Abstract
A gold mine located in western China is facing the problem of a low concentrate grade, significantly hindering its economic benefits. Preliminary assessments indicate that this is caused by gangue minerals that are prone to floating and sliming, necessitating suppression in the flotation process. The effect of fenugreek polysaccharide gum (FGM) upon the flotation separation of arsenopyrite (representative of Au-bearing minerals) and pyrophyllite (a typical gangue mineral) was investigated; its industrial potential was verified through actual ore flotation and pilot plant testing. Additionally, the selective inhibition mechanism of FGM on pyrophyllite was elucidated. The flotation tests of pure minerals indicated that pyrophyllite has a high natural floatability; thus, it cannot be separated from arsenopyrite at low alkaline pH (7–9); smaller pyrophyllite particle sizes, especially −0.038 mm fractions, significantly decreased the arsenopyrite recovery; FGM can eliminate this adverse effect to a large extent through its selective depression of the flotation of pyrophyllite. For real ore systems, FGM also exhibited superior performance compared with the commonly used silicate and SHMP; closed-circuit flotation tests showed that the gold grade of the concentrate increased by 3.90 g/t and the enrichment ratio increased by 2.53 with the addition of FGM. As of now, FGM has increased the profits by USD 1.715 M in the past two years by improving concentrate grade and recovery efficiency. According to the results of contact angle, zeta potential, Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS), the selective adsorption of FGM onto the pyrophyllite surface was the reason for the positive effect; the interaction primarily involved the Al sites on the pyrophyllite surface and the –OH on FGM molecules.
1. Introduction
A gold mine in Golmud, Qinghai Province of China, is associated with a high content of clays, such as pyrophyllite and mica. The main valuable mineral is auriferous arsenopyrite [1]. Currently, the concentrator is encountering the challenge of a low concentrate grade and a reduced recovery rate of the gold element within the concentrate [2]. It is hypothesized that a significant quantity of gangue minerals, which are prone to flotation and sliming, may be incorporated into the concentrate during flotation [3]. Some clay minerals similar to pyrophyllite are notably endowed with excellent natural floatability coupled with their low hardness characteristics [4,5]. Consequently, during the grinding phase preceding flotation, a substantial quantity of sliming particles derived from gangue minerals is generated [6]. Yongjun Peng clearly points out that the flotation industry is well aware of the difficulty in treating copper ores in the presence of clay minerals. Currently, the way to treat this type of ore is mainly to blend it with normal ore at a small proportion. Limited studies have been conducted to understand the role of clay minerals in sulfide flotation. Xumeng Chen also pointed this out and gave a unique insight into managing clay minerals in froth flotation [7,8].
These sliming particles originated from clay minerals and pose a significant challenge, as achieving an effective separation between the primary gold-bearing mineral, arsenopyrite, and the dominant gangue mineral, pyrophyllite, becomes exceedingly difficult without the incorporation of a suitable dispersant [9,10]. The application of a depressant is crucial to selectively inhibit the flotation of the gangue minerals, thereby facilitating the enhancement of the separation efficiency and purity of the gold-bearing concentrate.
To address the adverse effects of secondary slime, mineral processing professionals have embarked on extensive research endeavors [11,12]. Sodium hexametaphosphate ((NaPO3)6, SHMP) and sodium silicate were employed to mitigate the detrimental impacts of secondary slime, yet achieving an optimal balance between flotation performance indices and the quantity of chemicals utilized posed a significant challenge [13,14,15,16]. Depressants such as oxalic acid and sodium pyrosulfite were also used in similar cases; however, the required dosage of these reagents is substantial, resulting in elevated costs [17,18]. Therefore, the development of a high-efficiency, low-cost, and green depressant is pivotal in enabling the effective flotation separation of arsenopyrite and pyrophyllite, thereby enhancing the grade of the ore concentrate.
Fenugreek polysaccharide gum (FGM) is abundantly found in a variety of leguminous plants, including beans, bitter beans, and other related species [19]. It demonstrates environmental compatibility, outstanding solubility in water, and the ability to interact with a diverse range of metal ions [20,21,22]. As a result, it holds the potential to interact with the active binding sites on mineral surfaces, positioning it as a promising candidate for utilization as an environmentally friendly flotation agent. For instance, FGM has been successfully applied as a depressant in the flotation of sulfide ores, where it selectively inhibits the flotation of naturally hydrophobic talc through hydrogen bonding with its [SiO4] tetrahedron, significantly reducing talc’s hydrophobicity while minimally affecting the flotation of valuable minerals such as chalcopyrite [23]. Additionally, FGM has shown excellent selectivity in the separation of smithsonite from calcite, where it preferentially adsorbs on calcite surfaces, reducing the adsorption of cationic collectors and enabling efficient separation [24].
In this study, the influence of pyrophyllite with various particle sizes on the recovery of arsenopyrite was discussed. Subsequently, the effects of the new depressant FGM on mineral flotation behavior, actual ore flotation, and pilot plant tests were investigated to evaluate its industrial potential for this gold ore. Finally, the interaction mechanism of FGM on minerals was investigated.
2. Experimental
2.1. Samples and Reagents
The arsenopyrite and pyrophyllite utilized in the experimental procedure were sourced from the targeted mine of this investigation. These minerals, after being meticulously hand-picked for purity, underwent crushing operations and were subsequently subjected to fine grinding in a ceramic ball mill (Jilin Jitan Machinery Co., Ltd., Changchun, China). The grinding products of arsenopyrite were screened to obtain −0.074 + 0.037 mm and −0.037 mm fractions, and that of pyrophyllite was screened to obtain −0.1 + 0.074 mm, −0.074 + 0.037 mm, and −0.037 mm fractions for tests. Some −0.037 mm samples were further ground to −5 μm for surface property detection before and after treatment with reagent. The X-ray diffraction (XRD, Thermo Fisher Scientific, Waltham, MA, USA, Figure 1) analysis of the samples revealed a high purity level of the minerals, qualifying them for utilization as pure minerals in the tests. The ore utilized in the actual flotation process originated from a targeted concentrator located in northwestern China. Chemical analysis results of actual ore used in closed-circuit tests are shown in Table 1. Field test personnel specified the grinding fineness of the real ore and the reagent system.
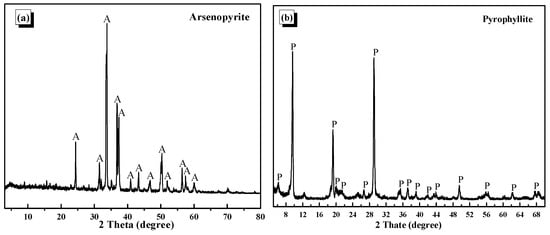
Figure 1.
X-ray diffraction spectrum of arsenopyrite (a) and (b) pyrophyllite.

Table 1.
Chemical analysis results of actual ore used in closed circuit tests.
FGM used in this experiment was obtained from Shandong Gukang Biotechnology Co., Ltd. (Jinan, China), and it was sold mainly as raw material for Chinese medicine [25,26]. In the flotation tests of pure mineral, analytical pure sulfuric acid and sodium hydroxide were used as pH regulators, sodium isobutyl xanthate (SIBX) as a collector, and terpineol (TP) as a frother; deionized water was used. In the actual ore flotation and pilot plant tests, sodium carbonate (Na2CO3) served as the pH regulator, cupric sulfate functioned as the activator, a mixture of SIBX and ammonium dibutyl dithiophosphate acted as the collectors, and 2# oil was utilized as the frother.
2.2. Flotation Test
2.2.1. Micro-Flotation Test
Micro-flotation experiments were conducted utilizing an XFG laboratory flotation apparatus (Jilin Jitan Machinery Co., Ltd., Changchun, China) equipped with a 60 mL flotation cell. During these micro-flotation trials, 2 g of mineral specimens were blended with an adequate amount of de-ionized water within the flotation cell. Subsequent to a pulping period of 2 min, various reagents were sequentially introduced: a pH adjuster, a depressant, a collector, and any additional agents as required. Following a 5-min flotation process, the resultant foam fraction and tailings were separately collected, filtered, dried to constant weight, and weighed. The recovery rate of the minerals was then calculated based on these measurements. Unless otherwise specified, the pure mineral size used was the −74 + 38 µm fraction.
2.2.2. Actual Ore Flotation Test
The 1.0 L XFD laboratory flotation machine (Jilin Jitan Machinery Co., Ltd., Changchun, China) was used to carry out the actual ore roughing and scavenging tests, and the 0.5 L one was used in the cleaning stage. According to the experimental flow chart, the flotation operation was carried out, and the products in each stage of flotation were sampled, weighed, filtered, and dried. Finally, the gold grade of the sample was determined, and the recovery was calculated.
First, the properties of FGM and several common inhibitors were studied by a one roughing–one sweeping open-circuit flotation test (Figure 2). It should be noted here that all dosage amounts are optimized by a large number of experiments.
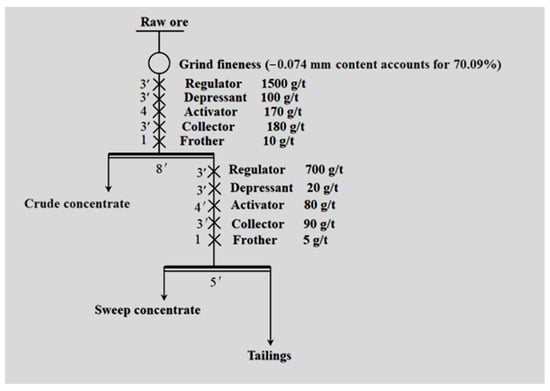
Figure 2.
Flotation test process of depressant type.
The closed-circuit flotation experiment was conducted according to the flow chart (one roughing–two sweepings–two cleanings, Figure 3).
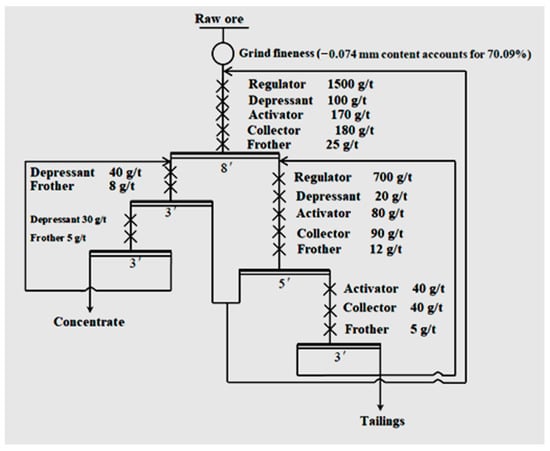
Figure 3.
Closed-circuit flotation test flow.
Finally, the industrial test was conducted in the target concentrator (Figure 4) according to the process of one roughing–three sweepings–three cleanings (Figure 5).

Figure 4.
A photo of the industrial test site.
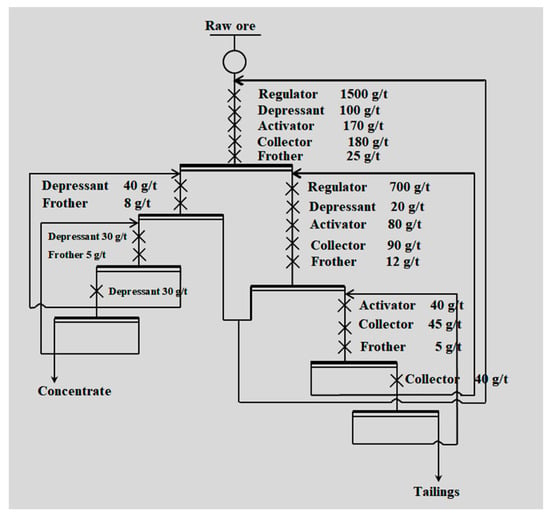
Figure 5.
Flotation flow chart of the industrial test.
2.3. Contact Angle
The Rame–Hart goniometer (Ramé-Hart Instrument Co., Raleigh, NC, USA) was utilized to ascertain the contact angle employing the droplet technique [27]. Cubic arsenopyrite and pyrophyllite specimens, each measuring 3 × 3 × 1 cm, were submerged in their respective reagent solutions for 3 min. Following this immersion, the mineral specimens underwent cleaning and drying procedures. To determine the contact angle, an image capturing both the prepared mineral sample and the water droplet was recorded and analyzed.
2.4. Zeta Potential Measurements
In this study, 30 mg of mineral powders were dispersed into 50 mL of aqueous KCl solution (1 mM) to form a suspension. This suspension was then subjected to magnetic stirring for conditioning. During the stirring process, the appropriate pH regulator and depressant were sequentially introduced into the beaker, followed by an additional 10 min of conditioning. Stirring was subsequently halted for a minimum of 30 min to facilitate precipitation. The zeta potential was measured using a Delsa 440sx Zeta Potential Analyzer manufactured by Malvern Instruments Ltd. (Worcestershire, UK).
2.5. FTIR Spectrometer
The FTIR spectra of mineral samples treated with flotation reagents were recorded with an IRffinity-1 Fourier Transform Infrared Spectrometer (Shimadzu, Kyoto, Japan) by the KBr diffuse reflection method. 30 mg pure samples (−5 μm) were ultrasonically dispersed in 100 mL deionized water. Then, the flotation reagents were added in sequence to a desired concentration (FGM = 60 mg/L). The pulp was conditioned with a magnetic stirrer (Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China) at 25 °C (pH = 6.5), and the conditioning time for each reagent was 30 min. After conditioning, the suspensions were centrifuged, and then the precipitation was washed three times with deionized water and subsequently dried. Lastly, the dry mineral samples were collected for infrared detection at room temperature. The wave number range of the spectra was 400–4000 cm−1. Each spectrum was recorded with 20 scans measured at 4 cm−1 resolution.
2.6. XPS Measurements
A Thermo Scientific K-Alpha spectrometer (manufactured by Thermo Fisher Scientific, Waltham, MA, USA) was used to acquire the XPS spectra of the pyrophyllite powders. The pressure in the analysis chamber was less than 5 × 10−7 mbar. Al Kα X-rays with 1486.6 eV of energy and 12 kV × 15 mA of power for narrow scans were employed for observations. The samples were dried in a vacuum oven (Shanghai Yidian Scientific Instrument Co., Ltd., Shanghai, China) and tested by the meter at an ambient temperature. The C1s peak was referenced to a binding energy (BE) for uncharged hydrocarbons at 284.8 eV. The detection was carried out in FAT mode, and the spectrometer was calibrated with Cu2p3/2/(932.67 eV), Ag3d5/(368.30 eV), and Au4f7/2/(84.00 eV) standard samples. XPS Peak Fit software (version 4.1) was used for data fitting [28].
3. Results and Discussion
3.1. Flotation Tests
3.1.1. Single Mineral Flotation Test
Single mineral flotation tests were conducted to investigate the floatability of minerals with collector SIBX. The results are shown in Figure 6. This exploratory experiment determined that the optimum dosage of SIBX was 0.15 mM. Arsenopyrite had great floatability in the pH of 2–9, but it decreased sharply when the pH was higher than 9. However, pyrophyllite had good floatability in the whole pH range. These results suggest that separating arsenopyrite from pyrophyllite by flotation under low alkalinity conditions is challenging. Generally, for this kind of situation, it is necessary to add appropriate gangue mineral depressants to enlarge the difference in the recoveries of two minerals [29].
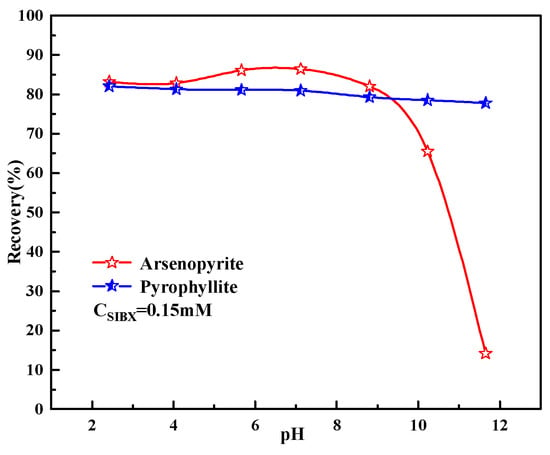
Figure 6.
Effects of pulp pH on mineral recovery.
In the presence of FGM, the recoveries of pyrophyllite on arsenopyrite were studied then, and the results are shown in Figure 7. As shown in Figure 7a, with the increase in FGM, the recovery of pyrophyllite decreased significantly. When the dosage of FGM was 60 mg/L, the recovery of pyrophyllite was only about 10%, while that of arsenopyrite still surpassed 70%. FGM exhibited selective depression on pyrophyllite against arsenopyrite. It provides the basic conditions for flotation separation [30].
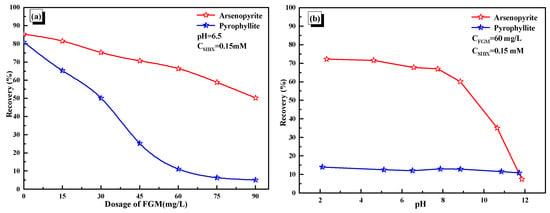
Figure 7.
(a) Effects of FGM dosage and pulp pH (b) on mineral recovery.
The results about the adaptability of FGM on pulp pH are shown in Figure 7b. In the whole pH range, FGM had a great depressant effect on pyrophyllite. These experimental results suggested that FGM had the potential to serve as a depressant for the flotation separation of arsenopyrite and pyrophyllite.
3.1.2. Mixed Mineral Flotation Test
The flotation of mixed minerals with different pyrophyllite mass ratios and particle sizes was conducted to verify the performance of FGM for a mineral system closer to the actual process. As shown in Figure 8a, adding pyrophyllite of −0.074 mm size decreased arsenopyrite floatability distinctly. Especially when the mass ratio of pyrophyllite of −0.037 mm was 80%, the recovery of arsenopyrite decreased from 86.51% to 25.13%. It could be inferred that the existence of pyrophyllite would significantly deteriorate the recovery of gold-bearing minerals.
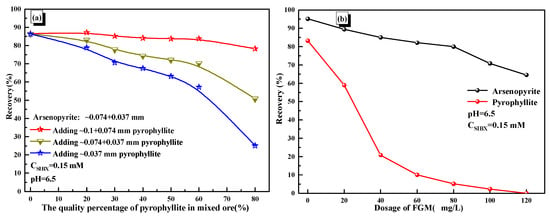
Figure 8.
(a) Effects of mass percentage of pyrophyllite on arsenopyrite recovery and (b) effects of FGM dosage on mineral recovery.
To weaken this adverse effect of pyrophyllite on arsenopyrite, the effect of FGM on the mixed mineral system was investigated. Here, the mass ratio between arsenopyrite (−0.074 + 0.037 mm) and pyrophyllite (−0.037 mm) was 1:1 at mass ratio. It could be seen from Figure 8b that the recovery of pyrophyllite decreased significantly with the increase in FGM, but arsenopyrite was less affected. Although FGM demonstrated effectiveness in the simulated ore system, its performance in more complex actual gold ore systems remains to be evaluated.
3.1.3. Actual Ore Test
The industrial potential of FGM was comparatively investigated with SHMP and silicate, which are the commonly used depressants [31,32], for actual ore flotation. The flotation process was shown in Figure 2, and the flotation results were shown in Table 2. It could be seen from Table 1 that the Au grade of the crude gold concentrate increased by 1.9 g/t after adding FGM, suggesting that its inhibition effect on gangue minerals was stronger than that of sodium silicate and SHMP at the same dosage.

Table 2.
Results of the actual ore flotation test.
On the basis of the preliminary test and reference to the field reagent system, the flotation closed-circuit test was carried out according to the flow shown in Figure 3. As shown in Table 3, after adding inhibitors, the concentrate grade increased from 14.60 g/t to 18.50 g/t, and the enrichment ratio increased by 2.53. Adding an appropriate dosage of depressant, the viscosity of pulp increased, but the frother strength decreased, which was helpful to the flotation of target minerals [33,34]. The closed-circuit flotation results showed that FGM had the potential to realize large-scale industrialization in high clay content gold ore.

Table 3.
Closed-circuit flotation test results.
3.1.4. Industrial Test
The industrial test of FGM was carried out in a gold concentrator in northwest China. The test flow was one roughing–three cleanings–three sweepings and the results are shown in Table 4. After the addition of depressant, the concentrate grade increased by 4.86 g/t, from 26.11 g/t to 30.97 g/t, and the enrichment ratio was increased by 1.99. There has also been a slight increase in recovery.

Table 4.
Industrial flotation test results.
The industrial test showed that FGM could realize industrialization. Up to now, FGM has increased the profits by USD 1.715 M in the past two years by improving concentrate grade and valuation coefficient.
3.2. Contact Angle Tests
The relationship between the amount of FGM and the contact angle of the minerals surface was studied to discover the interaction mechanism (pH = 6.5). The natural contact angles of the fresh surfaces of arsenopyrite and pyrophyllite are 64.9 and 53.8 degrees, respectively. The results show that the fresh surface wettability of arsenopyrite is slightly worse than that of pyrophyllite. Generally, the greater the value of the contact angle, the value of the wettability index will decrease accordingly.
As shown in Figure 9, with the increase in the dosage of FGM, the contact angle of the arsenopyrite decreases slightly, but it remains about 60° overall, which indicates that FGM has little effect on the hydrophobicity of arsenopyrite at this pH. On the contrary, the contact angle of pyrophyllite showed a significant downward trend, from 53° to below 40°. This reflected the ability of FGM to selectively decrease the hydrophobicity of pyrophyllite [35]. In other words, FGM can enhance the surface wettability of pyrophyllite and inhibit its flotation.
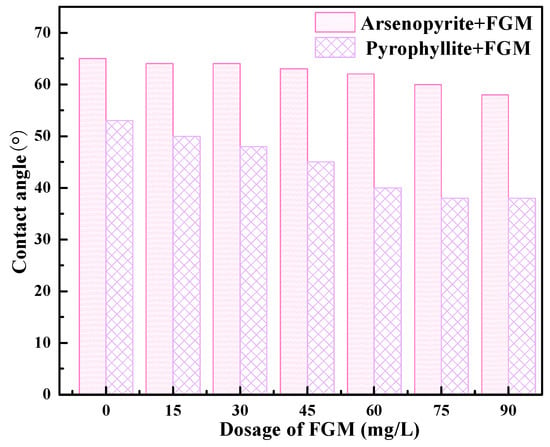
Figure 9.
The relationship between the dosage of FGM and the contact angle of the mineral surface.
3.3. Electrokinetic Potentials
When the mineral acted with the flotation reagent, the electrical characteristics of its surface would alter. By measuring the zeta potential of the mineral surface under the action of reagents, we could evaluate the adsorption capacity of the reagent [36].
Figure 10 showed the effect of FGM on the surface potential of arsenopyrite and pyrophyllite under different pH conditions. It could be seen that FGM would result in an increase in zeta potentials of pyrophyllite and arsenopyrite. It was because the adsorption of FGM on the mineral surface would replace the adsorption of OH− on the mineral surface [37]. At the same time, after the action of FGM, the increase in surface zeta potential of pyrophyllite was more than that of arsenopyrite, which showed that FGM was more likely to adsorb selectively on pyrophyllite, reducing the floatability.
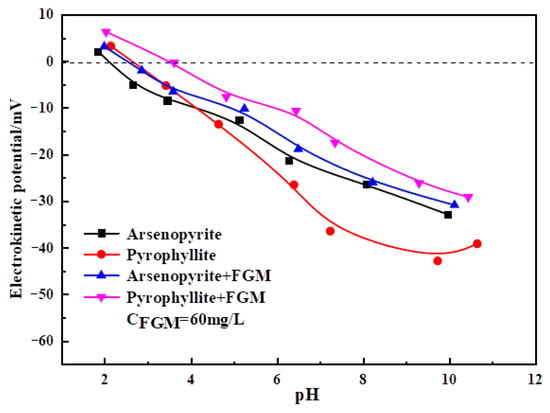
Figure 10.
Zeta potential values shown as a function of pH.
3.4. FTIR Measurements
Figure 11a,b shows the infrared spectra of arsenopyrite/FGM and pyrophyllite/FGM, respectively. In the infrared spectrum of FGM, it could be seen that there was an obvious peak at 3452 cm−1, which was caused by the stretching vibration of -OH. It was an important characteristic peak of natural polymer polysaccharides containing multiple hydroxyl groups, and it was also a key basis for judging polymer polysaccharides [38]. In addition, the peak at 2889 cm−1 was due to the stretching vibration of -CH2, and the peak at 1651 cm−1 was caused by the ring stretching vibration of the carbon–oxygen six-membered ring. The peaks at 1441 cm−1, 1162 cm−1, and 1018 cm−1 corresponded to the C-O stretching vibration in the ether -O- and the C-O stretching vibration in the primary alcohol group and the secondary alcohol group.
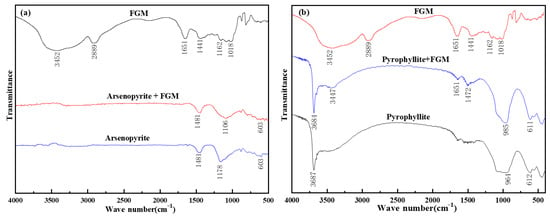
Figure 11.
(a) Infrared spectra of arsenopyrite and (b) pyrophyllite before and after the FGM treatment.
When the arsenopyrite was treated by FGM, the absorption peak at 1178 cm−1 shifted significantly, and it moved to a new position of 1106 cm−1 [39], while the peaks at other positions remained unchanged. The phenomenon revealed that the effect of FGM on arsenopyrite was relatively weak, more of a physical adsorption.
After the interaction between FGM and pyrophyllite, new absorption peaks appeared at 3447 cm−1 and 1472 cm−1. These new peaks correspond to the strong absorption peak of -OH in the FGM spectrum (located at 3452 cm−1), the ring stretching vibration peak of the carbon–oxygen six-membered ring, and the C-O stretching vibration peak of the ether group. At the same time, the broad peak of pyrophyllite at 964 cm−1 also moved to the right to 985 cm−1, which reflected the influence of C-O stretching vibration of the secondary alcohol group in FGM. In addition, other absorption peaks of pyrophyllite also had some deviation after interacting with FGM. These changes indicated that FGM was not only physically but also chemically adsorbed on the surface of pyrophyllite.
3.5. XPS Detection
The results of zeta potential and FTIR showed that the adsorption of FGM on the mineral surface had selectivity. In order to further explore the underlying mechanism of FGM on pyrophyllite, XPS detection was employed. The narrow area scanning of Al1s and Si2p on the surface of pyrophyllite with and without FGM adsorption was carried out, and the results are shown in Figure 12 and Table 5.
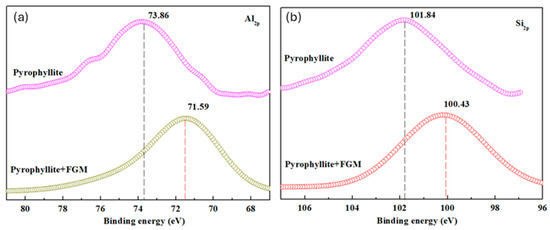
Figure 12.
The change in valence state of atoms (a) Al and (b) Si on the pyrophyllite surface before and after FGM action.

Table 5.
The change in binding energy of Al1s and Si2p peaks on the pyrophyllite surface before and after the action of polymer depressant FGM.
The binding energy of Al2p and Si2p on the mineral surface appeared to have a chemical shift, which was −2.27 eV and −1.41 eV after FGM interaction. According to the lattice chemical bond strength and dissociation characteristics of clay minerals, when the layered aluminosilicate clay minerals such as pyrophyllite were comminuted, the Si4+-O2− bond in the lattice was difficult to break, while the Al3+-O2− ion bond was relatively easy to break, which means that the surface of pyrophyllite was exposed to Al3+-O2−. FGM mainly reacted with Al3+ on the surface of pyrophyllite. It could be inferred that the chemical adsorption of FGM on pyrophyllite was mainly through the -OH active group in the FGM with Al3+ on the pyrophyllite surface [40]. Chemisorption, of course, is always accompanied by physical adsorption for polysaccharide drug molecules.
4. Conclusions
- (1)
- Pyrophyllite has good natural floatability, so it is difficult to separate arsenopyrite from it without a depressant. In addition, pyrophyllite with particle sizes less than 0.074 mm distinctly decreased the flotation of arsenopyrite. FGM can eliminate this adverse effect largely due to it selectively depressing the flotation of pyrophyllite.
- (2)
- For the actual ore flotation system, FGM still exhibited better selective depression performance on the gangue minerals compared with SHMP and silicate, resulting in a concentrate grade increase in laboratory closed-circuit flotation tests and industrial tests. FGM has the potential to achieve large-scale industrialization for the flotation of high-clay-content gold ore.
- (3)
- The selective adsorption of FGM onto the pyrophyllite (gangue mineral) surface was the foundation of the great performance exhibited for the pure mineral system and actual ore. This adsorption was established mainly through the interaction between the Al sites on the surface of pyrophyllite and the –OH on the molecular chain of FGM.
Author Contributions
Conceptualization, X.W. and P.M.; methodology, X.W. and Q.X.; software, K.Z.; validation, P.M., Q.X. and Z.W.; investigation, Q.X. and F.L.; resources, K.Z. and Z.W.; writing—original draft preparation, Q.X.; writing—review and editing, Z.W.; visualization, Z.W.; funding acquisition, K.Z. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program ‘Intergovernmental Cooperation in International Science and Technology Innovation’ Key Special Projects (No. 2023YFE0104100), the Sichuan Science and Technology Program of China (No. 2024YFHZ0243 and No. 2022YFS0453), and the National Science Foundation of China (No. 52474301).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We would like to thank Hui Yang (Y.H.), a student from Kunming University of Science and Technology, for her help with the FT-IR tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.; Zeng, Q.; Wei, Z.; Fan, H.; Yang, K.; Zhang, Z.; Li, X.; Liang, G.; Xia, F. Prospecting potential of the yanjingou gold deposit in the east kunlun orogen, nw china: Evidence from primary halo geochemistry and in situ pyrite thermoelectricity. Minerals 2021, 11, 1117. [Google Scholar] [CrossRef]
- Ming, P.; Li, F.; Chen, Z.; Xiong, Z.; Hu, M. Practice and Application of Regrinding and Re-election of Swept Concentrate from a Low-grade Difficult Gold Ore in Qinghai Province. Multipurp. Util. Miner. Resour. 2024, 45, 15–23. (In Chinese) [Google Scholar]
- Zhao, K.; Wang, X.; Yan, W.; Gu, G.; Wang, C.; Wang, Z.; Xu, L.; Peng, T. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Miner. Eng. 2019, 132, 134–141. [Google Scholar]
- Zhang, N.; Nguyen, A.V.; Zhou, C. A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv. Colloid Interface Sci. 2018, 254, 56–75. [Google Scholar] [PubMed]
- Pavlovic, M.; Dojcinovic, M.; Nikolic, J.; Terzic, A.; Pavicevic, V.; Drmanic, S.; Kurtanovic, E. Application of waste raw materials as a reinforcement for protective coatings based on pyrophyllite. Chem. Ind. Chem. Eng. Q. 2024. online-first. [Google Scholar]
- Somasundaran, P. An overview of the ultrafine problem. In Mineral Processing at a Crossroads: Problems and Prospects; Springer: Berlin/Heidelberg, Germany, 1986; pp. 1–36. [Google Scholar]
- Peng, Y.; Zhao, S. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar]
- Chen, X.; Peng, Y. Managing clay minerals in froth flotation—A critical review. Miner. Process. Extr. Metall. Rev. 2018, 39, 289–307. [Google Scholar]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar]
- Kashif, N. Grinding, Flotation and Drying Performance of the Clay-Containing Ores. Doctoral Dissertation, Curtin University, Bentley, Australia, 2024. [Google Scholar]
- Liu, S.; Chen, X.; Lauten, R.A.; Peng, Y.; Liu, Q. Mitigating the negative effects of clay minerals on gold flotation by a lignosulfonate-based biopolymer. Miner. Eng. 2018, 126, 9–15. [Google Scholar]
- Demeusy, B.; Madanski, D.; Gaydardzhiev, S. Laboratory flotation study of the DPM Krumovgrad gold ore (Bulgaria)—Effects from improved collector suite and ore desliming. Miner. Eng. 2024, 208, 108596. [Google Scholar]
- Wang, L.; Shen, L.; Sun, W.; Zhang, X.; Zhang, Y.; Wang, Y. Selective flotation separation of smithsonite from dolomite by using sodium hexametaphosphate as a depressant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129621. [Google Scholar] [CrossRef]
- Hoang, D.H.; Ebert, D.; Möckel, R.; Rudolph, M. Impact of Sodium Hexametaphosphate on the flotation of ultrafine magnesite from dolomite-rich desliming tailings. Minerals 2021, 11, 499. [Google Scholar] [CrossRef]
- Rao, D.S.; VijayaKumar, T.V.; Rao, S.S.; Prabhakar, S.; Raju, G.B. Effectiveness of sodium silicate as gangue depressants in iron ore slimes flotation. Int. J. Miner. Metall. Mater. 2011, 18, 515–522. [Google Scholar] [CrossRef]
- Long, T.; Zhao, H.; Wang, Y.; Yang, W.; Deng, S.; Xiao, W.; Lan, X.; Wang, Q. Synergistic mechanism of acidified water glass and carboxymethyl cellulose in flotation of nickel sulfide ore. Miner. Eng. 2022, 181, 107547. [Google Scholar] [CrossRef]
- Li, H.; Chai, W.; Cao, Y.; Yang, S. Flotation enhancement of low-grade bauxite using oxalic acid as surface pretreatment agent. Appl. Surf. Sci. 2022, 577, 151964. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.; Liu, D.; Chen, J. Selective alteration mechanisms of sodium tripolyphosphate towards serpentine: Implications for flotation of pyrite from serpentine. J. Mol. Liq. 2022, 368, 120687. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Fenugreek seed gum: Biological properties, chemical modifications, and structural analysis—A review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef]
- Nalbantova, V.; Benbassat, N.; Delattre, C. Fenugreek Galactomannan and Its Versatile Applications. Polysaccharides 2024, 5, 478–492. [Google Scholar] [CrossRef]
- De, A.; Mishra, S. Synthesis of fenugreek gum-based metal–organic framework (FG/Zr-AIPA MOF) composite beads for sequestration of heavy metal ions from aqueous solution. Environ. Sci. Pollut. Res. 2024, 31, 32571–32587. [Google Scholar] [CrossRef]
- Lgaz, H.; Chung, I.-M.; Salghi, R.; Ali, I.H.; Chaouiki, A.; El Aoufir, Y.; Khan, M.I. On the understanding of the adsorption of Fenugreek gum on mild steel in an acidic medium: Insights from experimental and computational studies. Appl. Surf. Sci. 2019, 463, 647–658. [Google Scholar] [CrossRef]
- Che, Y.; Chen, W.; Liu, S.; Zeng, G.; Liu, G. Exploring the highly efficient depressant role of fenugreek gum on talc in chalcopyrite flotation. Miner. Eng. 2024, 213, 108751. [Google Scholar]
- Luo, Y.; Zhang, G.; Li, C.; Mai, Q.; Liu, H.; Zhou, H.; Shi, Q. Flotation separation of smithsonite from calcite using a new depressant fenugreek gum. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123794. [Google Scholar]
- Zhang, Y.; Xu, Y.; Zhang, L.; Chen, Y.; Wu, T.; Liu, R.; Zhang, M. Licorice extract ameliorates hyperglycemia through reshaping gut microbiota structure and inhibiting TLR4/NF-κB signaling pathway in type 2 diabetic mice. Food Res. Int. 2022, 153, 110945. [Google Scholar] [PubMed]
- Ning, S.; Pei, B.; Ma, Y.; Li, J.; Liu, R.; Liu, D. Flotation separation of Galena from pyrite in a Low-alkalinity Media using Welan Gum as a new depressant. Miner. Process. Extr. Metall. Rev. 2024, 45, 790–802. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.A. Hydrogen storage in depleted gas reservoirs using nitrogen cushion gas: A contact angle and surface tension study. Int. J. Hydrogen Energy 2023, 48, 38782–38807. [Google Scholar]
- Moseenkov, S.I.; Kuznetsov, V.L.; Zolotarev, N.A.; Kolesov, B.A.; Prosvirin, I.P.; Ishchenko, A.V.; Zavorin, A.V. Investigation of amorphous carbon in nanostructured carbon materials (A Comparative Study by TEM, XPS, Raman Spectroscopy and XRD). Materials 2023, 16, 1112. [Google Scholar] [CrossRef]
- Dai, L.; Feng, B.; Zhang, L.; Chen, Y.; Bayoundoula, J. Selective flotation separation of spodumene and quartz with carboxylated chitosan as depressant. Miner. Eng. 2023, 203, 108343. [Google Scholar]
- Saim, A.K.; Darteh, F.K. Eco-friendly and biodegradable depressants in chalcopyrite flotation: A review. Miner. Process. Extr. Metall. Rev. 2023, 44, 492–510. [Google Scholar]
- Wang, Y.; Tian, J.; Han, H.; Sun, W.; Zhang, X. The enhanced flotation separation of magnesite and dolomite by introducing chelating reagent EDTA. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132969. [Google Scholar]
- Mahroomi, M.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M.; Ebrahimi, E. The flotation of apatite and calcite using different reagents: A comparative study. Int. J. Min. Geo-Eng. 2024, 58, 341–350. [Google Scholar]
- Molifie, A.; Becker, M.; Geldenhuys, S.; McFadzean, B. Investigating the reasons for the improvement in flotation grade and recovery of an altered PGE ore when using sodium silicate. Miner. Eng. 2023, 195, 108024. [Google Scholar] [CrossRef]
- Moudgil, B.M. Correlation between froth viscosity and flotation efficiency. Min. Metall. Explor. 1993, 10, 100–101. [Google Scholar]
- Zheng, Q.; Dong, L.; Shen, P.; Liu, D. A novel activation scheme for fine cassiterite flotation by using a metal ion modified collector. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136310. [Google Scholar]
- Liu, J.; Kong, D.; Xie, R.; Li, Y.; Zhu, Y.; Liu, C. Flotation behavior and mechanism of hydroxycitric acid as a depressant on the flotation separation of cassiterite from calcite. Miner. Eng. 2021, 170, 107046. [Google Scholar] [CrossRef]
- Xiao, Y.; Feng, J.; Yang, B.; Luo, H.; Zhou, F. The combination of calcium hypochlorite and fulvic acid as an efficient arsenopyrite depressant in Cu-As separation. Miner. Eng. 2024, 206, 108497. [Google Scholar]
- Nawrocka, A.; Krekora, M.; Niewiadomski, Z.; Miś, A. FTIR studies of gluten matrix dehydration after fibre polysaccharide addition. Food Chem. 2018, 252, 198–206. [Google Scholar]
- Dong, W.; Liu, R.; Wang, C.; Zhu, X.; Xie, Z.; Sun, W. Insight into selective depression of sodium thioglycallate on arsenopyrite flotation: Adsorption mechanism and constructure. J. Mol. Liq. 2023, 377, 121480. [Google Scholar]
- Zhao, S.M.; Wang, D.Z.; Hu, Y.H.; Liu, B.D.; Xu, J. The flotation behaviour of N-(3-aminopropyl)-dodecanamide on three aluminosilicates. Miner. Eng. 2003, 16, 1391–1395. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).