Abstract
The increasing demand for sustainable mining practices has driven the development of environmentally friendly reagents for mineral processing. This study investigates vitamin E sodium succinate (VE_SS), a novel bio-based collector, for its potential in hematite flotation. The performance of VE_SS was benchmarked against sodium oleate (NaOL), a widely used conventional collector in mineral processing. To assess the flotation performance of VE_SS, micro-flotation experiments were conducted using hematite, sourced from a mine, and silica, a common associated gangue mineral. These tests were complemented by comprehensive surface characterizations, including contact angle measurements, zeta potential analysis, Fourier-transform infrared (FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS), to investigate the adsorption mechanisms of VE_SS in comparison to NaOL. The results demonstrate that VE_SS effectively enhances hematite recovery, achieving levels comparable to NaOL. Furthermore, VE_SS exhibited reduced sensitivity to pH, addressing a key limitation of NaOL, which performs well in neutral to alkaline conditions but shows significantly lower recovery under acidic pH. These findings highlight the potential of VE_SS as a bio-based alternative to conventional collectors, contributing to the advancement of more sustainable mineral processing practices.
1. Introduction
Iron, one of the most abundant metallic elements in the Earth’s crust, plays a critical role in various economic sectors, including manufacturing, transportation, construction, and building. Hematite, a primary source of iron containing up to 69% metal iron, is one of the most economically significant iron minerals. However, the depletion of high-grade iron-ore reserves has necessitated the beneficiation of low-grade and refractory iron ores. These ores often consist of fine hematite particles closely associated with quartz, the predominant gangue mineral. Achieving sufficient liberation of hematite requires intensive grinding, which produces large quantities of fine particles and necessitates the use of substantial chemical reagents. Consequently, there is a critical need for selective and environmentally friendly flotation reagents to enhance separation efficiency while minimizing ecological impact [1,2].
The flotation process, a cornerstone of mineral processing for over a century, exploits the wettability contrast between minerals to achieve separation. Flotation of iron ores is typically performed via direct flotation (hematite floated) or reverse flotation (hematite depressed) [3,4]. The process involves two primary classes of reagents: collectors, which render target mineral surfaces hydrophobic, and depressants, which suppress gangue minerals [5]. While effective, traditional reagents often pose environmental risks, driving a shift towards greener alternatives.
Recent research has explored the use of eco-friendly and biodegradable reagents, including biosurfactants, as sustainable alternatives to traditional petrochemical-based reagents [6,7,8]. Biosurfactants, derived from biological sources such as plants and microbial processes, offer several advantages, including low toxicity, biodegradability, and compatibility with biological systems. Low-molecular-weight biosurfactants, such as glycolipids [9], saponins [10], fatty acids [11], and surfactins [12], have demonstrated efficacy as bio-based and environmentally friendly collectors. High-molecular-weight biosurfactants, including proteins [13], enzymes [14], and cellulose derivatives [15], are often used as depressants to enhance separation efficiency. Fatty acids, a prominent class of bio-based collectors, are commonly employed for iron oxide flotation due to their lower environmental impact. However, their selectivity and performance under varying flotation conditions, such as pH and ionic strength, require further investigation [3,16,17,18].
The development of environmentally friendly collectors is important for supporting sustainable mining practices and aligning with industry goals to reduce chemical usage and environmental impact. This study contributes to these efforts by investigating the use of a novel bio-based collector, vitamin E sodium succinate (VE_SS), in the flotation of hematite and silica under controlled laboratory conditions. Related compounds, such as vitamin E polyethylene glycol 6000 succinate, have recently been explored as bio-frothers in froth flotation [19], highlighting the potential of vitamin E derivatives in mineral processing. Furthermore, vitamin E polyethylene glycol 1000 succinate (TPGS), a non-ionic surfactant, has been widely used in drug delivery applications as a solubilizer, absorption and permeation enhancer, emulsifier, and surface stabilizer [20,21,22]. These compounds exhibit desirable properties such as biocompatibility and biodegradability, with their degradation occurring through ester hydrolysis, a well-established process that converts esters into carboxylic acids and alcohols. This degradation can occur in vivo, as demonstrated in drug delivery systems, or under alkaline conditions [23,24]. VE_SS, a sodium salt derivative of D-α-tocopherol succinate used in this study, is a well-known precursor to the well-established bio-based compound TPGS. During degradation, it decomposes into succinic acid and the natural product, vitamin E. This makes it an environmentally friendly alternative reagent compared to those currently being used. Although it is commonly used in the production of bio-based materials, this compound has yet to be explored as a reagent in the mineral industry, offering a unique opportunity to investigate its potential beyond its current applications.
In this study, single-mineral flotation experiments were conducted on hematite and silica, selected as representative minerals, to evaluate the performance of VE_SS. Micro-flotation tests and comprehensive mineral surface property characterization, including contact angle measurements, zeta potential analysis, Fourier-transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and X-ray fluorescence (XRF), were conducted. Sodium oleate (NaOL), a widely used fatty acid derivative, was employed as a reference collector, enabling a comparative assessment of VE_SS’s efficacy and selectivity.
2. Materials and Methodology
2.1. Materials and Reagents
The hematite sample was received from a commercial mine and 200 G size silica sample was sourced from Holcim product. Both samples were wet-sieved to obtain +38 µm and −38 µm size fractions after which they were dried. The +38 µm fraction was used for conducting the micro-flotation tests. X-ray diffraction (XRD) and X-ray fluorescence (XRF) analysis, performed using a PANalytical X’Pert series diffractometer (Malvern Panalytical Ltd., Malvern, UK) and a Bruker S8 TIGER series II (Bruker AXS GmbH, Karlsruhe, Germany), respectively, showed that the contents of both hematite and silica exceeded 90%, indicating their high purity as represented in Table 1 and Table 2. The data for chemical analysis of hematite and silica indicate that the purity of hematite and silica was 93.56% and 97.77%, respectively. X-ray diffraction (XRD) spectra of hematite and silica are presented in Figure 1, indicating the phases present in each sample. The results indicate that the iron-ore sample is mainly hematite and the silica is quartz.

Table 1.
Elemental composition (wt.%) of the iron-ore sample through XRF.

Table 2.
Elemental composition (wt.%) of silica sample.
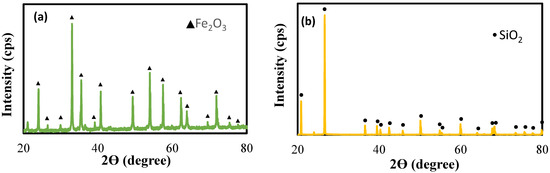
Figure 1.
X-ray diffraction patterns of (a) hematite and (b) silica particles.
Sodium oleate (NaOL) was purchased from Sigma-Aldrich (St. Louis, MO, USA) as the proposed alternative collector, VE_SS was derived from the natural product α-tocopherol commonly known as vitamin E. VE_SS is available as a solid and is not readily soluble in water. To dissolve the collector, 1 mL each of ethanol and Milli-Q water was added to the collector and the solution was then brought to the required volume using Milli-Q water. The pH of the suspension was adjusted using sodium hydroxide (NaOH) and hydrochloric acid (HCl). NaCl was used as background electrolyte for particle surface charge measurements. Milli-Q water with a resistivity of 18 mΩ/cm was used for all the experiments.
Synthesis of Vitamin E Sodium Succinate (VE_SS)
The precursor to VE_SS, D-α-tocopherol succinate, was synthesized by placing D-α-tocopherol (20 g, 46.43 mmol) and succinic anhydride (9.6 g, 95.93 mmol) in a 100 mL round bottom flask and dissolving it using methyl ethyl ketone (30 mL) at room temperature. For the stirring reaction, triethylamine (6.79 mL, 48.75 mmol) was added dropwise, and the mixture was further stirred for 16 h at room temperature. After the TLC (silica, toluene: glacial acetic acid (v/v 19:1)) of the crude mixture indicated that all starting material had been consumed, the reaction was diluted with isopropanol (30 mL) and washed with diluted 5% sulfuric acid (40 mL) followed by water until the pH value of the solution was neutral. The combined organic layer was further washed with brine and dried over MgSO4 and concentrated in vacuo affording a yellow liquid. The crude mixture was purified by column chromatography on silica gel using hexane and ethyl acetate (v/v 1:1) as eluent to afford D-α-tocopherol succinate (22.15 g, 90%) as a pale-yellow solid.
1H-NMR (400 MHz, CDCl3) δ 2.93 (t, J = 6.8 Hz, 2H), 2.84 (t, J = 6.8 Hz, 2H), 2.59 (t, J = 6.8 Hz, 2H), 2.09 (s, 3H), 2.02 (s, 3H), 1.98 (s, 3H), 1.85–1.71 (m, 2H), 1.56–1.50 (m, 3H), 1.43–1.05 (m, 21H), 0.88–0.84 (m, 12H); 13C NMR (100 MHz, CDCl3) δ 178.3, 170.8, 149.5, 140.4, 126.7, 125.0, 123.0, 117.4, 75.1, 39.4, 37.5, 37.4(8), 37.4(3), 37.4(1), 37.3, 32.8, 32.7, 29.0, 28.6, 28.0, 24.8, 24.5, 22.8, 22.7, 21.0, 20.6, 19.8, 19.7, 19.6(8), 19.6(5), 12.9, 12.0, 11.8.
To prepare VE_SS, D-α-tocopherol succinate (5 g, 9.42 mmol, 1 equivalent) was dissolved in 95 mL of acetone. Separately, NaOH (377 mg, 9.42 mmol, 1 equivalent) was dissolved in 5 mL of distilled water and added dropwise to the stirring acetone solution. After 10 min, a white precipitate began to form, and the reaction mixture was stirred overnight at room temperature to ensure complete reaction.
To induce further precipitation, excess acetone was added to the mixture. The solution was decanted, and the resulting compound was dried in a vacuum oven (SalvisLab, Rotkreuz, Switzerland), yielding VE_SS as the final product. The formation of VE_SS was confirmed by FTIR analysis (the data is presented in Section 3.4). In D-α-tocopherol succinate the FTIR spectrum showed characteristic absorption bands at 2922 (CH, medium), 2866 (CH, weak), 1752 (C=O, medium), and 1711 (C=O, medium). In VE_SS, the spectrum showed similar stretching bands at 2922 (CH, medium) and 2851 (CH, weak), while the carbonyl stretching frequencies shifted to 1744 cm−1 (medium) and 1567 cm−1 (medium). A shift in the carbonyl stretching frequency was seen from 1711 cm−1 in D-α-tocopherol succinate to 1567 cm−1 in VE_SS. The shift of the carbonyl band from 1711 cm−1 in D-α-tocopherol succinate to 1567 cm−1 in VE_SS indicates the successful conversion of the succinate to its sodium salt form. The structure of VE_SS is illustrated in Figure 2.
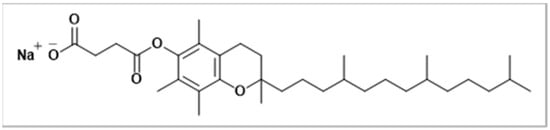
Figure 2.
Structure of vitamin E sodium succinate (VE_SS).
2.2. Micro-Flotation Experiments
The micro-flotation experiments were conducted in a modified 250 mL mechanical flotation cell. All experiments were conducted at 3 wt.% solids with Milli-Q water and the pulp was mixed at 2500 rpm. The pulp was initially mixed for 1 min, followed by pH adjustment for 2 min using the pH regulators. Subsequently, the collector was then added to the pulp and the pH was readjusted where necessary. The pulp was conditioned with the collector for 5 min during which the pH was continuously monitored using TPS pH meter. After the conditioning period, the cell was injected with 1 L/min gas and the concentrate was collected using an automated froth scraper for an additional 5 min. Concentrate and tailings samples were dried in an oven and processed for further analysis. The dried weight of the samples was used to determine the mass yield using Equation (1), which, in this case, is equivalent to recoveries for single minerals. Each test was repeated multiple times to ensure data reliability. It should be noted that, similar to sodium oleate, the new collector VE_SS serves as both a collector and a frother; therefore, no additional frother was used in these experiments.
2.3. Method for Zeta Potential Measurement
Froth flotation is a surface-chemistry-dependent process; therefore, understanding the surface chemistry of the particles is essential for efficient separation. Particle interactions with each other or with chemicals in a solution are influenced by electrokinetic effects, which can be measured in terms of zeta potential [25]. Zeta potential measurement is one of the relevant techniques used to study the interaction of collectors and mineral surfaces under specified conditions [26,27]. The zeta potential on the mineral’s surface was measured using a Malvern Zetasizer Pro-Blue (Malvern Panalytical Ltd., Malvern, UK). Zeta potential measurement against pH with and without a collector was determined. The suspension was produced by conditioning 50 mg of the sample in a 10 mL solution using 1.0 mM NaCl as a background electrolyte. The pH modifiers were used to adjust the suspension pH for each test. The tests were conducted at room temperature, and the results reported represent the mean of at least three separate measurements.
2.4. Method for Contact Angle Measurement
When evaluating the floatability of the target minerals and their separation from the associated gangue minerals, surface wettability plays a critical role. The separation of valuables from gangue is based on differences in their natural or induced hydrophobicity. In the true or direct flotation process, hydrophobic minerals attach readily to air bubbles and are carried to the top of the pulp, forming the froth zone, while hydrophilic gangue remains in the pulp and is discarded as tailings. Thus, there is a quantitative link between surface wettability and floatability, as the surface chemistry and wettability of minerals play a crucial role in controlling the bubble-particle adhesion process.
The wettability of a mineral is defined by the contact angle formed at the point where the mineral particle, water, and air bubble co-exist, also known as the three-phase contact line (TPC). This point represents the intersection of the water–bubble interface, the mineral–air interface, and the mineral–water interface. The contact angle provides valuable information about the wetting characteristics of solid mineral surfaces [28].
Contact angles were determined using the Sessile Drop Method using an Optical Contact Angle device (OCA 25L-PMC; DataPhysics Instruments GmbH, Filderstadt, Germany) to characterize the surface wettability of minerals. Mineral samples were conditioned with the required amount of collectors at the desired pH, to replicate flotation conditions. The conditioned samples were then filtered using a vacuum filter and dried in an oven at 60 °C for 1 h. Then, the treated samples were compressed into tablets using a hydraulic press before measuring the contact angles.
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
FTIR analysis was performed using a Cary 650 FTIR spectrometer (Agilent Technologies, Santa Clara, CA, USA) to characterize the functional groups of the sample within the 600–4000 nm wavelength range. The sample preparation involved mixing 0.5 g of mineral with 50 mL of distilled water. A collector was added to the mixture at a concentration of 10 mg/L, and the pH of the solution was adjusted to its optimal value based on the collector used. The mixture was stirred at room temperature for 30 min after which the sample was obtained by filtration and thoroughly washed with distilled water. Another sample was prepared under the same condition without the addition of the collector and was used as the control. These samples were then dried overnight in a vacuum oven. Finally, the dried mineral sample was mixed with dry potassium bromide (KBr) to form an FTIR sample pellet for spectrum measurements. The KBr pellet made from dried KBr was used to correct the background during the FTIR analysis.
2.6. X-Ray Photoelectron Spectroscopy (XPS)
The XPS analysis was performed using a Nexsa surface analysis system (Thermo Scientific, Waltham, MA, USA) equipped with a hemispherical analyzer. The incident radiation consisted of monochromatic Al Kα X-rays (1486.6 eV) operating at 72 W (6 mA and 12 kV) with a spot size of 400 μm × 250 μm for a 400 μm setting. Both survey (wide) and high-resolution (narrow) scans were acquired using analyzer pass energies of 150 eV and 50 eV, respectively, with a step size of 1.0 eV and a dwell time of 10 ms. The analysis chamber’s base pressure was maintained below 5.0 × 10−⁹ mbar. To mitigate surface charging, a low-energy dual-beam (ion and electron) flood gun was utilized. Data processing was carried out using the Avantage software (version 6.7.0). XPS sample preparation involved mixing 0.5 g of mineral sample with 50 mL of distilled water, both with and without the addition of 5 × 10−4 mol/L of collector, in a 100 mL Schott bottle. The pH of the mixture was adjusted to the optimal value based on the collector used. The mixture was agitated at 130 rpm for 4 h at room temperature using a shaker. Afterward, the mineral samples were filtered, washed with distilled water, dried, and subsequently analyzed using XPS.
3. Results and Discussion
3.1. Micro-Flotation
To determine the optimal dosage of the novel collector VE_SS, single-mineral flotation tests were conducted on hematite at pH 9, the reported optimal pH for NaOL [18]. Figure 3 presents the effect of VE_SS concentration on hematite flotation recovery. It was seen that increasing the collector concentration up to 80 ppm (~0.15 mM) led to a rise in yield, while further increasing the concentration to 165 ppm (~0.30 mM—even lower than the chosen optimal molar concentration of NaOL at 120 ppm (~0.39 mM) resulted in minimal additional improvement. Therefore, 80 ppm was selected as the optimal concentration for VE_SS in this study. It should be noted that as micro-flotation experiments were conducted on a single mineral, the identified optimal concentration should not be assumed as universally optimal for other systems. The required dosage may vary depending on the mineral composition and the presence of other reagents. The selection of 80 ppm VE_SS for the remainder of the study was primarily intended to investigate the fundamental flotation behavior of vitamin E sodium succinate rather than to directly equate its molar concentration with that of NaOL.
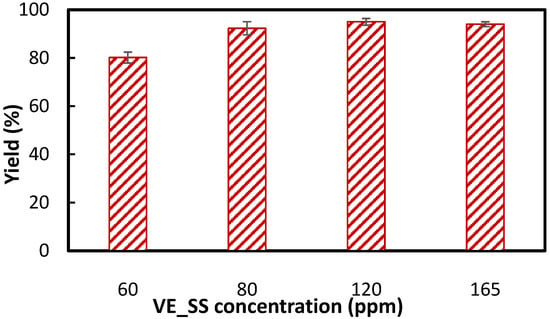
Figure 3.
Hematite recovery using VE_SS at different concentrations at pH 9, the reported optimal pH for NaOL.
To investigate the influence of pH and determine the optimal pH for VE_SS, additional single-mineral flotation tests were conducted on both hematite and silica across a range of pH levels. Figure 4 illustrates the effect of pH on the recovery of hematite and silica using 80 ppm VE_SS. The results indicate that VE_SS performs effectively across a wide pH range, except under highly alkaline conditions (pH 11), where a significant drop in recovery was observed. This behavior closely correlates with the foaming ability of VE_SS at different pH levels.
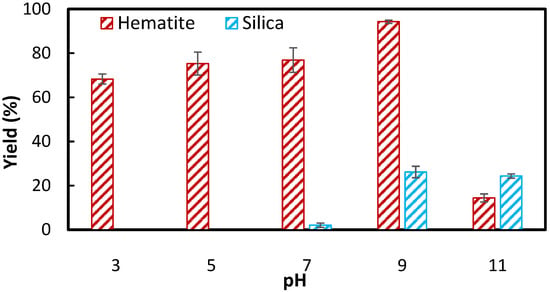
Figure 4.
Hematite and silica recovery using 80 ppm (~0.15 mM) VE_SS as a function of pH.
To further explore this relationship, separate foam column experiments were conducted in the absence of particles to examine foamability (using the maximum foam height) and foam stability (using the foam half-life—the time required for foam to decay to half of its initial height). Using an air flow rate of 2 L/min, air injection was stopped after 40 s, and the foam height was recorded over time. The corresponding results are presented in Figure 5. The data show that no foam formation occurred at pH levels below 7, while both foamability and foam stability significantly increased at higher pH.
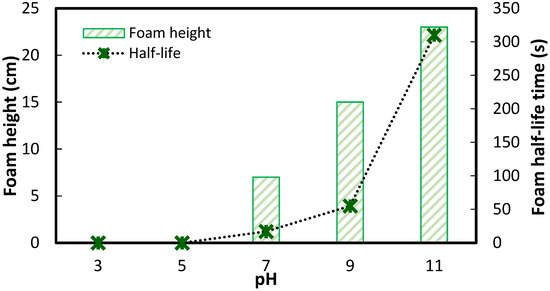
Figure 5.
Foaming properties of 80 ppm (~0.15 mM) VE_SS as a function of pH.
Consistent with the foam characterization results, minimal frothing was observed during flotation experiments at pH values below 7, suggesting that any recovery under these acidic conditions in Figure 4 can be attributed solely to the inherent hydrophobicity of the particles, enabling them to rise to the surface of the cell without froth assistance. In contrast, increased foamability of VE_SS at higher pH resulted in elevated silica yield, as hydrophilic silica, representing gangue, relies on water recovery and entrainment to be recovered. At lower pH, the absence of foam precludes silica recovery, while at higher pH, enhanced foamability and stability facilitate its capture. These findings align with the established behavior of NaOL, which also exhibits pH-dependent froth generation [29,30,31].
For better comparison, Figure 6 presents hematite and silica recovery using 80 ppm VE_SS and 120 ppm NaOL across various pH levels. The results indicate that NaOL, like VE_SS, performs effectively in neutral to alkaline conditions, with significantly lower recovery under acidic pH due to the formation of different oleate species in the solution [18]. In both cases, hematite recovery increased with rising pH, reaching a peak at pH 9 before declining. This optimum floatability at pH 9 is consistent with literature reports indicating low NaOL adsorption in highly acidic or alkaline media [18,26,32]. At acidic pH, NaOL is reported to have a poor water solubility and low surface activity [33], leading to the precipitation of oleic acid on the mineral surface and its physical adsorption [34]. In contrast, in slightly alkaline conditions, the chemisorption of oleate onto the mineral surfaces is the dominant mechanism, potentially resulting in the formation of multilayers on the surface of the mineral [34]. With a further increase in pH to 11, the adsorption of NaOL significantly decreased to a less than 20% hematite yield in Figure 6, which can be related to reduced surface activity [33] and increased competition from hydroxide ions (OH−) over the adsorption of anionic oleate species onto the surface of the hematite particles [32].
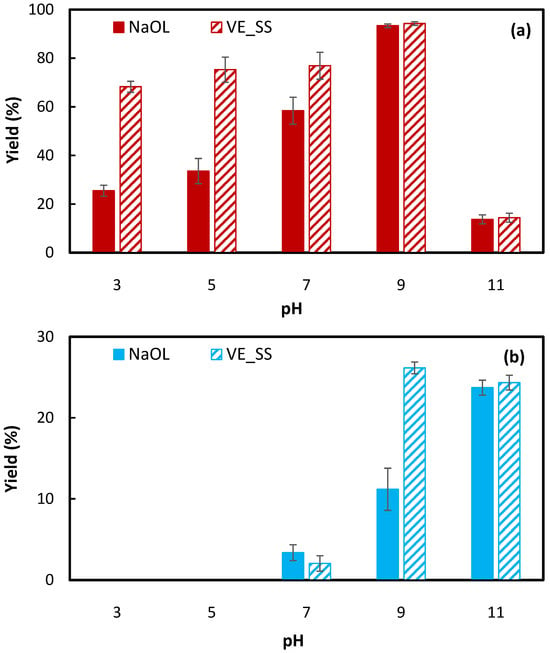
Figure 6.
Comparison of (a) hematite and (b) silica recovery using 80 ppm (0.15 mM) VE_SS and 120 ppm (0.39 mM) NaOL at different pH.
Considering both hematite and silica recovery, pH 7 was identified as the optimum pH for VE_SS, yielding approximately 76.9% compared to only a 2.0% silica yield. Additionally, while VE_SS followed the same general trend as NaOL, it exhibited significantly lower sensitivity to pH variations below 9, providing a broader operational range for effective flotation. This suggests that VE_SS could be a versatile collector under varying pH conditions, making it advantageous for practical applications where pH control may be challenging.
3.2. Zeta Potential Analysis
3.2.1. Zeta Potential of Hematite
To provide further insights into the adsorption mechanism on the mineral surface, zeta potential measurements were carried out to characterize the interactions between mineral surface and flotation reagents. It has been reported that the primary adsorption mechanism between hematite and sodium oleate is chemisorption, which results in the shift of the isoelectric point (IEP) and charge reversal of hematite [35]. Han, Yin, Yang, Wang, Yao, and Zhu [27] investigated this mechanism using atomic force microscopy (AFM), FTIR, and XPS, reporting that the iron atoms exposed on the surface were the main active centers for the chemisorption of NaOL. Figure 7 presents zeta potential measurements for hematite and silica with and without collectors at different pH levels.
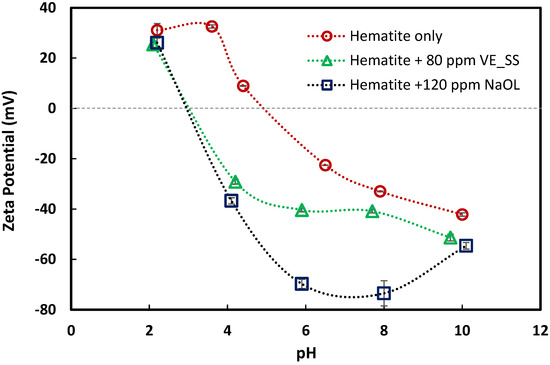
Figure 7.
Zeta potential of hematite as a function of pH in 1.0 mM NaCl background electrolyte with and without collectors.
The zeta potential of hematite sample is known to decrease in the presence of sodium oleate, resulting in a corresponding shift in the IEP. However, the outcomes of such investigations are directly influenced by the origin and purity of the sample. Synthetic iron samples have a relatively higher IEP compared to the natural ores. These differences are mainly due to the amount of silica present. A summary of reported zeta potential results for synthetic and natural iron samples, specifically hematite, magnetite, and goethite, are reported by [25,36,37].
In this study, the hematite sample had an IEP of 5. Upon conditioning with both collectors, its IEP shifted by approximately 2 units into the acidic range and both collectors exhibited a similar adsorption trend. The findings for NaOL align with previously reported results for certain natural iron ores [35]. Overall, the IEP values observed here fall within the range documented in the literature [37].
3.2.2. Zeta Potential of Silica
It is commonly reported that the zeta potential of silica remains predominantly negative across a wide pH range, with the exception of extremely acidic conditions. Some studies have also reported the absence of an IEP for silica [36,37], which aligns with the findings for the silica sample used in this study. Similar to the hematite samples, variations in the IEP are influenced by the origin of the sample. In this study, silica samples were used as received, without undergoing any cleaning treatment, which likely resulted in a lower IEP compared to most synthetic samples. Synthetic silica samples typically exhibit an IEP about pH 2, as reported in previous studies [26,38].
Figure 8 shows the corresponding zeta potential data of silica with and without collectors at different pH values. Upon conditioning silica with both collectors, minimal changes in the zeta potential were observed, suggesting negligible adsorption.
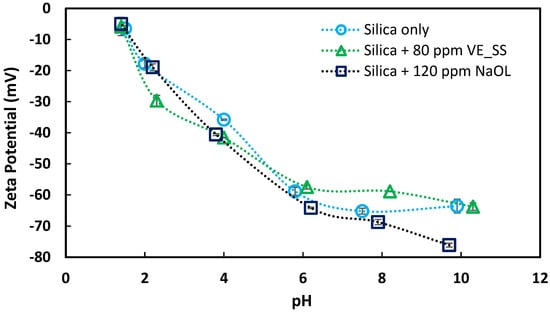
Figure 8.
Zeta potential of silica as a function of pH in 1.0 mM NaCl background electrolyte with and without collectors.
3.3. Contact Angle Analysis
Figure 9 shows the advancing contact angle of hematite samples conditioned with 80 ppm VE_SS at the optimum pH level, compared to NaOL at its optimum concentration of 120 ppm and pH of 9. The advancing contact angle of untreated hematite was measured at approximately 35°. For hematite conditioned with 120 ppm NaOL, the contact angle was measured at 113°, and for 80 ppm VE_SS, the contact angle measured at pH 7 was 78°. These results demonstrate the effective adsorption of both collectors on the hematite surface, significantly increasing its hydrophobicity and thereby enhancing flotation efficiency.
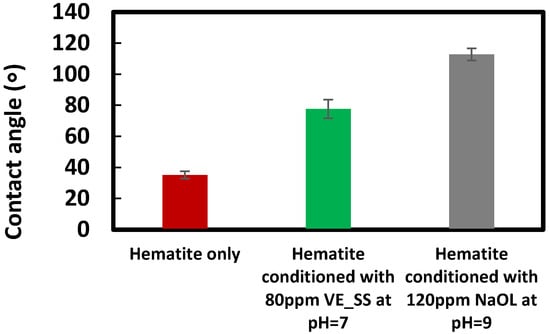
Figure 9.
Effect of collectors on the advancing contact angle of hematite at their optimum concentrations and pH values.
3.4. FTIR and XPS Analysis
FTIR spectra were acquired to assess the collectors’ adsorption on hematite and silica at their optimal pH values: pH 7 for VE_SS and pH 9 for NaOL, as shown in Figure 10. Both collectors exhibit distinct strong stretches between 2800 and 3000 cm−1, corresponding to CH stretches. VE_SS also shows a characteristic carbonyl (C=O) stretch at 1567 cm−1, while NaOL displays a carbonyl stretch at 1560 cm−1, both corresponding to the carboxylate anion functionality.
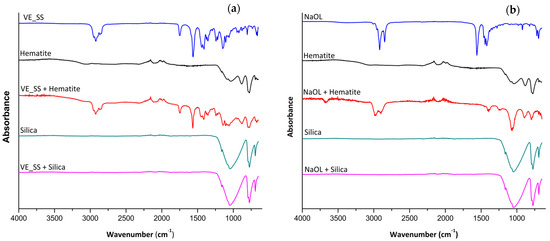
Figure 10.
FTIR of (a) VE_SS against hematite and silica at pH 7 and (b) NaOL against hematite and silica at pH 9.
Similar trends were observed when comparing the FTIR spectrum of the hematite and silica before and after treatment with the collectors, VE_SS and NaOL. Peaks corresponding to the collectors were present in the hematite samples treated with VE_SS and NaOL, indicating adsorption. In contrast, no such peaks were observed in the silica samples treated with the collectors, suggesting that no adsorption of collectors on the silica surface was detected.
Further analysis of the adsorption interaction of VE_SS or NaOL on hematite and silica was conducted by XPS analysis (Figure 11). A distinct change was observed in the surface energy corresponding to the iron (Fe 2p) signal after the adsorption of the collector VE-SS (Figure 11b) and NaOL (Figure 11d) at their respective optimal pH levels. In the presence of VE_SS, the Fe 2p3/2 peak shifted from 711.15 eV to 711.64 eV, and the Fe 2p1/2 from 725.13 eV to 726.53 eV. Similarly, with NaOL, the Fe 2p3/2 peak shifted from 711.87 eV to 712.32 eV, and the Fe 2p1/2 from 724.73 eV to 726.44 eV, indicating strong interaction and binding with the hematite surface. Additionally, an increase in the carbon (C 1s) signal and the disappearance of the sodium (Na 1s) signal—displaced as the counterion during collector binding—further confirmed the adsorption of the collectors onto the hematite surface.
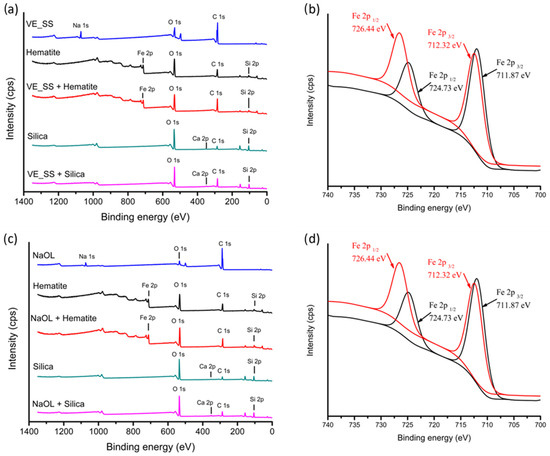
Figure 11.
(a) XPS survey of VE_SS against hematite and silica surface at pH 7; (b) high-resolution XPS spectra for Fe 2p of hematite surface in the absence (black) and presence (red) of VE_SS; (c) XPS survey NaOL against hematite and silica surface at pH 9; and (d) high-resolution XPS spectra for Fe 2p of hematite surface in the absence (black) and presence (red) of NaOL.
In the case of the silica surface, a slight increase in the carbon (C 1s) signal was also observed in the XPS survey during the adsorption of VE_SS and NaOL. However, this adsorption is predominantly attributed to the presence of lime in the sample. XPS detected calcium (Ca 2p) signals, suggesting that lime facilitated the binding of the collector to the silica surface at higher pH levels [39]. These findings align with the minor recovery of silica shown in Figure 6, particularly when the pH was above 7, where lime-induced adsorption became more significant.
4. Conclusions
This study evaluated the adsorption and flotation performance of vitamin E sodium succinate (VE_SS), a novel bio-based collector derived from vitamin E, for hematite recovery using froth flotation. The performance of VE_SS was benchmarked against sodium oleate (NaOL), a widely used collector in mineral processing. Single-mineral flotation experiments were conducted with hematite and silica, a common gangue mineral associated with hematite ore, under varying pH levels and collector concentrations to identify the optimal conditions for VE_SS. Comprehensive characterization techniques, including zeta potential analysis, contact angle measurements, XPS, and FTIR spectroscopy, were employed to investigate the adsorption mechanisms of VE_SS on the hematite surface.
The results indicated that increasing both the concentration and pH enhanced the recovery of hematite and silica. Increasing the pH to 9 achieved a hematite recovery exceeding 94%, but it also resulted in increased gangue entrainment, with silica recovery exceeding 26%. The optimal conditions for the selective separation of hematite using VE_SS were identified as an 80 ppm collector concentration and a pH of 7, yielding a hematite recovery of 76.9% with minimal silica recovery of 2.0%.
The results demonstrated that VE_SS exhibits a promising performance comparable to NaOL, with the added advantage of a significantly reduced sensitivity to pH. Lowering pH from 9 to 3 resulted in a reduction in hematite recovery from 94 ± 0.5% for both collectors to a 68.2% yield for VE_SS and a 25.5% yield for NaOL. Therefore, unlike NaOL, which performs effectively within a narrow pH range, VE_SS maintained high flotation performance across a broader range of conditions.
While this research focused on single-mineral flotation, the effectiveness of VE_SS in mixed-mineral flotation systems is being explored and will be presented in a forthcoming publication.
Author Contributions
Conceptualization, R.M., T.D.S.P., T.H., P.A., S.H.T. and M.F.; methodology, R.M., T.D.S.P., T.H., P.A., S.H.T. and M.F.; formal analysis, R.M., T.D.S.P., T.H., P.A., S.H.T. and M.F.; investigation, R.M., T.D.S.P., T.H., P.A., S.H.T. and M.F.; writing—original draft preparation, R.M., T.D.S.P., T.H. and P.A.; writing—review and editing, R.M., T.D.S.P., T.H., P.A., S.H.T. and M.F.; supervision, T.H., S.H.T. and M.F.; project administration, S.H.T. and M.F.; funding acquisition, S.H.T. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Research Council for the ARC Centre of Excellence for Enabling Eco-Efficient Beneficiation of Minerals grant number CE200100009.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, B.; Fu, Y.-F.; Yin, W.-Z.; Sheng, Q.-Y.; Zhu, Z.-L.; Yin, X.-M. Selective collection performance of an efficient quartz collector and its response to flotation separation of malachite from quartz. Miner. Eng. 2021, 172, 107174. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Song, S.; Gao, H.; Niu, F.; Zhang, J.; Ai, G. Selective separation of hematite from quartz with sodium oleate collector and calcium lignosulphonate depressant. J. Mol. Liq. 2021, 322, 114502. [Google Scholar] [CrossRef]
- Kulkarni, R.; Somasundaran, P. Flotation chemistry of hematite/oleate system. Colloids Surf. 1980, 1, 387–405. [Google Scholar] [CrossRef]
- Ma, M. Froth flotation of iron ores. Int. J. Min. Eng. Miner. Process. 2012, 1, 56–61. [Google Scholar] [CrossRef]
- Araujo, A.; Viana, P.; Peres, A. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Jain, G.; Havskjold, H.; Dhar, P.; Ertesvåg, H.; Chernyshova, I.; Kota, H.R. Green foam-based methods of mineral and ion separation. In Multidisciplinary Advances in Efficient Separation Processes; ACS Publications: Washington, DC, USA, 2020; pp. 265–301. [Google Scholar]
- Oulkhir, A.; Lyamlouli, K.; Danouche, M.; Ouazzani, J.; Benhida, R. A critical review on natural surfactants and their potential for sustainable mineral flotation. Rev. Environ. Sci. Bio/Technol. 2023, 22, 105–131. [Google Scholar] [CrossRef]
- Amani, P.; Amiralian, N.; Athukoralalage, S.S.A.; Firouzi, M. Eco-efficient pickering foams: Leveraging sugarcane waste-derived cellulose nanofibres. J. Mater. Chem. A 2023, 11, 24379–24389. [Google Scholar] [CrossRef]
- Behera, S.K.; Mulaba-Bafubiandi, A.F. Microbes assisted mineral flotation a future prospective for mineral processing industries: A review. Miner. Process. Extr. Metall. Rev. 2017, 38, 96–105. [Google Scholar] [CrossRef]
- Asna, A.; Songli, A.; Nabilah, R.; Ikhsan, S.; Rickiadi, M.F.; Wahyuningsih, T. The Effect of Aloe Vera Bioreagent as a Frother and Collector in the Gold Ore Flotation Process on Increasing Grade and Recovery. J. Metall. Eng. Process. Technol. 2024, 4, 1–9. [Google Scholar] [CrossRef]
- Quast, K. Flotation of hematite using 18-carbon fatty acids. Miner. Eng. 2021, 160, 106647. [Google Scholar] [CrossRef]
- Schlebusch, I.; Pott, R.W.M.; Tadie, M. The ion flotation of copper, nickel, and cobalt using the biosurfactant surfactin. Discov. Chem. Eng. 2023, 3, 7. [Google Scholar] [CrossRef]
- Bu, X.; Chen, F.; Chen, W.; Ding, Y. The effect of whey protein on the surface property of the copper-activated marmatite in xanthate flotation system. Appl. Surf. Sci. 2019, 479, 303–310. [Google Scholar] [CrossRef]
- Yehia, A.; Khalek, M.A.; Ammar, M. Cellulase as a new phosphate depressant in dolomite-phosphate flotation. Physicochem. Probl. Miner. Process. 2017, 53, 1092–1104. [Google Scholar]
- Laitinen, O.; Hartmann, R.; Sirviö, J.A.; Liimatainen, H.; Rudolph, M.; Ämmälä, A.; Illikainen, M. Alkyl aminated nanocelluloses in selective flotation of aluminium oxide and quartz. Chem. Eng. Sci. 2016, 144, 260–266. [Google Scholar] [CrossRef]
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Han, Y.; Parra-Álvarez, N.; Claremboux, V.; Kawatra, S. Flotation of iron ores: A review. Miner. Process. Extr. Metall. Rev. 2021, 42, 184–212. [Google Scholar] [CrossRef]
- Joseph-Soly, S.; Quast, K.; Connor, J.N. Effects of Eh and pH on the oleate flotation of iron oxides. Miner. Eng. 2015, 83, 97–104. [Google Scholar] [CrossRef]
- Amani, P.; Hsia, T.; Thang, S.H.; Firouzi, M. Assessment of a bio-inspired frothing agent derived from Vitamin E in mineral processing. Miner. Eng. 2024, 218, 108974. [Google Scholar] [CrossRef]
- Sikorska, M.; Borowy-Borowski, H.; Zurakowski, B.; Walker, P.R. Derivatised α-tocopherol as a CoQ10 carrier in a novel water-soluble formulation. Biofactors 2003, 18, 173–183. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Luiz, M.T.; Di Filippo, L.D.; Alves, R.C.; Araújo, V.H.S.; Duarte, J.L.; Marchetti, J.M.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Yan, A.; Von Dem Bussche, A.; Kane, A.B.; Hurt, R.H. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon 2007, 45, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.J.; Kawatra, S.K. Factors Affecting Zeta Potential of Iron Oxides. Miner. Process. Extr. Metall. Rev. 2013, 34, 269–303. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of flotation characteristics of monazite, hematite, and quartz using anionic collectors. Int. J. Miner. Process. 2017, 158, 55–62. [Google Scholar] [CrossRef]
- Han, H.; Yin, W.; Yang, B.; Wang, D.; Yao, J.; Zhu, Z. Adsorption behavior of sodium oleate on iron minerals and its effect on flotation kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129108. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Iwasaki, I.; Cooke, S.; Choi, H. Flotation characteristics of hematite, goethite, and activated quartz with 18-carbon aliphatic acids and related compounds. Am. Inst. Min. Metall. Pet. Engineers. Trans. 1960, 217, 237–244. [Google Scholar]
- Atrafi, A.; Gomez, C.O.; Finch, J.A.; Pawlik, M. Frothing behavior of aqueous solutions of oleic acid. Miner. Eng. 2012, 36–38, 138–144. [Google Scholar] [CrossRef]
- Shu, X.; Meng, Y.; Wan, L.; Li, G.; Yang, M.; Jin, W. pH-Responsive aqueous foams of oleic acid/oleate solution. J. Dispers. Sci. Technol. 2014, 35, 293–300. [Google Scholar] [CrossRef]
- Bai, S.; Li, J.; Bi, Y.; Yuan, J.; Wen, S.; Ding, Z. Adsorption of sodium oleate at the microfine hematite/aqueous solution interface and its consequences for flotation. Int. J. Min. Sci. Technol. 2023, 33, 105–113. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic− molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Quast, K. Literature review on the interaction of oleate with non-sulphide minerals using zeta potential. Miner. Eng. 2016, 94, 10–20. [Google Scholar] [CrossRef]
- Quast, K. The use of zeta potential to investigate the interaction of oleate on hematite. Miner. Eng. 2016, 85, 130–137. [Google Scholar] [CrossRef]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-dependent surface charging and points of zero charge: III. Update. J. Colloid Interface Sci. 2006, 298, 730–741. [Google Scholar] [CrossRef]
- Khandaker, S.; Willott, J.D.; Webber, G.B.; Wanless, E.J. Adsorption of polyacrylamides on mineral oxides: Effect of solution pH and polymer molecular weight. Miner. Eng. 2024, 206, 108547. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Yuan, J.; Yu, A.; Wen, S.; Bai, S. Insights into the influence of calcium ions on the adsorption behavior of sodium oleate and its response to flotation of quartz: FT-IR, XPS and AMF studies. Miner. Eng. 2023, 204, 108437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).