Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review
Abstract
1. Introduction
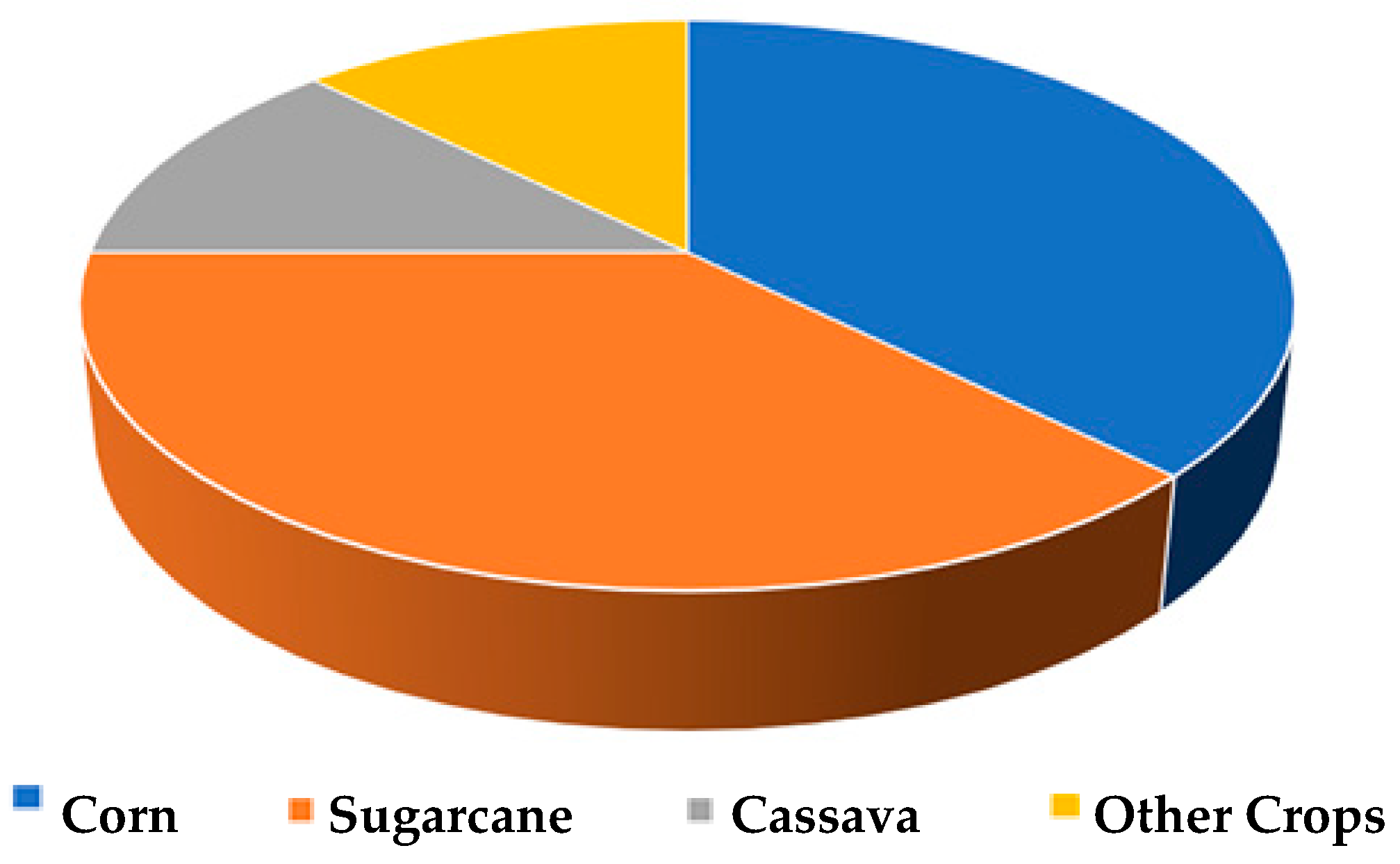
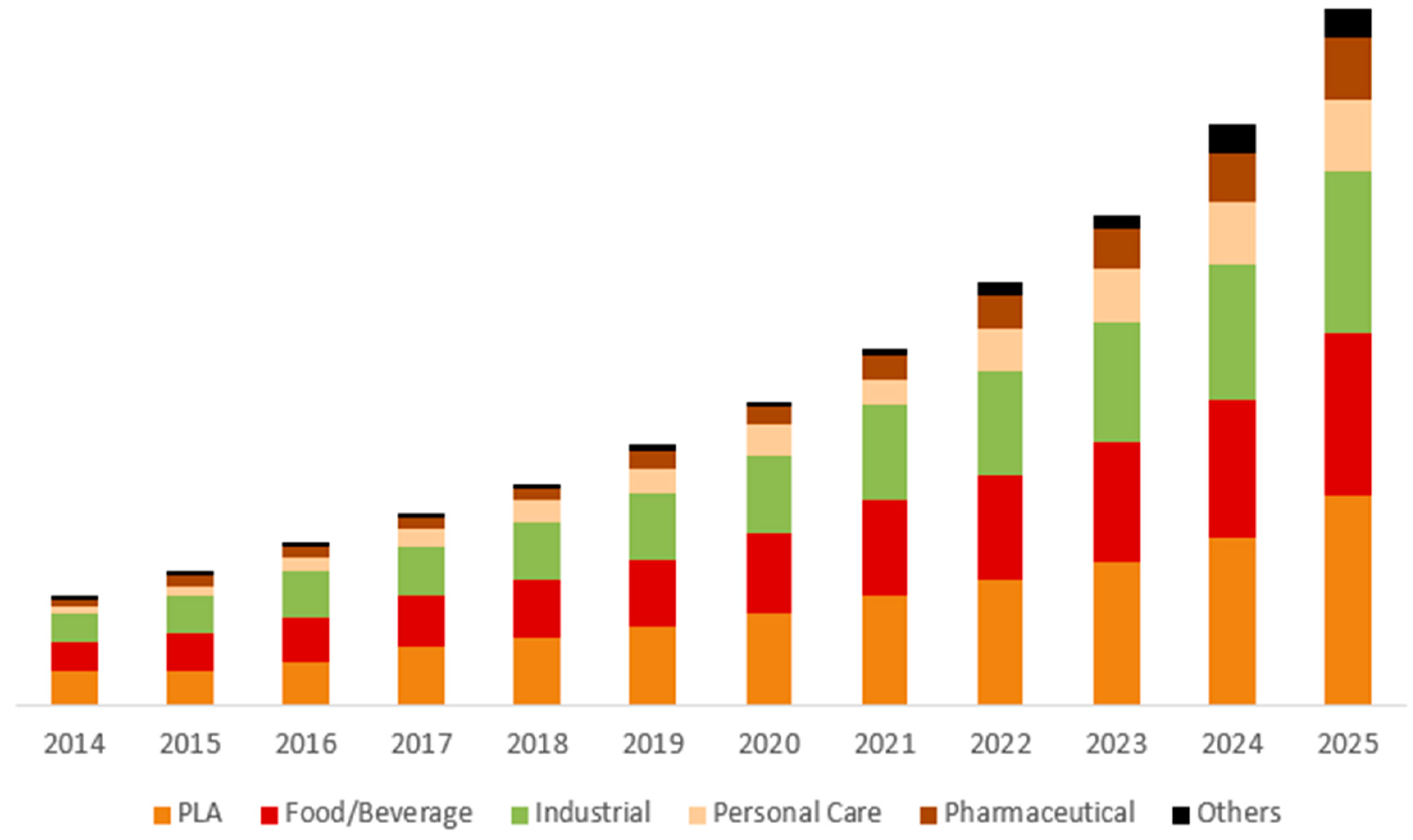
1.1. PLA Production, General Properties, and Present Market
1.2. Chemical Structure of PLA and Chemical Modification Possibilities
1.3. The Use of PLA in 3D Printers and the Present Drawbacks
1.4. Reasons for the Surface Modification of PLA
2. Surface Modification of PLA Solids
2.1. Surface Modification with Chemical Methods Giving Covalent Attachment
2.1.1. Alkaline Surface Hydrolysis and Atom Transfer Polymerization (ATRP)
2.1.2. Photografting by UV Light
2.2. Surface Modification of PLA with Plasma Treatment
2.2.1. Advantages of Plasma Treatment
2.2.2. Surface Modification of PLA with Plasma Treatment
2.2.3. Surface Modification of PLA with Plasma and Chemical Reaction
3. 3D Printed PLA Objects
3.1. PLA in Extrusion-Based Printing Technologies
3.2. Use of Powder-Based Techniques and Photopolymerization in 3D Printing of PLA
4. Surface Modification of 3D Printed PLA Objects
4.1. The Use of Polydopamine Coatings for the Surface Modification of 3D-Printed PLA Objects in Biomedical Applications
4.2. The Use of Other Materials for the Surface Modification of 3D-Printed PLA Objects in Biomedical Applications
4.3. The Use of Other Methods for the Surface Modification of 3D-Printed PLA Objects
5. Conclusions
Funding
Conflicts of Interest
References
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial app. Polym. Degrad. Stab. 1998, 3910, 145–152. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mat. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.A.; Lim, L.T.; Selke, S.E.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 10, pp. 293–307. ISBN 978-0-470-29366-9. [Google Scholar]
- Ren, J. Biodegradable Poly (Lactic Acid): Synthesis, Modification, Processing and Applications; Springer Science & Business Media, Tsinghua University Press: Beijing, China, 2011; pp. 1–204. ISBN 978-7-302-23601-6. [Google Scholar]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides—Methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio) degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef]
- Dorgan, J.R.; Lehermeier, H.J.; Palade, L.I.; Cicero, J. Polylactides: properties and prospects of an environmentally benign plastic from renewable resources. Macromol. Symp. 2001, 175, 55–66. [Google Scholar] [CrossRef]
- Cicero, J.A.; Dorgan, J.R.; Garrett, J.; Runt, J.; Lin, J.S. Effects of molecular architecture on two-step, melt-spun poly (lactic acid) fibers. J. Appl. Polym. Sci. 2002, 86, 2839–2846. [Google Scholar] [CrossRef]
- Radano, C.P.; Baker, G.L.; Smith, M.R. Stereoselective polymerization of a racemic monomer with a racemic catalyst: direct preparation of the polylactic acid stereocomplex from racemic lactide. J. Am. Chem. Soc. 2000, 122, 1552–1553. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Jürgens, C.; Wolter, D. Polylactides-synthesis, characterization and medical application. Macromol. Symp. 1996, 103, 85–102. [Google Scholar]
- Fambri, L.; Pegoretti, A.; Fenner, R.; Incardona, S.D.; Migliaresi, C. Biodegradable fibres of poly (l-lactic acid) produced by melt spinning. Polymer 1997, 38, 79–85. [Google Scholar] [CrossRef]
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Sawyer, D.J. Bioprocessing–no longer a field of dreams. Macromol. Symp. 2003, 201, 271–282. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices-A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Bergsma, J.E.; De Bruijn, W.C.; Rozema, F.R.; Bos, R.R.M.; Boering, G. Late degradation tissue response to poly (l-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Incardona, S.D.; Fambri, L.; Migliaresi, C. Poly-l-lactic acid braided fibres produced by melt spinning: characterization and in vitro degradation. J. Mater. Sci. Mater. Med. 1996, 7, 387–391. [Google Scholar] [CrossRef]
- Xia, X.; Liu, W.; Zhou, L.; Hua, Z.; Liu, H.; He, S. Modification of flax fiber surface and its compatibilization in polylactic acid/flax composites. Iran. Polym. J. (English Ed.) 2016, 25, 25–35. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Wu, C.S. Modulation, functionality, and cytocompatibility of three-dimensional printing materials made from chitosan-based polysaccharide composites. Mater. Sci. Eng. C 2016, 69, 27–36. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Review of Multifarious Applications of Poly (Lactic Acid). Polym. Plast. Technol. Eng. 2016, 55, 1057–1075. [Google Scholar] [CrossRef]
- Ambone, T.; Joseph, S.; Deenadayalan, E.; Mishra, S.; Jaisankar, S.; Saravanan, P. Polylactic Acid (PLA) Biocomposites Filled with Waste Leather Buff (WLB). J. Polym. Environ. 2017, 25, 1099–1109. [Google Scholar] [CrossRef]
- Ratner, B.D. Surface modification of polymers: chemical, biological and surface analytical challenges. Biosens. Bioelectron. 1995, 10, 797–804. [Google Scholar] [CrossRef]
- Burg, K.J.L.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Loebsack, A.B.; Roland, W.D.; Mooney, D.J.; Halberstadt, C.R. Parameters affecting cellular adhesion to polylactide films. J. Biomater. Sci. Polym. Ed. 1999, 10, 147–161. [Google Scholar] [CrossRef]
- Mochizuki, M. Textile applications. In Poly (Lactic Acid) Synthesis, Structures, Properties, Processing, and Applications, 1st ed.; Auras, R.A., Lim, L.T., Selke, S.E., Tsuji, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 10, pp. 469–476. ISBN 978-0-470-29366-9. [Google Scholar]
- Gruber, P.; O’Brien, M. Polylactides NatureWorksTM PLA. In Biopolymers; Doi, Y., Steinbuchel, A., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 4, pp. 235–249. [Google Scholar]
- Serizawa, S.; Inoue, K.; Iji, M. Kenaf-fiber-reinforced poly(lactic acid) used for electronic products. J. Appl. Polym. Sci. 2006, 100, 618–624. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market (accessed on 6 February 2019).
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/polylactic-acid-market (accessed on 6 February 2019).
- Kimura, Y.; Shirotani, K.; Yamane, H.; Kitao, T. Ring-Opening Polymerization of 3(S)-[(Benzyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione: A New Route to a Poly(α-hydroxy acid) with Pendant Carboxyl Groups. Macromolecules 1988, 21, 3338–3340. [Google Scholar] [CrossRef]
- Tymrak, B.M.; Kreiger, M.; Pearce, J.M. Mechanical properties of components fabricated with open-source 3-D printers under realistic environmental conditions. Mater. Des. 2014, 58, 242–246. [Google Scholar] [CrossRef]
- Sun, Q.; Rizvi, G.M.; Bellehumeur, C.T.; Gu, P. Effect of processing conditions on the bonding quality of FDM polymer filaments. Rapid Prototyp. J. 2008, 14, 72–80. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Van Puyvelde, P. 3D Printing of Poly(lactic acid). Adv. Polym. Sci. 2018, 282, 139–158. [Google Scholar]
- Tran, P.; Ngo, T.D.; Ghazlan, A.; Hui, D. Bimaterial 3D printing and numerical analysis of bio-inspired composite structures under in-plane and transverse loadings. Compos. Part B Eng. 2017, 108, 210–223. [Google Scholar] [CrossRef]
- Melnikova, R.; Ehrmann, A.; Finsterbusch, K. 3D printing of textile-based structures by Fused Deposition Modelling (FDM) with different polymer materials. IOP Conf. Ser. Mater. Sci. Eng. 2014, 62. [Google Scholar] [CrossRef]
- Caulfield, B.; McHugh, P.E.; Lohfeld, S. Dependence of mechanical properties of polyamide components on build parameters in the SLS process. J. Mater. Process. Technol. 2007, 182, 477–488. [Google Scholar] [CrossRef]
- Garcia, C.R.; Correa, J.; Espalin, D.; Barton, J.H.; Rumpf, R.C.; Wicker, R.; Gonzalez, V. 3D Printing of anisotropic metamaterials. Prog. Electromagn. Res. Lett. 2012, 34, 75–82. [Google Scholar] [CrossRef]
- The Voice of 3D Printing/Additive Manufacturing. Available online: https://3dprint.com/210552/filaments-directory-2018-survey (accessed on 6 February 2019).
- Groenendyk, M.; Gallant, R. 3D printing and scanning at the Dalhousie University Libraries: A pilot project. Libr. Hi Tech. 2013, 31, 34–41. [Google Scholar] [CrossRef]
- King, D.L.; Babasola, A.; Rozario, J.; Pearce, J.M. Mobile Open-Source Solar-Powered 3-D Printers for Distributed Manufacturing in Off-Grid Communities. Challenges Sustain. 2014, 2, 18–27. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of PLA-PHBHHx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. Part A 2009, 88, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly(l-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523. [Google Scholar] [CrossRef]
- Gault, R.; Pham, D. A comparison of rapid prototyping technologies. Int. J. Mach. Tools Manuf. 1998, 38, 1257–1287. [Google Scholar]
- Yan, X.; Gu, P.E.N.G. A review of rapid prototyping technologies and systems. Comput. Aided Des. 1996, 28, 307–318. [Google Scholar] [CrossRef]
- Wittbrodt, B.; Pearce, J.M. The effects of PLA color on material properties of 3-D printed components. Addit. Manuf. 2015, 8, 110–116. [Google Scholar] [CrossRef]
- El Habnouni, S.; Darcos, V.; Garric, X.; Lavigne, J.P.; Nottelet, B.; Coudane, J. Mild methodology for the versatile chemical modification of polylactide surfaces: Original combination of anionic and click chemistry for biomedical applications. Adv. Funct. Mater. 2011, 21, 3321–3330. [Google Scholar] [CrossRef]
- Wang, S.; Cui, W.; Bei, J. Bulk and surface modifications of polylactide. Anal. Bioanal. Chem. 2005, 381, 547–556. [Google Scholar] [CrossRef]
- Jiao, Y.P.; Cui, F.Z. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Moraczewski, K.; Rytlewski, P.; Malinowski, R.; Zenkiewicz, M. Comparison of some effects of modification of a polylactide surface layer by chemical, plasma, and laser methods. Appl. Surf. Sci. 2015, 346, 11–17. [Google Scholar] [CrossRef]
- Yang, J.; Shi, G.; Bei, J.; Wang, S.; Cao, Y.; Shang, Q.; Yang, G.; Wang, W. Fabrication and surface modification of macroporous poly(l-lactic acid) and poly(l-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 2002, 62, 438–446. [Google Scholar] [CrossRef]
- Xie, X.; Gengenbach, T.R.; Griesser, H.J. Changes in wettability with time of plasma-modified perfluorinated polymers. J. Adhes. Sci. Technol. 1992, 6, 1411–1431. [Google Scholar]
- Occhiello, E.; Morra, M.; Morini, G.; Garbassi, F.; Humphrey, P. Oxygen-plasma-treated polypropylene interfaces with air, water, and epoxy resins: Part I. Air and water. J. Appl. Polym. Sci. 1991, 42, 551–559. [Google Scholar] [CrossRef]
- Safinia, L.; Datan, N.; Höhse, M.; Mantalaris, A.; Bismarck, A. Towards a methodology for the effective surface modification of porous polymer scaffolds. Biomaterials 2005, 26, 7537–7547. [Google Scholar] [CrossRef]
- Yang, J.; Wan, Y.; Tu, C.; Cai, Q.; Bei, J.; Wang, S. Enhancing the cell affinity of macroporous poly(l-lactide) cell scaffold by a convenient surface modification method. Polym. Int. 2003, 52, 1892–1899. [Google Scholar] [CrossRef]
- Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar]
- Luo, N.; Stewart, M.J.; Hirt, D.E.; Husson, S.M.; Schwark, D.W. Surface modification of ethylene-co-acrylic acid copolymer films: Addition of amide groups by covalently bonded amino acid intermediates. J. Appl. Polym. Sci. 2004, 92, 1688–1694. [Google Scholar] [CrossRef]
- Zhang, P.; He, C.; Craven, R.D.; Evans, J.A.; Fawcett, N.C.; Wu, Y.; Timmons, R.B. Subsurface formation of amide in polyethylene-co-acrylic acid film: A potentially useful reaction for tethering biomolecules to a solid support. Macromolecules 1999, 32, 2149–2155. [Google Scholar] [CrossRef]
- Janorkar, A.V.; Luo, N.; Hirt, D.E. Surface modification of an ethylene-Acrylic acid copolymer film: Grafting amine-terminated linear and branched architectures. Langmuir 2004, 20, 7151–7158. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Cui, Y.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Luo, J. Surface modification of poly(d,l-lactic acid) with chitosan and its effects on the culture of osteoblasts in vitro. J. Biomed. Mater. Res. 2002, 60, 398–404. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, M.; Wu, J.; Shen, J. Covalent immobilization of chitosan/heparin complex with a photosensitive hetero-bifunctional crosslinking reagent on PLA surface. Biomaterials 2002, 23, 4657–4665. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of Biomacromolecules onto Aminolyzed Poly(l-lactic acid) toward Acceleration of Endothelium Regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.; Noel, S.; Liberelle, B.; El Ayoubi, R.; Ajji, A.; De Crescenzo, G. Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering. Biomatter 2016, 6, e1231276. [Google Scholar] [CrossRef]
- Alippilakkotte, S.; Sreejith, L. Benign route for the modification and characterization of poly(lactic acid) (PLA) scaffolds for medicinal application. J. Appl. Polym. Sci. 2018, 135, 46056. [Google Scholar] [CrossRef]
- Xu, F.J.; Yang, X.C.; Li, C.Y.; Yang, W.T. Functionalized polylactide film surfaces via surface-initiated ATRP. Macromolecules 2011, 44, 2371–2377. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, F.; Yu, X.M.; Gao, A.L.; Xue, L.X. Surface zwitterionization of hemocompatible poly(lactic acid) membranes for hemodiafiltration. J. Membrane Sci. 2015, 475, 469–479. [Google Scholar] [CrossRef]
- Ma, H.; Davis, R.H.; Bowman, C.N. Novel sequential photoinduced living graft polymerization. Macromolecules 2000, 33, 331–335. [Google Scholar] [CrossRef]
- Rahane, S.B.; Kilbey, S.M.; Metters, A.T. Kinetics of surface-initiated photoiniferter-mediated photopolymerization. Macromolecules 2005, 38, 8202–8210. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Bae, G.Y.; Jang, J.; Jeong, Y.G.; Lyoo, W.S.; Min, B.G. Superhydrophobic PLA fabrics prepared by UV photo-grafting of hydrophobic silica particles possessing vinyl groups. J. Colloid Interf. Sci. 2010, 344, 584–587. [Google Scholar] [CrossRef]
- Edlund, U.; Källrot, M.; Albertsson, A.C. Single-step covalent functionalization of polylactide surfaces. J. Am. Chem. Soc. 2005, 127, 8865–8871. [Google Scholar] [CrossRef]
- Källrot, M.; Edlund, U.; Albertsson, A.C. Surface functionalization of degradable polymers by covalent grafting. Biomaterials 2006, 27, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Steffens, G.C.M.; Nothdurft, L.; Buse, G.; Thissen, H.; Höcker, H.; Klee, D. High density binding of proteins and peptides to poly(d,l-lactide) grafted with polyacrylic acid. Biomaterials 2002, 23, 3523–3531. [Google Scholar] [CrossRef]
- Höglund, A.; Hakkarainen, M.; Edlund, U.; Albertsson, A.C. Surface modification changes the degradation process and degradation product pattern of polylactide. Langmuir 2010, 26, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Modification of poly(lactic acid) films: Enhanced wettability from surface-confined photografting and increased degradation rate due to an artifact of the photografting process. Macromolecules 2004, 37, 9151–9159. [Google Scholar] [CrossRef]
- Guo, B.; Finne-Wistrand, A.; Albertsson, A.C. Electroactive hydrophilic polylactide surface by covalent modification with tetraaniline. Macromolecules 2012, 45, 652–659. [Google Scholar] [CrossRef]
- Gutiérrez-Villarreal, M.H.; Guzmán-Moreno, J.G. Surface graft polymerization of N-vinylcaprolactam onto polylactic acid film by UV irradiation. J. Polym. Res. 2013, 20. [Google Scholar] [CrossRef]
- Nugroho, R.W.N.; Pettersson, T.; Odelius, K.; Höglund, A.; Albertsson, A.C. Force interactions of nonagglomerating polylactide particles obtained through covalent surface grafting with hydrophilic polymers. Langmuir 2013, 29, 8873–8881. [Google Scholar] [CrossRef] [PubMed]
- Loh, I.H.; Sheu, M.S. Plasma surface modification in biomedical applications. Mater. Res. Soc. Symp. Proc. 1995, 414, 43–52. [Google Scholar] [CrossRef]
- D’Agostino, R. Plasma Deposition, Treatment and Etching of Polymers; Academic Press: Boston, MA, USA, 1990; ISBN 9780122004308. [Google Scholar]
- Chapman, B. Glow Discharge Processes; Wiley-Interscience: New York, NY, USA, 1980; ISBN 0-471-07828-X. [Google Scholar]
- Favia, P.; D’Agostino, R. Plasma treatments and plasma deposition of polymers for biomedical applications. Surf. Coatings Technol. 1998, 98, 1102–1106. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Improving cell affinity of poly (d,l-lactide) film modified by anhydrous ammonia plasma treatment. Polym. Adv. Technol. 2002, 13, 220–226. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, J.; Yang, J.; Bei, J.; Wang, S. Cell adhesion on gaseous plasma modified poly-(l-lactide) surface under shear stress field. Biomaterials 2003, 24, 3757–3764. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Pinheiro, L.M.P.; Nascente, P.A.P.; Ferreira, M.J.; Duek, E.A.R. Plasma surface treatments of poly(l-lactic acid) (PLLA) and poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV). Mater. Sci. Eng. C 2009, 29, 806–813. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Desmet, T.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Plasma modification of polylactic acid in a medium pressure DBD. Surf. Coatings Technol. 2010, 204, 3272–3279. [Google Scholar] [CrossRef]
- Guowei, Z.; Junping, G.; Qiang, G.; Yashao, C. Surface modification of biodegradable poly(d,l-lactic acid) by nitrogen and nitrogen/hydrogen plasma for improving surface hydrophilicity. Plasma Sci. Technol. 2011, 13, 230–234. [Google Scholar]
- Jiao, Y.; Xu, J.; Zhou, C. Effect of ammonia plasma treatment on the properties and cytocompatibility of a poly(l-lactic acid) film surface. J. Biomater. Sci. Polym. Ed. 2012, 23, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.; Declercq, H.; De Geyter, N.; Cornelissen, R.; Dubruel, P.; Leys, C.; Beaurain, A.; Payen, E.; Morent, R. Plasma surface modification of polylactic acid to promote interaction with fibroblasts. J. Mater. Sci. Mater. Med. 2013, 24, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Tenn, N.; Follain, N.; Fatyeyeva, K.; Poncin-Epaillard, F.; Labrugère, C.; Marais, S. Impact of hydrophobic plasma treatments on the barrier properties of poly(lactic acid) films. RSC Adv. 2014, 4, 5626–5637. [Google Scholar] [CrossRef]
- Bolbasov, E.N.; Rybachuk, M.; Golovkin, A.S.; Antonova, L.V.; Shesterikov, E.V.; Malchikhina, A.I.; Tverdokhlebov, S.I. Surface modification of poly (l-lactide) and polycaprolactone bioresorbable polymers using RF plasma discharge with sputter deposition of a hydroxyapatite target. Mater. Lett. 2014, 132, 281–284. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J.; Dörsam, E. Effects of argon low temperature plasma on PLA film surface and aging behaviors. Vacuum 2017, 145, 278–284. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Yang, J.; Wan, Y.; Yang, J.; Bei, J.; Wang, S. Plasma-treated, collagen-anchored polylactone: Its cell affinity evaluation under shear or shear-free conditions. J. Biomed. Mater. Res. Part A 2003, 67, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Chen, J.; Gao, S.; Chang, J.; Zhang, J.; Kang, E.T. Immobilization of chitosan onto poly-l-lactic acid film surface by plasma graft polymerization to control the morphology of fibroblast and liver cells. Biomaterials 2004, 25, 1059–1067. [Google Scholar] [CrossRef]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Wu, J.; He, Q.; Zhou, X.; Lu, G.; Shang, L.; Boey, F.; Venkatraman, S.S.; Zhang, H. Surface modification of smooth poly(l-lactic acid) films for gelatin immobilization. ACS Appl. Mater. Interfaces. 2012, 4, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, V.; Niaros, V.; Giotitsas, C. Open source 3D printing as a means of learning: An educational experiment in two high schools in Greece. Telemat. Informatics. 2014, 32, 118–128. [Google Scholar] [CrossRef]
- Baden, T.; Chagas, A.M.; Gage, G.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open Labware: 3-D Printing Your Own Lab Equipment. PLoS Biol. 2015, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Applications of Open Source 3-D Printing on Small Farms. Org. Farming 2015, 1, 19–35. [Google Scholar] [CrossRef]
- Rankin, T.M.; Giovinco, N.A.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Three-dimensional printing surgical instruments: Are we there yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef]
- Zhang, J.W.; Peng, A.H. Process-Parameter Optimization for Fused Deposition Modeling Based on Taguchi Method. Adv. Mater. Res. 2012, 538, 444–447. [Google Scholar] [CrossRef]
- Drummer, D.; Cifuentes-Cuéllar, S.; Rietzel, D. Suitability of PLA/TCP for fused deposition modeling. Rapid Prototyp. J. 2012, 18, 500–507. [Google Scholar] [CrossRef]
- Too, M.H.; Leong, K.F.; Chua, C.K.; Du, Z.H.; Yang, S.F.; Cheah, C.M.; Ho, S.L. Investigation of 3D non-random porous structures by fused deposition modelling. Int. J. Adv. Manuf. Technol. 2002, 19, 217–223. [Google Scholar] [CrossRef]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Torres, J.; Cole, M.; Owji, A.; DeMastry, Z.; Gordon, A.P. An approach for mechanical property optimization of fused deposition modeling with polylactic acid via design of experiments. Rapid Protyp. J. 2016, 22, 387–404. [Google Scholar] [CrossRef]
- Lanzotti, A.; Grasso, M.; Staiano, G.; Martorelli, M. The impact of process parameters on mechanical properties of parts fabricated in PLA with an open-source 3-D printer. Rapid Prototyp. J. 2015, 21, 604–617. [Google Scholar] [CrossRef]
- Stephen, A.O.; Dalgarno, K.W.; Munguia, J. Quality assurance and process monitoring of fused deposition modelling made parts. High Value Manuf. Adv. Res. Virtual Rapid Prototyp. 2013, 31–35. [Google Scholar]
- Afrose, M.F.; Masood, S.H.; Iovenitti, P.; Nikzad, M.; Sbarski, I. Effects of part build orientations on fatigue behaviour of FDM-processed PLA material. Prog. Addit. Manuf. 2016, 1, 21–28. [Google Scholar] [CrossRef]
- Xinhua, L.; Shengpeng, L.; Zhou, L.; Xianhua, Z.; Xiaohu, C.; Zhongbin, W. An investigation on distortion of PLA thin-plate part in the FDM process. Int. J. Adv. Manuf. Technol. 2015, 79, 1117–1126. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Kim, E.; Shin, Y.J.; Ahn, S.H. The effects of moisture and temperature on the mechanical properties of additive manufacturing components: Fused deposition modeling. Rapid Prototyp. J. 2016, 22, 887–894. [Google Scholar] [CrossRef]
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds. Organogenesis 2013, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Ortiz-Hernandez, M.; Engel, E.; Planell, J.A.; Navarro, M. Relevance of PEG in PLA-based blends for tissue engineering. Mater. Sci. Eng. C 2014, 38, 55–62. [Google Scholar] [CrossRef]
- Zalipsky, S.; Harris, J.M. Introduction of chemistry and biological applications of poly (ethylene) glycol. In Poly(ethylene glycol): Biomedical and Biotechnological Applications, ACS Symposium Series; Harris, J.M., Zalipsky, S., Eds.; American Chemical Society: Washington, WA, USA, 1997; Volume 680, pp. 45–57. [Google Scholar]
- Inada, Y.; Furukawa, M.; Sasaki, H.; Kodera, Y.; Hiroto, M.; Nishimura, H.; Matsushima, A. Biomedical and biotechnological applications of PEG- and PM-modified proteins. Trends Biotechnol. 1995, 13, 86–91. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Lee, S.H.; Wang, M.; Cheung, W.L.; Ip, W.Y. Selective laser sintering of porous tissue engineering scaffolds from poly(l-lactide)/carbonated hydroxyapatite nanocomposite microspheres. J. Mater. Sci. Mater. Med. 2008, 19, 2535–2540. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, L.A.; Ma, P.X. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials 2006, 27, 3973–3979. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, L. Towards Scientifically Based Screening Criteria for Polymer Laser Sintering. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2016. [Google Scholar]
- Bai, J.; Goodridge, R.D.; Hague, R.J.M.; Okamoto, M. Processing and characterization of a polylactic acid/nanoclay composite for laser sintering. Polym. Compos. 2015, 38, 2570–2576. [Google Scholar] [CrossRef]
- Wang, W.L.; Cheah, C.M.; Fuh, J.Y.H.; Lu, L. Influence of process parameters on stereolithography part shrinkage. Mater. Des. 1996, 17, 205–213. [Google Scholar] [CrossRef]
- Storey, R.F.; Warren, S.C.; Allison, C.J.; Wiggins, J.S.; Pucket, A.D. Synthesis of bio-absorbable networks from methacrylate-endcapped polyesters. Polymer 1993, 34, 4365–5372. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Hou, Q.P.; Feijen, J. Preparation of biodegradable networks by photo-crosslinking lactide, epsilon-caprolactone and trimethylene carbonate-based oligomers functionalized with fumaric and monoethyl ester. Biomaterials 2005, 26, 2795–2802. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Jubbell, J.A. Bioerodible hydrogels based on photo-polymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Hinczewski, C.; Corbel, S.; Chartier, T. Ceramic suspensions suitable for stereolithography. J Eur. Ceram Soc. 1998, 18, 583–590. [Google Scholar] [CrossRef]
- Lynge, M.E.; Van der Westen, R.; Postma, A.; Stadler, B. Polydopamine-a nature-inspired polymer coating for biomedical science. Nanoscale 2011, 3, 4916–4928. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interf. 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly (dopamine) coating of 3D printed poly (lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 56, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chen, Y.W.; Shie, M.Y.; Fang, H.Y. Poly(Dopamine)-assisted immobilization of Xu Duan on 3D printed poly(Lactic Acid) scaffolds to up-regulate osteogenic and angiogenic markers of bone marrow stem cells. Materials 2015, 8, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Guo, M.; Wang, Z.; Shao, N.; Zhang, P.; Huang, Y. Degradable Three Dimensional-Printed Polylactic Acid Scaffold with Long-Term Antibacterial Activity. ACS Sustain. Chem. Eng. 2017, 6, 2047–2054. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; Thiré, R.M.D.S.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Günter, C.; Taubert, A. Co-Deposition of a Hydrogel/Calcium Phosphate Hybrid Layer on 3D Printed Poly (Lactic Acid) Scaffolds via Dip Coating: Towards Automated Biomaterials Fabrication. Polymers 2018, 10, 275. [Google Scholar] [CrossRef]
- Pokharna, P.; Ghantasala, M.K.; Rozhkova, E. Surface Modification and Characterization of Polylactic Acid (PLA) 3D Printed Structures for Cell Culture Applications. Tech. Connect Briefs 2018, 24–27. [Google Scholar]
- Wang, J.; Nor Hidayah, Z.; Razak, S.I.A.; Kadir, M.R.A.; Nayan, N.H.M.; Li, Y.; Amin, K.A.M. Surface entrapment of chitosan on 3D printed polylactic acid scaffold and its biomimetic growth of hydroxyapatite. Compos. Interf. 2019, 26, 465–478. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, A.; Shen, J. Surface engineering of poly (dl-lactic acid) by entrapment of biomacromolecules. Macromol. Rapid Commun. 2002, 23, 819–823. [Google Scholar] [CrossRef]
- Quirk, R.A.; Shakesheff, K.M.; Tendler, S.J.B.; Davies, M.C.; Chan, W.C. Controlling Biological Interactions with Poly(lactic acid) by Surface Entrapment Modification. Langmuir 2007, 17, 2817–2820. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Lee, K.M.; Park, H.; Kim, J.; Chun, D.M. Fabrication of a superhydrophobic surface using a fused deposition modeling (FDM) 3D printer with poly lactic acid (PLA) filament and dip coating with silica nanoparticles. Appl. Surf. Sci. 2019, 467, 979–991. [Google Scholar] [CrossRef]
- Cheng, C.; Gupta, M. Surface functionalization of 3D-printed plastics via initiated chemical vapor deposition. Beilstein J. Nanotechnol. 2017, 8, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Gleason, K.K. Initiated chemical vapor deposition of linear and cross-linked poly(2-hydroxyethyl methacrylate) for use as thin-film hydrogels. Langmuir 2005, 21, 8930–8939. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gleason, K.K. Initiated chemical vapor deposition of poly(1H,1H,2H,2H-perfluorodecyl acrylate) thin films. Langmuir 2006, 22, 10047–10052. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, E.H.; Erbil, H.Y. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. https://doi.org/10.3390/colloids3020043
Baran EH, Erbil HY. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids and Interfaces. 2019; 3(2):43. https://doi.org/10.3390/colloids3020043
Chicago/Turabian StyleBaran, Eda Hazal, and H. Yildirim Erbil. 2019. "Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review" Colloids and Interfaces 3, no. 2: 43. https://doi.org/10.3390/colloids3020043
APA StyleBaran, E. H., & Erbil, H. Y. (2019). Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids and Interfaces, 3(2), 43. https://doi.org/10.3390/colloids3020043





