Abstract
Silylation of clay minerals from Cherkasy deposit (Ukraine) montmorillonite (layer silicate) and palygorskite (fibrous silicate) was performed using organosilane (3-aminopropyl)triethoxysilane (APTES). Solvents with different polarity (ethanol, toluene) were used in synthesis. The structure of modified minerals was characterized by complex of methods (X-ray powder diffraction, Fourier transform infrared spectroscopy, nitrogen adsorption-desorption at −196 °C and thermal analysis). Studies of adsorption characteristics of APTES-modified clay minerals were carried out in relation to uranium (VI). The results indicated that modified montmorillonite and palygorskite were effective materials for water purification from UO22+.
1. Introduction
One of the urgent problems in the field of environmental safety at present is the protection of surface and ground waters from pollution with heavy metals and radionuclides, large amounts of which are contained in industrial wastewater. Sorption technologies are widely used for the removal of the specified pollutants. In the majority of cases, these technologies ensure attaining the scientifically-based standards specifying the maximum permissible content of pollutants in water. The natural and waste water treatment technologies involve a wide use of different layer silicates, including clay minerals [1,2,3,4]. Advantages of their application include the low cost of initial raw materials along with sufficiently high sorption characteristics in relation to inorganic toxicants. However, such sorbents feature insufficient selectivity in a number of cases, especially those requiring the removal of trace amounts of pollutants from the water having complex chemical composition and containing components-complexing agents of natural and technogenic origin (humic substances, EDTA (ethylenediaminetetraacetic acid), NTA (nitrilotriacetic acid), etc.) [5].
The synthetic silica-based inorganic sorbents are obtained using different methods of the sorbent surface functionalization for enhancing the selectivity. Such methods, primarily, involve the use of silicone compounds containing active acid groups (SH, COOH, P(O)(OH)2), basic groups (primary, secondary and tertiary amino groups, pyridine) and their combination [6,7,8,9,10].
In recent years, the method of surface functionalization with silicone compounds for the purpose of enhancing the selectivity has been used for clay minerals of different types of structure: with aluminosilicalayer 1:1 of kaolinite group minerals [11,12]; with layer 2:1 of montmorillonite group minerals [13,14,15,16], fibrous silicates of palygorskite group [17,18,19] and synthetic clay minerals [20,21]. At the same time, due to the structural peculiarities of clay minerals, the processes of their surface functionalization have a series of significant distinctions from those of silica-based sorbents [22]. This is primarily related to the swelling ability of aluminosilicate framework of a number of clay minerals in polar media that stipulates the possibility of penetration of modifier molecules into interlayer space with their subsequent fixing on tetrahedral and octahedral sheets of structural layers [23,24]. Moreover, the modification of internal surface of aluminosilicate framework was achieved also for clay minerals that do not swell under ordinary conditions, such as kaolinite, by using dimethyl sulfoxide as an intermediate reagent [25]. Formerly, it was also shown that the chemical nature of the solvents (polarity and dielectric constant) used in silylation reaction has significant influences on the process [15]. Furthermore, the dryness conditions of the reaction media may prevent the lateral polymerization of organosilane (3-aminopropyl)triethoxysilane (APTES) molecules and support the efficient silane grafting [20]. Among the clay minerals commonly used for water purification montmorillonite and palygorskite are the most popular because of their high specific surface area associated with their selectivity for heavy metal ions [26]. In the lamellar montmorillonite structure two silicon tetrahedral sheets sandwiching a central aluminum octahedral sheet. Palygorskite is fibrous clay mineral whose structure can be described as pyroxene-like ribbons laterally linked by basal oxygen atoms [27].
Uranium compounds belong to the class of most dangerous toxicants. They can be found in water both in the form of uranyl cations and in the form of poorly sorbed carbonate complexes or complexes with dissolved fulvic acids or complexing substances of technogenic origin [28]. The treatment of water contaminated with these compounds is a complex task that is stipulated by both the variety of chemical forms of uranium in real water systems and strict standards specifying the permissible content of this element in water [29]. Silanol centers on the surface of clay particles show middle sorption capacity for metal ions of actinide group of elements [30]. Grafting of aminosilanes onto the surface of clays is of great interest due to the chelating effect towards actinides (uranium). Taking into account the importance of environmental studies based in natural clay minerals, the adsorption of uranyl ions on two phyllosilicates, montmorillonite and palygorskite, modified with organosilane (3-aminopropyl)triethoxysilane, has been studied. The main purpose of this work was to verify and to understand the effect of chemical modification of two most popular in water purification practice clay minerals (montmorillonite and palygorskite) by aminosilanes on U(VI) adsorption.
2. Materials and Methods
2.1. Raw Materials
The used clay minerals were montmorillonite and palygorskite from Cherkassy deposit, Ukraine. The structural formula for montmorillonite and palygorskite as determined from chemical analysis are [31]:
Ca0,12Na0,03K0,03)0,18(Al1,39Mg0,13Fe3+0,44)1,96[(Si3,88·Al0,12)4,0O10](OH)2·2,6H2O and
(Al1,49Fe3+0,83Mg1,54)[(Si7,43Al0,58)8,0O20](OH)2·(OH2)3,15.
Their cation-exchange capacity (CEC) are 0.71 and 0.24 meq/100 g clay [31].
Na-montmorillonite (Na-MMT) and Na-palygorskite (Na-Pg) were obtained by triple treatment of 1 M NaCl. Solid phases were separated from the solutions, washed by deionized water and dried at 105 °C. Washing of the Na-form of clay minerals was repeated to achieve the complete removal of Cl-ions as indicated by the AgNO3 test of the supernatant solutions.
The organosilane agent (3-aminopropyl)triethoxysilane (APTES) was purchased from Aldrich.
2.2. Silylation with APTES
Two solvents with different polarity were chosen in this study. The polar-protic solvent used was ethanol-water solution (3:1) and the nonpolar solvent was toluene. 6.0 g of dry clay minerals (Na-MMT or Na-Pg) were dispersed in 100 mL of solvent with the use of ultrasound for 10 min. 6.0 mL of APTES was added dropwise during vigorous stirring. The clay-toluene mixtures were stirred at argon atmosphere at 45 °C for 2 h. The clay-ethanol-water mixtures were stirred at 80 °C for 5 h. Modified samples of montmorillonite (APTES-MMT) and palygorskite (APTES-Pg) were filtered and washed with solvent six times and then dried at 105 °C.
2.3. Characterization Methods
X-ray powder diffraction (XRD) patterns were recorded on X-ray diffractometer DRON-4-07 (Russia) in the range of 3–60° (2θ) using CuKα irradiation.
Fourier transform infrared spectra (FTIR) of the samples were obtained on Fourier spectrometer Thermo Nicolet Nexus in the range of 4000–450 cm–1 at twenty-fold scanning. The FTIR spectra were obtained by using KBr pellet technique (1:100).
Low-temperature nitrogen adsorption isotherms were determined on a volumetric automatic apparatus (Quantachrome, Nova 2200e) at −196 °C.
Curves of differential thermal analysis (DTA-TG) were obtained using apparatus Derivatograph–C (F. Paulik, J. Paulic, L. Erdey) in the range of temperature 20–1000 °C at the heating rate 10 min−1.
2.4. Adsorption Experiments
The adsorption experiments were carried out at 25 °C. The reaction mixture (50 mL) containing 0.05 g of adsorbent (background electrolyte 0.01 M NaCl) and desired amount of U(VI) ions (UO2SO4·3H2O solution) were prepared. Uranium concentration between 50 and 500 μmol/L were used. After stirring the suspensions for 1 h in an automatic shaker the solid and liquid phases were separated by the use of centrifuge at 6000 rpm for 30 min.
The amount of U (VI) adsorbed on the samples of pristine and modified clay minerals was calculated as a difference between the initial concentration and the equilibrium concentrations. The equilibrium amount of metal adsorption from aqueous solution was determined using the formula:
where q is the amount of metal ions sorbed at equilibrium expressed in μmol/g of sorbent; m is the adsorbent mass (g); Co and Ce are the initial and the equilibrium concentrations of metal ions respectively (μmol/L); V is the volume of solution (L).
q = (Co − Ce)V/m
The concentration of uranium was determined by Spectrophotometer UNICO 2100UVat 665 nm by using the U-arsenazo (III) complex.
A kinetic study was carried out to obtain value of necessary duration of batch experiments for sorption isotherm determination. Kinetic experiments were conducted on samples of pristine and modified in ethanol minerals by uranium sulfate solution with the same initial concentration of 100 μmol/L for different selected times ranging from 5 to 120 min.
3. Results
The successful modification of clay surface using APTES is confirmed by FTIR spectra of initial and silylated samples (Figure 1a). The spectra of montmorillonite modified by both the polar protic solvents (ethanol) and nonpolar solvents (toluene) as compared with the initial sample reveal the presence of weak bands at 2800–2900 cm–1 corresponding to the stretching vibration of the methylene groups of successfully grafted APTES and the bands at 1490–1450 cm–1 corresponding to –NH2 and –CH2-bending vibration of modifier molecules [32]. The palygorskite spectra (Figure 1b) also register the bands indicating the grafting of APTES molecules to the surface of mineral particles, namely: typical bands of amino groups at 1480–1450 cm–1 and bands at 2920 cm–1 corresponding to C–H stretching vibration of APTES [33].
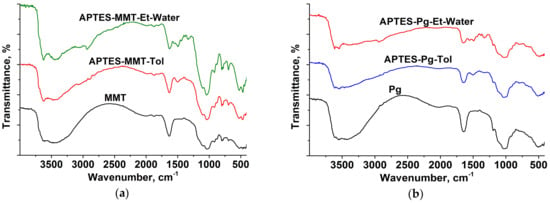
Figure 1.
FTIR spectra of pristine and APTES-modified clay minerals: (a) montmorillonite, (b) palygorskite.
XRD patterns of the initial and modified montmorillonite are presented in Figure 2a. The shift of basal reflection from 1.29 nm in nontreated samples to 1.6–1.7 nm in modified clays is the evidence of an increased distance between structural packets of mineral due to intercalation of APTES molecules into interlayer space. In the case of palygorskite, XRD patterns are identical for initial and treated samples, which is determined by the rigidity of this layer-ribbon silicate and the possible interaction of modifier molecules only with an external surface of mineral particles (Figure 2b).
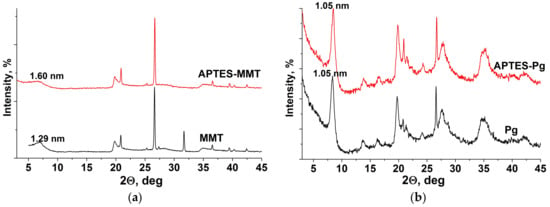
Figure 2.
XRD patterns of pristine and APTES-modified clay minerals: (a) montmorillonite, (b) palygorskite.
Findings of the thermal analysis of modified montmorillonite and palygorskite show that unlike nontreated clays, for which thermogravimetry (TG) curves at temperatures up to 800–900 °C reveal only mass loss associated with the removal of different forms of bound water [1], the treated APTES samples reveal mass loss in the temperature interval 300–500 °C that are determined by the thermal decomposition of grafted APTES (Figure 3). The mass loss in this temperature range corresponding to the removal of gaseous products of modifier decomposition amounts up to 10 % for the modified palygorskite and montmorillonite.
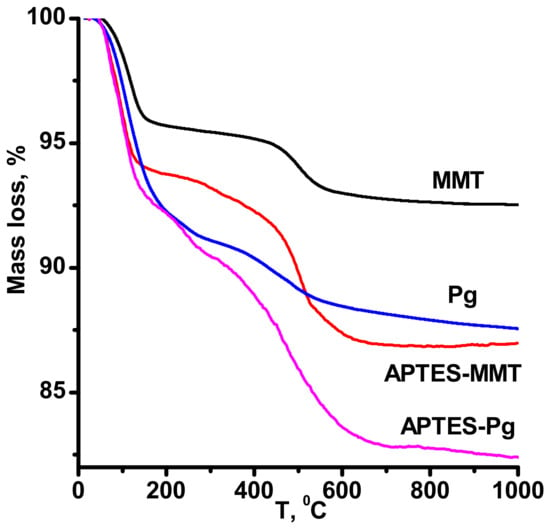
Figure 3.
Thermogravimetry (TG) of pristine and APTES-modified in toluene clay minerals.
Isotherms of nitrogen sorption-desorption (Figure 4) for all samples have the waveform intrinsic to layered and fibrillar silicates and belong to the II type isotherms in accordance with classification IUPAC [34]. In the range of values p/p0 > 0.35–0.4, the clearly pronounced hysteresis loops can be observed on isotherms indicating the presence of a well-developed structure of H3 type meso and macropores typical for such samples. Mesopore-size distributions were obtained from the low-temperature nitrogen adsorption data according currently used Barret-Joyner-Halenda (BJH) method [35]. As can be seen from the nitrogen isotherms based calculation of the characteristics of porous structure, the specific surface area of samples sharply decreases after the surface modification (Table 1). Such reduction is stipulated by practically complete filling of micropores with APTES molecules and the resultant blocking of the access of nitrogen molecules to these pores. The maximum in the pore-size distribution of the pristine montmorillonite is centered at 1.41 nm (Figure 5). This value slightly increases after functionalization to 2.13 nm. The pore size distribution for palygorskite is centered around two maxima: the slight maximum at 1.90 nm and large maximum at 6.26 nm. These values remain almost unchanged after functionalization.
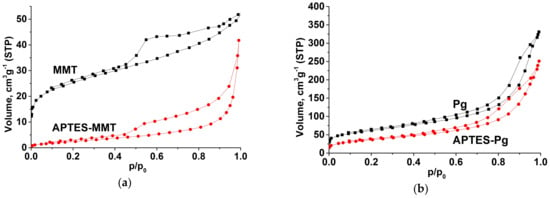
Figure 4.
Nitrogen adsorption-desorption isotherms of pristine and APTES-modified clay minerals: (a) montmorillonite, (b) palygorskite.

Table 1.
Surface characteristics of pristine and APTES-modified in toluene clay minerals.
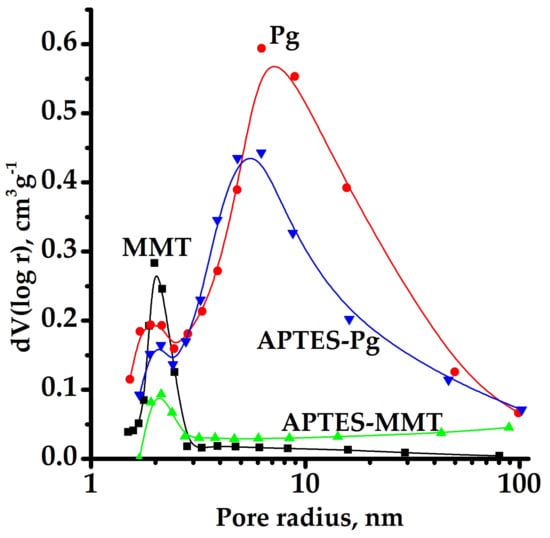
Figure 5.
Mesopore-size distributions for pristine and APTES-modified in toluene clay minerals.
Kinetic curves of sorption of uranium ions on all the samples indicate a sufficiently fast attainment of sorption equilibrium within 15–20 min (Figure 6). In all next sorption experiments, the duration was established as 1 h.
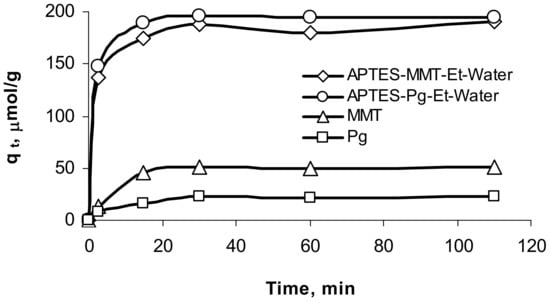
Figure 6.
Kinetic study of sorption of uranium ions on pristine and modified minerals.
Sorption isotherms of uranium ions show that the sorption characteristics of samples of montmorillonite and palygorskite (Figure 7) modified with APTES in water-alcohol medium and in toluene are much higher as compared to those for pristine minerals.
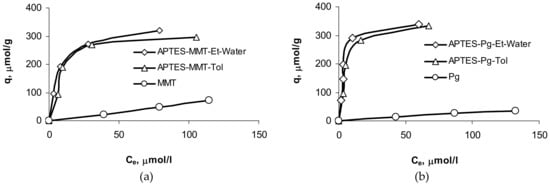
Figure 7.
Adsorption of U(VI) on pristine and APTES-modified clay minerals: (a) montmorillonite, (b) palygorskite.
To estimate the adsorption capacity of the modified clays isotherms of all samples were expressed in terms of Freundlich and Langmuir models. The obtained data are presented in Table 2.

Table 2.
Langmuir and Freundlich parameters for the adsorption of uranyl ions onto pristine and APTES-modified minerals.
The results of the R square indicate that uranium sorption data on pristine and amino-functionalized clays is better defined by the Langmuir equation with a regression coefficient more than 0.95 for all samples. Freundlich model gives a poor presentation for all isotherms (R2 < 0.92).
4. Discussion
The surface modification processes of clays are determined by structure and morphology of their particles. Montmorillonite particles (platelets) are relatively thin, usually containing 10÷30 stacked layers; however, the individual layer width is much smaller (e.g., 1 nm). The microstructure of montmorillonite gels and powders is mainly controlled by face-to-face interactions [35]. The sorption of metal ions on the surface of layer silicates takes place at active sites of the two main types: with participation of exchange cations localized on basal surfaces of particles and determined by the presence of nonstoichiometric isomorphous substitutions in tetrahedral and octahedral sheets of structural layers, and also on Si–OH and Al-OH groups that are located on side edges of particles at places of disrupted Si–O–Si and Al–O–Al structured sheets. Modification of layered silicates with formation of strong chemical bonds between atoms of the surface and silane substances takes place primarily at the side edges and structural defects on basal surfaces of particles. The fraction of sorption sites at side edges for clay minerals of the montmorillonite group amounts to 10–15% [1]. Therefore, the APTES molecules in the process of modification block slit-shaped micropores between flat structural layers of montmorillonite preventing the access of nitrogen molecules to these pores in experiments on determination of the porous structure characteristics of sorbents.
For the fibrous palygorskite silicate, the effect of modification on the value of specific surface area shows itself to a smaller degree as compared to that for montmorillonite. The molecules of modifier cover the external surface of palygorskite particles with partial blocking of zeolite channels in their structure. At the same time, micropores between rodlike particles due to their disordered arrangement in structured aggregates are not fully blocked by APTES molecules, and the access of nitrogen molecules to these pores is partially preserved. The estimation of the amount of bound silane based on data of thermal analysis (up to 0.5 mmol/g of montmorillonite and up to 0.8 mmol/g of palygorskite) is the evidence of sufficiently close-packed arrangement of APTES molecules on the surface of minerals.
One of the main factors controlling U adsorption on natural minerals is the chemical form of uranium in water. Uranium (VI) ions can form a series of complexes of different composition in aqueous solutions at different pH values. At close to neutral values of pH that correspond to natural water, the main form of U(VI) consists of uranyl ions UO22+ and its hydroxo complexes UO2OH+, (UO2)2(OH)22+, (UO2)3(OH)42+, (UO2)3(OH)5+, (UO2)4(OH)7+ and others. With the rise of pH values and concentrations of CO2 dissolved in water that corresponds to ground water, we can observe an enhanced content of neutral or even negatively charged forms of hydrolysis products UO2(OH)2, UO2CO3, (UO2)2CO3(OH)3- and others that are poorly sorbed on negatively charged particles of natural silicates (montmorillonite and palygorskite) [36].
The analysis of adsorption data with the use of Langmuir model show that the values of equilibrium adsorption constant KL, which is related to the affinity of binding sites are much more for modified samples than for pristine ones. The values of maximum amount of uranium per unit weight of adsorbent for complete monolayer coverage (qm) are also much more for modified clays.
The sketch in Scheme 1 illustrates the possible structure of grafted APTES and proposed models of complexation of uranyl ions with attached ligand. An amino group in the structure of APTES is at the end of the molecule chain. Uranyl ions interact with nitrogen atoms of one or two APTES molecules, which are covalently bonded to clay mineral surfaces.
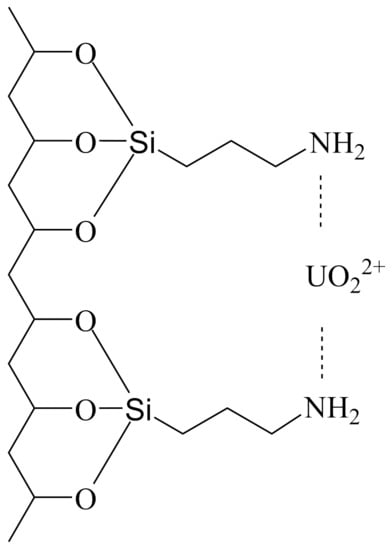
Scheme 1.
Proposed modes of complexation of uranyl ions on functionalized montmorillonite and palygorskite.
First of all, the great effect of amino-functionalized siloxane (APTES) on sorption of uranyl ions is due to strong affinity of these ligands for UO22+ ions [36] and to the high content of amino groups on the surface of modified clay particles. Hence, the several-fold rise of sorption of uranium ions on modified clays can be stipulated not only by the donor interaction of nitrogen atom of one amino group with an atom of sorbed metal, but also by the participation in formation of surface complex of the second amino group from close-spaced another molecule of APTES.
Amino-functionalized montmorillonite and palygorskite provides a promising environmental remediation material employed to treat groundwater and wastewater containing U(VI). Comparison of sorption capacity of obtained sorbents and other low-cost adsorbents clearly proves the effectiveness of the applied approach. The maximum sorption capacity of modified montmorillonite and palygorskite were 83.3 and 90.2 mg/g, which correspond 350 and 379 μmol/g of sorbed 238U respectively, and were higher than the reported low-cost uranium adsorbent based on activated carbon from sawdust and its amine form: 57.3 and 76.7 mg/g, respectively [37], and nanoscale zerovalent iron supported on Na-bentonite [38].
5. Conclusions
Modification of clay samples using APTES has been successfully carried out in polar-protic (water-alcohol) and nonpolar solvents (toluene) making it possible to attain a reasonably high degree of surface filling.
The study of sorption processes of uranium ions from aqueous solutions reveals that the sorption equilibrium is reached on modified samples within 15–20 min. The sorption values for such samples are several times as high as the values for initial clays that is determined by formation of sufficiently strong complexes of uranium ions with amino groups of APTES molecules grafted on the surface. The obtained sorbents can be effectively used for the removal of trace amounts of uranium compounds from polluted surface and ground waters.
Author Contributions
Conceptualization, B.K.; Methodology, V.T. and I.K.; Software, L.S.; Validation, B.K. and I.K.; Formal Analysis I.K. and V.T.; Investigation, I.K., V.T., and Y.K.; Writing-Original Draft Preparation, B.K., L.S.; Writing-Review & Editing, L.S.
Funding
This research received no external funding.
Acknowledgments
Author is grateful to the staff of Institute for Sorption and Problems of Endoecology and Igor Sikorsky Kyiv Polytechnic Institute for the help and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bergaya, F.; Theng, B.K.; Lagaly, G. (Eds.) Handbook of Clay Science; Developments in Clay Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; Volume 1. [Google Scholar]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review. J. Environ. Manag. 2017, 191, 35–57. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Vilarrasa-García, E.; Jiménez-Jiménez, J.; Rodriguez-Castellón, E. Synthesis, Characterization, Uses and Applications of Porous Clays Heterostructures: A Review. Chem. Rec. 2018, 18, 1–21. [Google Scholar] [CrossRef]
- Kami´nska, G. Removal of Organic Micropollutants by Grainy Bentonite-Activated Carbon Adsorbent in a Fixed Bed Column. Water 2018, 10, 1791. [Google Scholar] [CrossRef]
- Bogolepov, A.A.; Pshinko, G.N.; Kornilovich, B.Yu. The impact of complexing agents on the processes of sorption treatment of waters containing uranium. J. Water Chem. Technol. 2007, 29, 9–14. [Google Scholar] [CrossRef]
- Najafi, M.; Yousefi, Y.; Rafati, A.A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012, 85, 193–205. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tomita, M.; Kato, K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J. Asian Ceram. Soc. 2015, 3, 70–76. [Google Scholar] [CrossRef]
- Dána, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Repo, E. Synthesis of mesoporous and microporous amine and non-amine functionalized silica gels for the application of rare earth elements (REE) recovery from the waste water-understanding the role of pH, temperature, calcination and mechanism in Light REE and Heavy REE separation. Chem. Eng. J. 2017, 322, 56–65. [Google Scholar] [CrossRef]
- Diagboya, P.N.E.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, P.; He, H.-P.; Qin, Z. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite. Appl. Clay Sci. 2012, 62–63, 8–14. [Google Scholar] [CrossRef]
- Fatimah, I. Preparation, characterization and physicochemical study of 3-amino propyl trimethoxy silane-modified kaolinite for Pb(II) adsorption. J. King Saud Univ. 2017, 30, 250–257. [Google Scholar] [CrossRef]
- Balomenou, G.; Stathi, P.; Enotiadis, A.; Gournis, D.; Deligiannakis, Y. Physicochemical study of amino-functionalized organosilicon cubes intercalated in montmorillonite clay: H-binding and metal uptake. J. Colloid Interface Sci. 2008, 325, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Losey, B.; Mohseni, R.; Zlotnikov, E.; Vasiliev, A. Adsorption of heavy metal ions on mesoporous silica-modified montmorillonite containing a grafted chelate ligand. Appl. Clay Sci. 2012, 59–60, 115–120. [Google Scholar] [CrossRef]
- Su, L.; Tao, Q.; He, H.; Zhu, J.; Yuan, P.; Zhu, R. Silylation of montmorillonite surfaces: Dependence on solvent nature. J. Colloid Interface Sci. 2013, 391, 16–20. [Google Scholar] [CrossRef]
- Fang, L.; Wang, L.; Zhou, T.; Liu, L.; Li, M. Preparation and characterization of Fe,Co,Si-pillared montmorillonites with aminosilanes as silicon pillars precursor. Appl. Clay Sci. 2017, 141, 88–94. [Google Scholar] [CrossRef]
- Xue, A.; Zhou, Sh.; Zhao, Yi.; Lu, X. Adsorption of reactive dyes from aqueous solution by silylated palygorskite. Appl. Clay Sci. 2010, 48, 638–640. [Google Scholar] [CrossRef]
- Guerra, D.L.; Silva, E.M.; Airoldi, C. Application of modified attapulgites as adsorbents for uranyl uptake from aqueous solution—Thermodynamic approach. Process Saf. Environ. Prot. 2010, 88, 53–61. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Y.; Tan, X.; Wang, L.; Sun, Y.; Lin, D.; Sun, Y.; Qin, X.; Wang, Q. Heavy metal adsorbents mercapto and amino functionalized palygorskite: Preparation and characterization. Colloids Surf. A 2013, 426, 98–105. [Google Scholar] [CrossRef]
- Borrego, T.; Andrade, M.; Pinto, M.L.; Silva, A.R.; Carvalho, A.P.; Rocha, J.; Freire, C.; Pires, J. Physicochemical characterization of silylated functionalized materials. J. Colloid Interface Sci. 2010, 344, 603–610. [Google Scholar] [CrossRef]
- Sousa, C.; Pereira, C.; Rodriguez-Borges, J.E.; Freire, C. L-Serine functionalized clays: Preparation and characterization. Polyhedronics 2015, 102, 121–129. [Google Scholar] [CrossRef]
- He, H.; Tao, Q.; Zhu, J.; Yuan, P.; Shen, W.; Yang, S. Silylation of clay mineral surfaces. Appl. Clay Sci. 2013, 71, 15–20. [Google Scholar] [CrossRef]
- Silva, A.A.; Dahmouche, K.; Soares, B.G. Nanostructure and dynamic mechanical properties of silane-functionalized montmorillonite/epoxy nanocomposites. Appl. Clay Sci. 2011, 54, 151–158. [Google Scholar] [CrossRef]
- Asgari, M.; Abouelmagd, A.; Sundararaj, U. Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl. Clay Sci. 2017, 146, 439–448. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Cheng, H.; Zhang, Yu.; Li, X.; Frost, R.L. Intercalation of γ-aminopropyl triethoxysilane (APTES) into kaolinite interlayer with methanol-grafted kaolinite as intermediate. Appl. Clay Sci. 2015, 114, 484–490. [Google Scholar] [CrossRef]
- Murray, H.H. Applied Clay Mineralogy; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444517012. [Google Scholar]
- Galan, E.; Singer, A. (Eds.) Developments in Palygorskite-Sepiolite Research; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780444536075. [Google Scholar]
- Nguyen-Trung, C.; Begun, G.M.; Palmer, D.A. Aqueous uranium complexes. 2. Raman spectroscopic study of the complex formation of the dioxouranium (VI) ion with a variety of inorganic and organic ligands. Inorg. Chem. 1992, 31, 5280–5287. [Google Scholar] [CrossRef]
- Kornilovych, B.; Wireman, M.; Ubaldini, S.; Guglietta, D.; Koshik, Yu.; Caruso, B.; Kovalchuk, I. Uranium Removal from Groundwater by Permeable Reactive Barrier with Zero-Valent Iron and Organic Carbon Mixtures: Laboratory and Field Studies. Metals 2018, 8, 408. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1997; ISBN 978-0023674129. [Google Scholar]
- Tarasevich, Y.I. Poverhnostnyie yavleniya na dispesnyih materialah [The Superficial Phenomena on Disperse Materials]; Naukova dumka: Kyiv, Ukraine, 2011; p. 390. ISBN 978-966-00-1137-3. [Google Scholar]
- Zhou, L.; Chen, H.; Jiang, X.; Lu, F.; Zhou, Y.; Yin, W.; Ji, X. Modification of montmorillonite surfaces using a novel class of cationic gemini surfactants. J. Colloid Interface Sci. 2009, 332, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.A.; Ciuffi, K.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Clay Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. Res. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780080970356. [Google Scholar]
- Kornilovich, B.Y.; Pshinko, G.N.; Bogolepov, A.A. Effects of EDTA and NTA on sorption of U(VI) on the clay fraction of soil. Radiochemistry 2006, 48, 584–588. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Mohammaden, T.F.; El-Aassy, I.K.; Gad, H.M.H.; Hassan, A.M.; Mahmoud, M.A. Decontamination of Uranium-Polluted Groundwater by Chemically-Enhanced, Sawdust-Activated Carbon. Colloids Interfaces 2017, 1, 2. [Google Scholar] [CrossRef]
- Sheng, G.; Shao, X.; Li, Y.; Li, J.; Dong, H.; Cheng, W.; Gao, X.; Huang, Y. Enhanced Removal of Uranium(VI) by Nanoscale Zerovalent Iron Supported on Na-Bentonite and an Investigation of Mechanism. J. Phys. Chem. A 2014, 118, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).