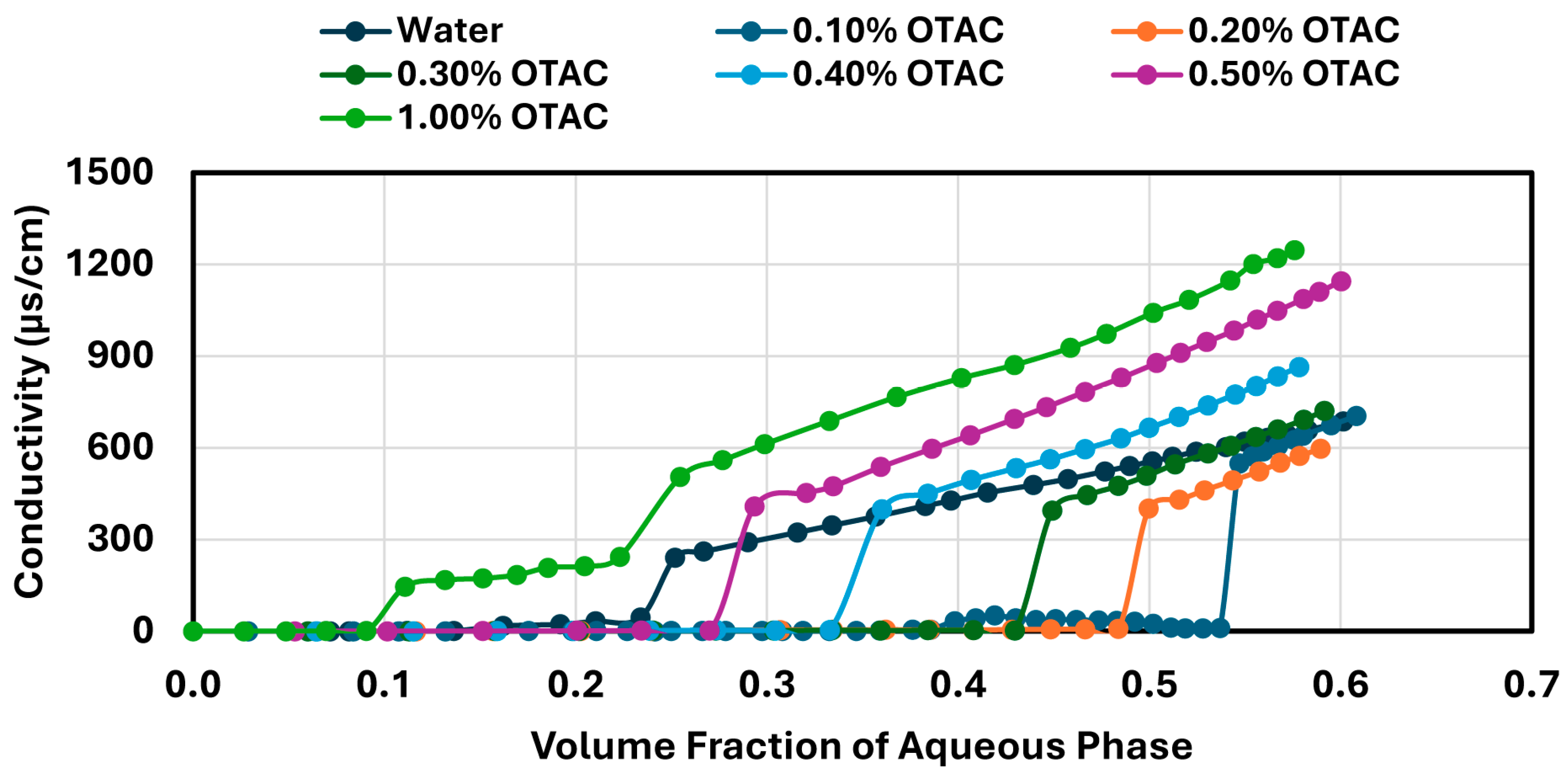
Phase inversion was investigated by gradually adding the aqueous phase, containing either NCC or surfactants (anionic, cationic, nonionic, or zwitterionic), to a known volume of oil (WO-15, 600 mL) under continuous agitation using a Gifford–Wood rotor–stator homogenizer. Additions were performed at 2–3 min intervals to ensure adequate mixing between steps. The aqueous phase contained fixed stabilizer concentration and was incrementally added up to approximately 1500 mL, resulting in a maximum final aqueous volume fraction of about 0.7. Conductivity measurements were taken after each addition, including after the inversion point, to fully capture the conductivity profile throughout the emulsification process. The inversion point was determined by a sharp increase in conductivity, corresponding to the transition from a water-in-oil (W/O) to an oil-in-water (O/W) emulsion. The homogenizer speed was initially set at 4000 rpm and gradually increased up to 5000 rpm as needed to maintain a consistent mixing intensity, compensating for the higher viscosity as the emulsion approached inversion. The stator geometry and addition protocol were kept constant for all tests to maintain comparable energy input and minimize variation in droplet size distribution across samples. While droplet size was not measured directly, this approach ensured uniform shearing conditions throughout the emulsification process.
2.4. Stability Experiments
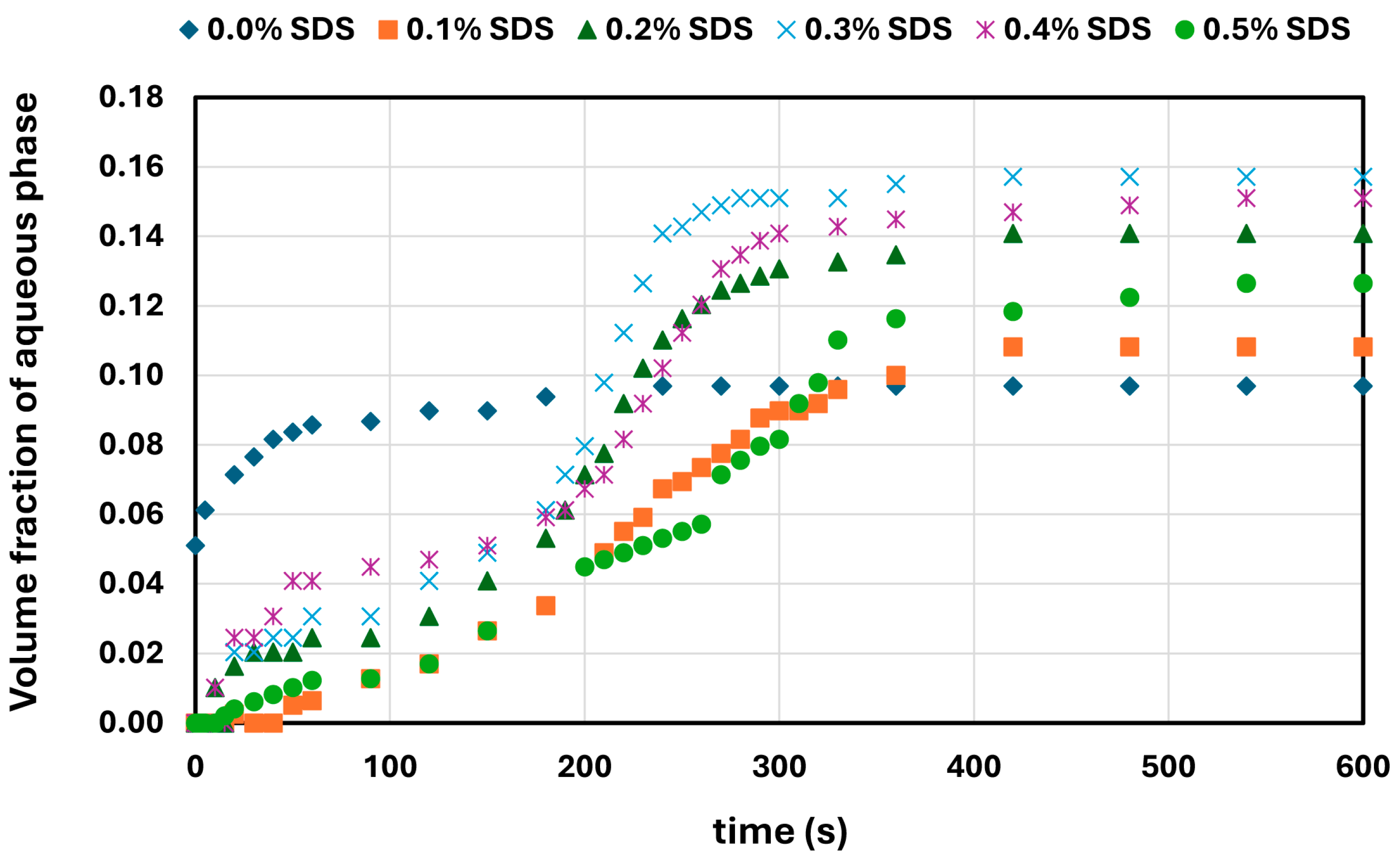
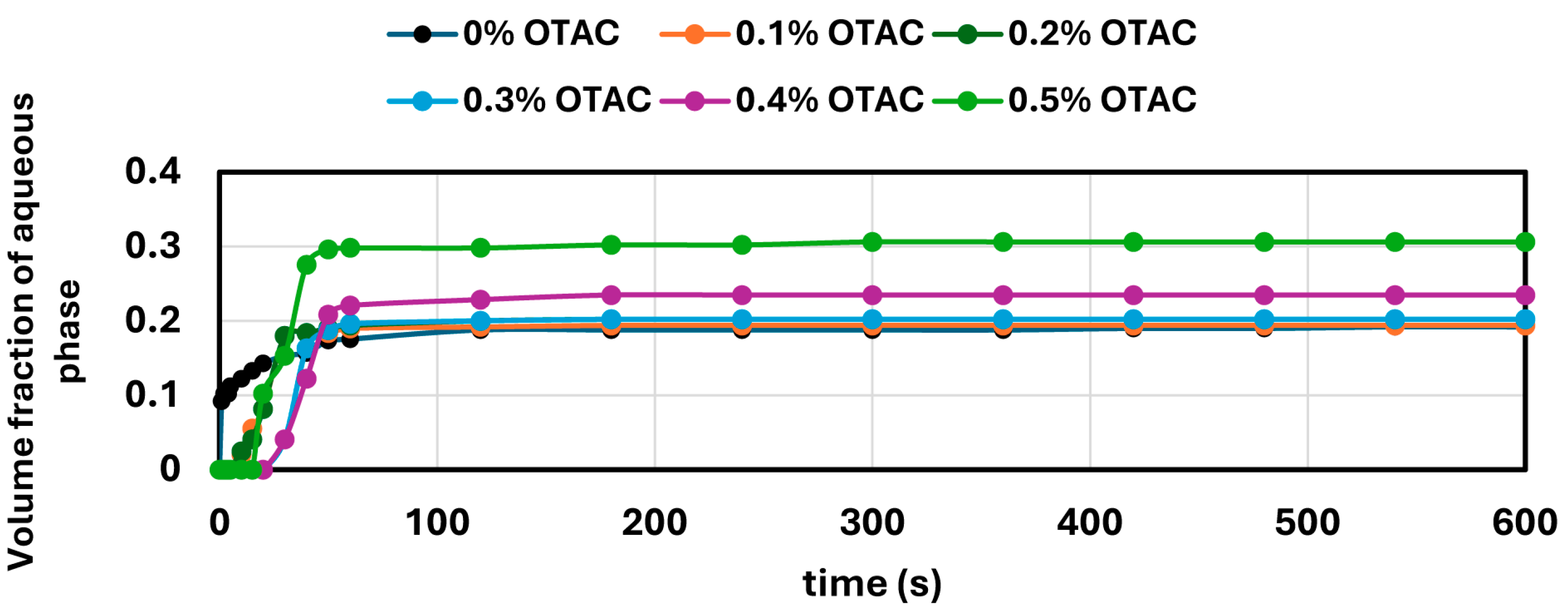
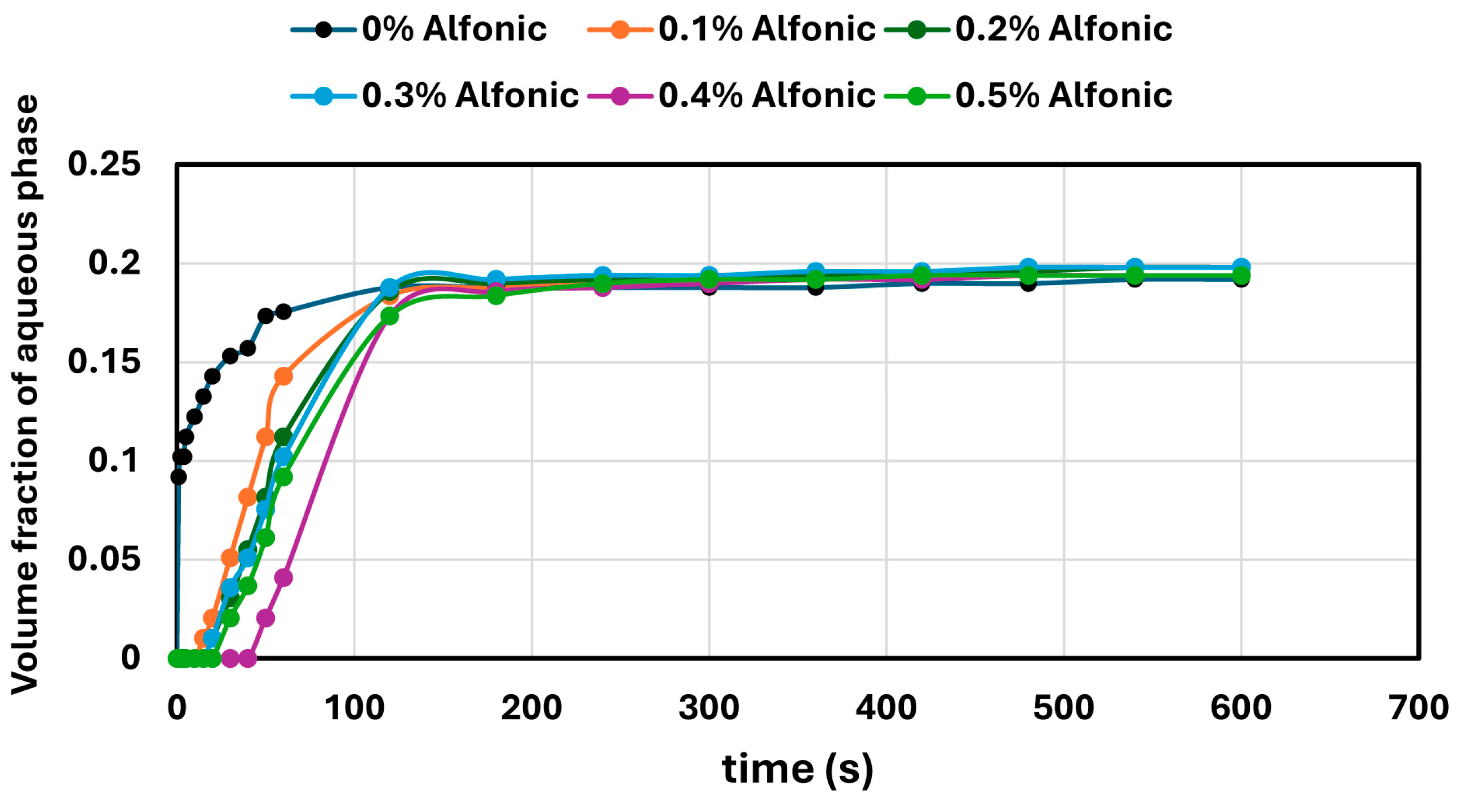
W/O emulsions containing a fixed amount of aqueous phase (generally 20 vol%, except in the case of SDS, where 10 vol% was used) were prepared by homogenizing oil with aqueous solutions of NCC or surfactants at high speed for 5 min. After emulsification, the samples were transferred to 500 mL graduated cylinders and stored at ambient conditions. Emulsion stability was assessed by monitoring the volume of coalesced aqueous phase over time. The extent of destabilization was quantified as the volume ratio of the separated aqueous phase to the total emulsion volume.
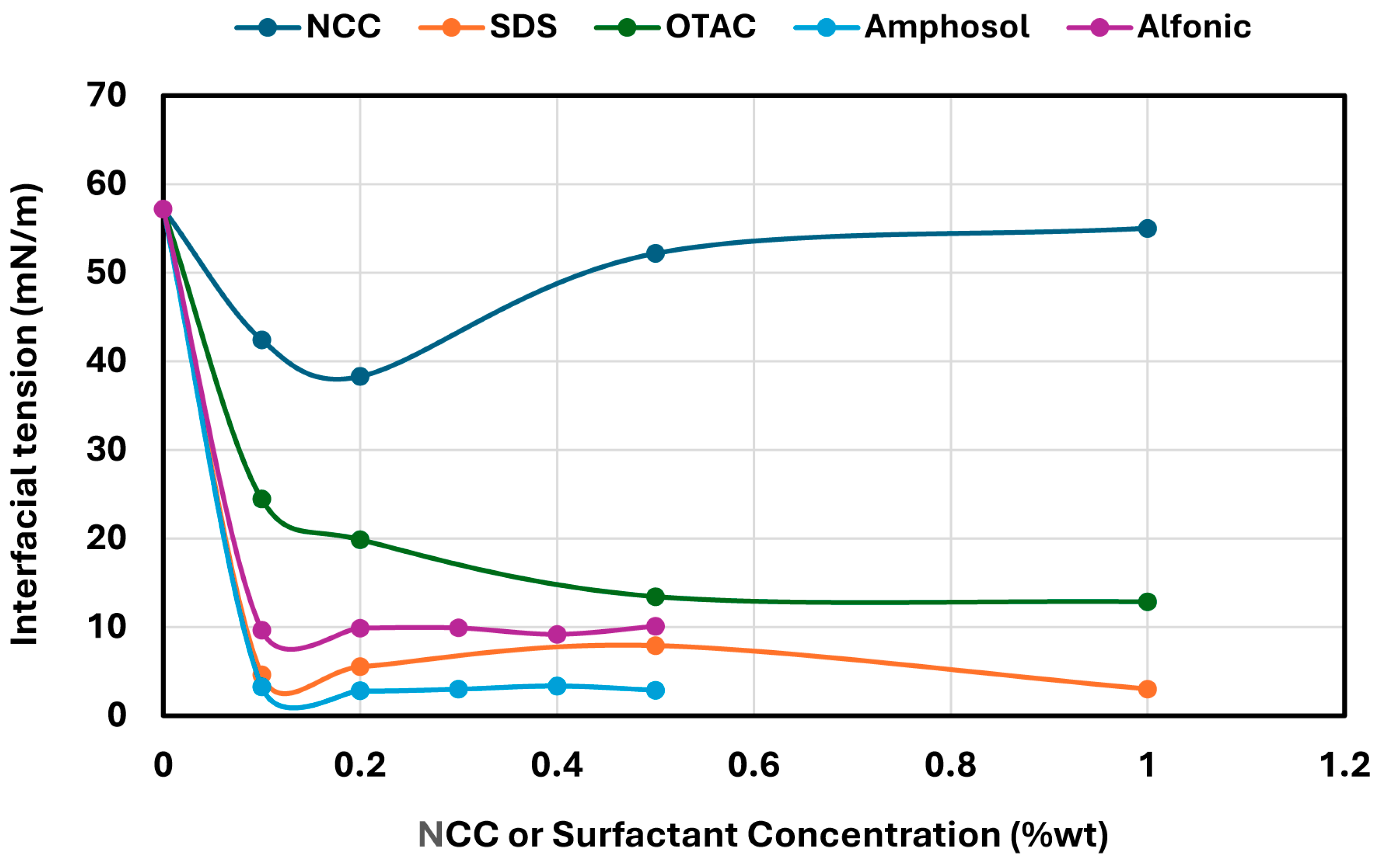
2.5. Interfacial and Surface Tension Measurements
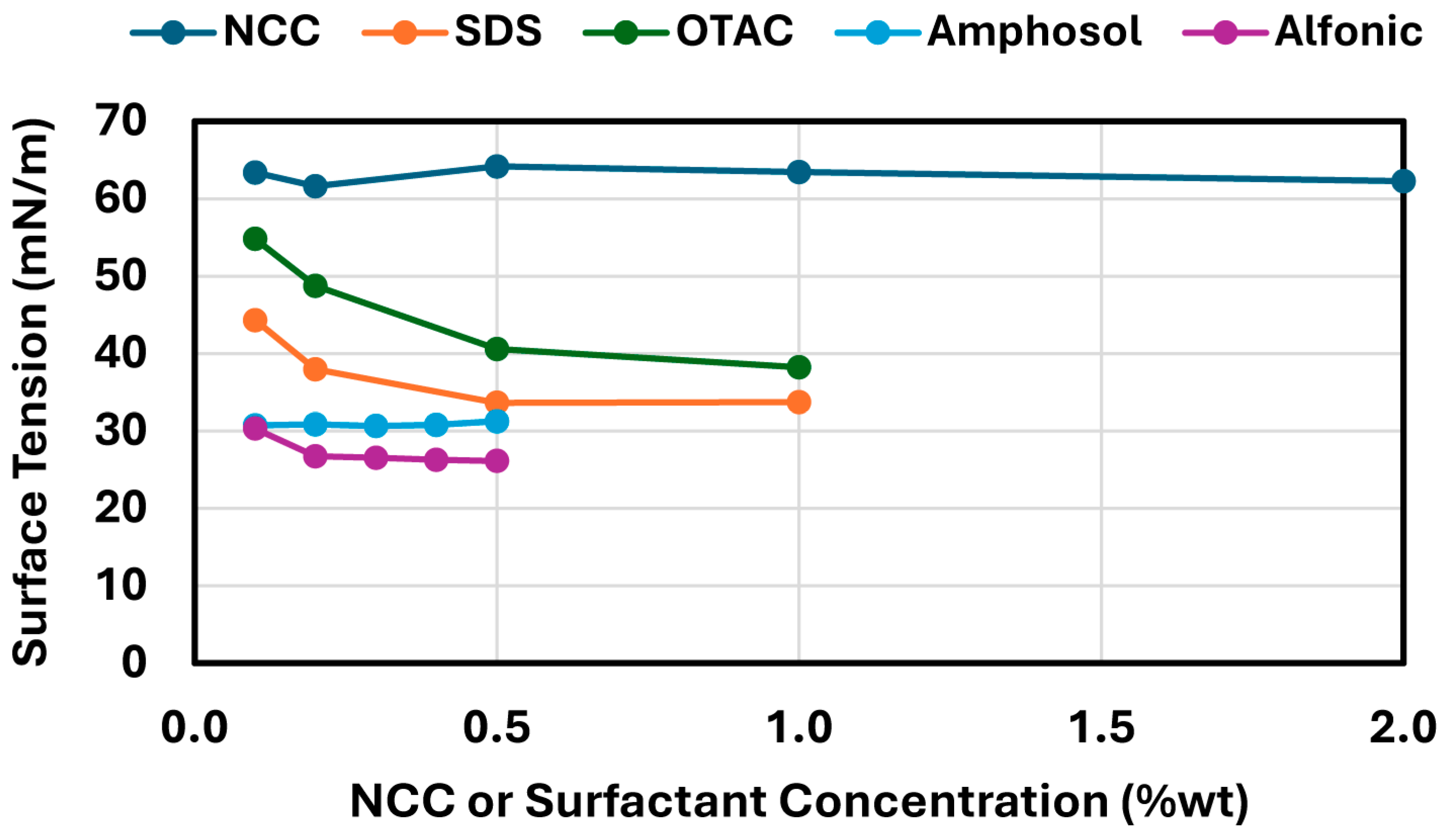
Interfacial and surface tension measurements were performed using the pendant drop method with a smartphone-based pendant drop tensiometer (Droplet Lab, Markham, ON, Canada). Drop shape analysis was carried out by fitting the droplet profile using the Young–Laplace equation with axisymmetric drop shape analysis (ADSA) [
34]. A pendant droplet of the aqueous phase (typically 10–20 µL), containing nanocrystalline cellulose (NCC) or surfactants (anionic, cationic, nonionic, or zwitterionic), was formed at the tip of a stainless-steel needle (1.8 mm diameter) connected to a 500 µL Hamilton
® gastight syringe (Model 1750 TPLT, Hamilton, Reno, NV, USA). The droplet was dispensed using a screw-driven plunger for precise control of flow rate and drop formation. Back illumination was provided by an LED light to ensure consistent optical accuracy. For interfacial tension measurements, the droplet was dispensed into a sealed quartz cuvette containing WO-15 oil; for surface tension, the droplet was suspended in air. Each measurement was repeated 10 times per solution to ensure precision and reproducibility, and average values were reported. All measurements were conducted at ambient temperature (22 ± 1 °C).
4. Conclusions
This work examined the catastrophic phase inversion and coalescence stability behavior of W/O emulsions stabilized by nanocrystalline cellulose (NCC) and compared them with emulsions stabilized by four different molecular surfactants, namely sodium dodecyl sulfate (SDS), octadecyltrimethylammonium chloride (OTAC), C12–14 alcohol ethoxylate (Alfonic), and cetyl betaine (Amphosol). NCC-stabilized emulsions exhibited an increase in the critical aqueous phase volume fraction for phase inversion as NCC concentration increased. This trend was accompanied by improved coalescence resistance, with minimal aqueous phase separation observed at concentrations ≥0.2 wt%. The behavior plateaued at higher concentrations, indicating limited further gains in stability.
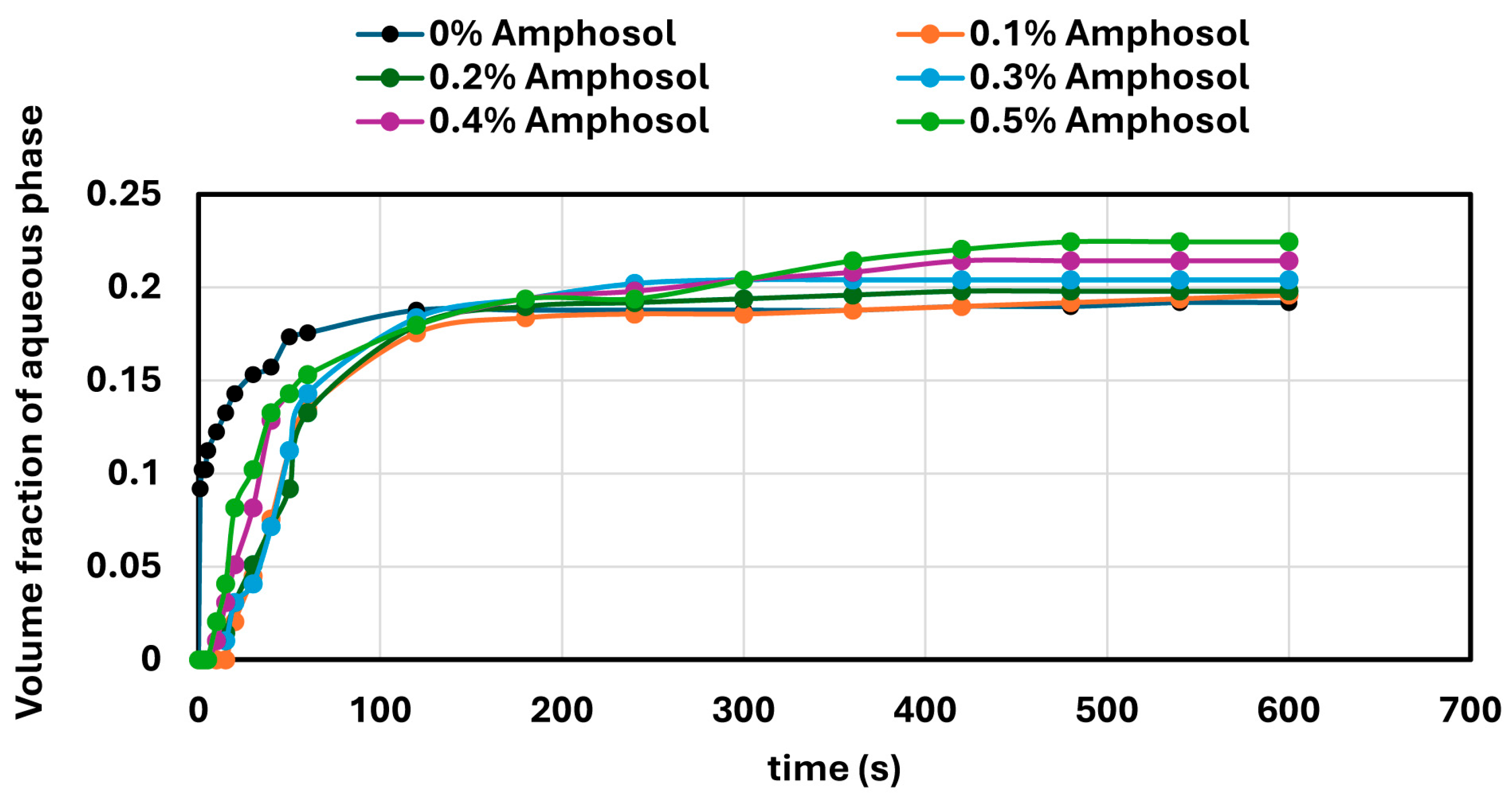
In contrast, surfactant-stabilized systems exhibited non-monotonic inversion behavior. At low concentrations, all surfactants delayed inversion relative to the surfactant-free control, consistent with improved interfacial stabilization. However, as the surfactant concentration increased beyond a threshold, the inversion point shifted to lower aqueous phase fractions. This behavior correlated with interfacial tension measurements, where an initial sharp decrease in IFT was followed by a plateau, indicating that excess surfactant does not contribute to additional stabilization but rather becomes more available under shear conditions. Coalescence stability also improved with increasing surfactant concentration, though the extent of improvement varied by the surfactant type. SDS, OTAC, and Alfonic showed strong concentration-dependent stabilization. Amphosol displayed the highest coalescence stability at low concentrations (0.1–0.2 wt%), with slightly diminished performance at higher levels.
Together, these findings highlight the fundamental differences in the stabilization mechanisms of particle and surfactant systems. While surfactants modulate interfacial tension, NCC appears to stabilize emulsions through a distinct, concentration-dependent mechanism that maintains emulsion integrity over extended internal phase ranges. Importantly, this work demonstrates that NCC stabilization markedly delays catastrophic phase inversion, allowing for emulsions to remain stable at higher internal phase fractions than comparable surfactant systems. This mechanistic insight could help guide industrial formulation strategies for high internal phase emulsions in applications such as food, cosmetics, and enhanced oil recovery, where controlling phase inversion is critical to ensure product stability and performance. Moreover, the use of NCC, a bio-based and renewable material, offers an opportunity to improve the sustainability of emulsion formulations by reducing reliance on synthetic surfactants.
It should be noted that this work was conducted under controlled laboratory conditions using a well-defined oil–water system. The results assume consistent droplet size distributions generated under uniform mixing. Variations in droplet size or polydispersity could affect the onset of catastrophic phase inversion. Interfacial rheology and time-dependent interfacial tension effects were not evaluated in this study but may influence stabilization mechanisms under dynamic process conditions. Future studies should investigate the role of interfacial rheology, droplet size distribution, and more complex oil–water systems to confirm the generality of these trends under industrially relevant conditions.