Abstract
In this work, adsorption of rhodamine B (RB) and acid yellow 17 (AY17) was investigated on ordered mesoporous carbon material obtained by soft-templating method with hydrochloric acid (ST-A). For comparison, the adsorption process on commercial activated carbon CWZ-22 was also carried out. The sorbents were characterized by nitrogen adsorption/desorption isotherms and scanning electron microscopy. Langmuir and Freundlich adsorption isotherm models were applied to simulate the equilibrium data of RB and AY17. Adsorption isotherm data could be better described by the Langmuir model than the Freundlich model. The adsorption kinetics of RB and AY17 on studied carbons could be well depicted by using pseudo-second-order kinetic modeling. The adsorption capacity increased with temperature increase in the range of 298–315 K. In the whole diffusion process, the intraparticle diffusion was involved, but not the whole rate-controlling step. The calculated thermodynamic parameters, including Gibbs free energy (∆G), enthalpy (∆H), and entropy (ΔS) suggested that adsorption processes of RB and AY17 on ST-A and CWZ-22 were endothermic and spontaneous.
1. Introduction
Water is needed for life. Different pollutants (including dyes) from a constantly evolving industry get into water [1]. In the textile and dye industry, synthetic dyes are very often used and, unfortunately, have a harmful effect on human and animal health [2]. These colorful compounds affect the change in the color of water and, thus, have a detrimental effect on fauna and flora [3]. Many of them, even at low concentrations, have toxic and carcinogenic effects and can affect water organisms and the digestive system of humans [4]. They are extremely resistant to the effects of temperature, light, and other factors, such as chemicals, and are therefore extremely difficult to remove [5]. Many techniques [6,7,8,9] used in wastewater treatment are known, but adsorption, due to its many advantages, is most commonly used. The adsorption process is primarily simple and has many economic advantages, but it is worth considering that it catches toxic substances at very low concentrations [1,10].
Rhodamine B and acid yellow 17 are widely used in food, paper and textile dyes industry, among others. Due to the carcinogenic nature, the use of RB was prohibited. However, this has changed and although RB carries a risk to human and animal health, it is being used again, especially in the textile industry. AY17 is also used for the production of personal care, laundry, and cleaning agents [11,12].
Nowadays, the key is to exploit selective and efficient adsorbents that will adsorb dyes from the aquatic environment in the shortest possible time and with the highest efficiency [3,13]. In recent years, many studies have been carried out on the adsorption of dyes on various carbon adsorbents [14,15,16,17,18,19,20,21,22,23,24,25,26], on waste materials (vegetable waste, fruit waste, agricultural and industry waste, natural inorganic materials, and bioadsorbents) [27,28,29,30,31,32], and on synthetic carbon materials such as carbon nanotubes [33,34], ordered mesoporous carbons (OMCs) [35,36,37,38,39,40,41,42], and others.
OMCs, due to their unique properties, are gaining more and more interest in adsorption processes. They are characterized by high specific surface area, large pore volume, and good mechanical stability [3].
In this work, ordered mesoporous carbon material ST-A and commercial adsorbent (activated carbon CWZ-22) have been studied for the removal of RB and AY17 from aqueous solutions. The surface properties of applied adsorbents were characterized by the methods: nitrogen adsorption isotherms and scanning electron microscopy (SEM). The efficiency of the adsorption process was analyzed based on the effect of contact time, initial dye concentration, and temperature. Adsorption isotherm models (Langmuir and Freundlich), were used to analyze the experimental data. Three various kinetic models (pseudo-first-order, pseudo-second-order, and intraparticle diffusion model) for the adsorption of dyes are also presented. In addition, thermodynamic parameters were calculated to better explain the adsorption process.
2. Materials and Methods
2.1. Materials
RB (C28H31ClN2O3) with molar weight 479.01 g·mol−1 and AY17 (C16H10Cl2N4Na2O7S2) with molar weight 551.29 g·mol−1 (Sigma-Aldrich, Darmstadt, Germany) were used as the adsorbates. The maximum wavelength of the dyes are 553 and 402 nm, respectively. The structural formulas of dyes are presented in Figure 1a,b.
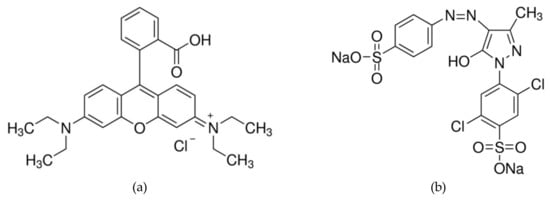
Figure 1.
Structural formulas of rhodamine B (RB) (a) and acid yellow 17 (AY17) (b).
Mesoporous carbon, obtained by the soft-templating method with hydrochloric acid, was prepared using a slightly modified synthesis procedure described in the work of Choma et al. [43], and used as the adsorbent. During the synthesis of the adsorbent, silver nanoparticles were not added and the Pluronic F127 triblock copolymer was used instead Lutrol F127. The remaining stages of the synthesis were analogous to [43]. For comparison, the adsorption process on commercial activated carbon CWZ-22 from GRYFSKAND (Hajnowka, Poland) was also carried out.
2.2. Characterization of Adsorbents
Low-temperature nitrogen adsorption/desorption isotherms were measured at −196 °C on an ASAP 2020 volumetric analyzer (Micromeritics Inc., Norcross, GA, USA) in the Structural Research Laboratory of Jan Kochanowski University in Kielce. Before the measurements, all samples were degassed at 200 °C for at least 2 h. Using experimental low-temperature nitrogen adsorption isotherms for studied adsorbents, standard parameters of the porous structure were determined [44,45,46,47,48,49,50]. Brunauer–Emmett–Teller (BET) method was involved for investigated carbon materials specific surface area determination. SBET was calculated in the range of relative pressure from 0.05 to 0.2, considering the surface occupied by a single molecule of nitrogen in an adsorptive monolayer (cross-sectional area equal 0.162 nm2) [44]. The sum of micropores volume (Vmi) and mesopores (Vme), being the total pore volume (Vt), was determined from one point of nitrogen adsorption isotherm, corresponding to the relative pressure p/p0 equal to 0.99 [45]. The pore size distribution functions (PSDs) were calculated from the adsorption branch of isotherms using the Kruk-Jaroniec-Sayari (KJS) method [49] based on the Barrett–Joyner–Halenda (BJH) calculation procedure for cylindrical pores [50].
SEM images were taken using a scanning electron microscope manufactured by Zeiss (Germany) mod. Ultra Plus, EDS Bruker Quantax 400. It is a high-resolution low energy microscope with field emission Schottky cathode. The applied voltage of measurements was 2 kV.
The functional acidic and basic groups on the studied carbon materials were determined using Boehm’s titration method [51,52]. According to Boehm’s method, sodium bicarbonate can neutralize carboxyl groups; sodium carbonate can neutralize carboxyl and lactone groups; sodium hydroxide can neutralize carboxyl, lactone, and phenol groups; sodium ethoxide can neutralize carboxyl, lactone, phenolic, and carbonyl groups; and hydrochloric acid can neutralize total basic groups. The obtained carbon materials (0.2 g) were dispersed in the following solutions: sodium bicarbonate, sodium carbonate, sodium hydroxide, sodium ethoxide, and hydrochloric acid. Then, each solution with carbon material was shaken for 48 h at room temperature. The sample was then filtered, and 10 mL of filtrate were titrated with 0.1 mol dm−1 HCl to determine acidic groups and 0.05 mol dm−1 NaOH to determine total basic groups.
2.3. Adsorption Experiments
For adsorption investigations, ST-A (with grain size from 0.2 to 0.8 mm) and commercial activated carbon were applied. Before experiments, all carbon materials were dried in the laboratory dryer at temperature 373 K to constant masses. The parameters presented in Table 1 were applied for adsorption isotherms and adsorption kinetic experiments. Concentrations of RB and AY17 were determined in solution at a maximum adsorption wavelength of 553 and 402 nm respectively, before and after adsorption, using the spectrophotometric method by spectrophotometer SP-830 Plus from Metertech. Adsorption processes were performed in a 100 mL Erlenmeyer’s flask. To each flask, 0.1 g of studied carbon and 50 cm3 of RB or AY17 with defined concentration were added. Next, the flasks were placed in the incubator for specified time (Table 1). The adsorption capacities of studied carbon for RB and AY17 were calculated applying Equation (1):
where qe is the RB or AY17 adsorption capacity (mg g−1); C0 is the concentration of RB or AY17 in solution before adsorption (mg dm−3); Ce is the concentration of RB or AY17 in solution after adsorption (mg dm−3); V is the volume of the dye solution used for adsorption process (dm3); and m is the carbon mass (g).

Table 1.
The adsorption isotherms and kinetics—conditions and runs.
3. Results and Discussion
3.1. Characterization of Adsorbents
Nitrogen adsorption isotherms are shown in Figure 2a. According to IUPAC classification of adsorption isotherms [53], the experimental isotherm for studied ST-A material is type IV, which is characteristic for mesoporous solids. The H1 hysteresis loops confirm the presence of accessible mesopores. The isotherm for CWZ-22 carbon is type I. A type I isotherm indicates high adsorption in the range of low relative pressures, i.e., refers to adsorbents with highly developed microporosity (porosity, which forms pores with linear dimensions less than 2 nm). In the area of medium and high relative pressures, the isotherm for CWZ-22 carbon has a course almost parallel to the abscissae axis, which indicates that mesoporosity (pores with dimensions of 2 to 50 nm) is poorly developed [54]. The type H4 hysteresis loop for CWZ-22 carbon is associated with narrow slit pores.
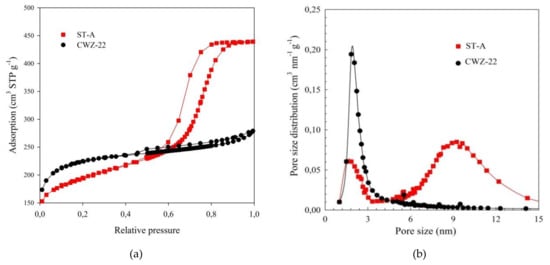
Figure 2.
Nitrogen adsorption isotherms (a) and pore size distribution functions (PSDs) (b) for studied carbon materials.
Pore size distribution functions (PSDs) for the adsorbents studied are shown in Figure 2b. The PSD curve for the carbon ST-A exhibit two maxima. The first, occurring for pore sizes 1.83 nm, corresponds to the width of micropores, whereas the second, for pore sizes 9.24 nm, corresponds to the width of mesopores. The PSD curve for the carbon CWZ-22 exhibit one maximum for pore sizes 1.82nm, which corresponds to the width of micropores. The values wmi and wme are presented in Table 2.

Table 2.
Structural parameters of the studied carbon materials.
Structural parameters determined from adsorption isotherms are shown in Table 2. The ST-A adsorbent is characterized by a slightly lower specific surface area (SBET) in comparison to CWZ-22 (670 and 775 m2/g, respectively). However, the total pore volume (Vt) for carbon ST-A is much larger: 0.68 cm3/g in comparison to carbon CWZ-22: 0.43 cm3/g. Micropore volume (Vmi) changes in the range from 0.17 (ST-A) to 0.23 cm3/g (CWZ-22). The synthesized carbon ST-A has a much larger volume of mesopores. The mesoporosity expressed as a percentage (Table 2) confirms that studied carbon ST-A is, in fact, mesoporous, and commercial active carbon CWZ-22 is microporous.
The SEM images confirm the properties of the studied carbons (Figure 3). The ST-A carbon presented interesting mesoporous structure with visible canals of mesopores (Figure 3a). Commercial carbon CWZ-22 is characterized by a much less developed porosity in comparison with the obtained carbon ST-A, which is observed in the image (Figure 3b).
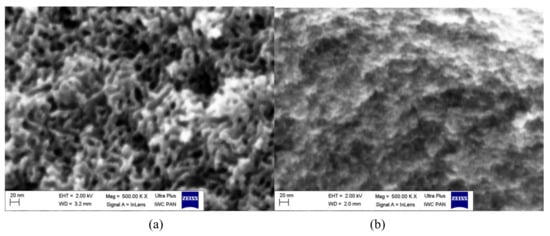
Figure 3.
SEM images of carbons: ST-A (a), CWZ-22 (b).
Table 3 lists the different functional groups available on studied adsorbents. The obtained carbon (ST-A) has more acidic properties and the commercial carbon (CWZ-22), on the contrary, has more basic properties.

Table 3.
Functional groups available on studied carbons.
The ST-A carbon, due to the fact that it has acidic groups on its surface (phenolic and carbonyl), adsorbs the basic dye RB. It is the opposite of CWZ-22 because, due to the presence of more basic groups on its surface, it better adsorbs AY 17, which is an acidic dye. This is confirmed by the data in Table 4. Electrostatic interactions of the dye–adsorbent play an important role during the processes.

Table 4.
Langmuir and Freundlich isotherm parameters for the RB and AY17 adsorption on studied carbon.
3.2. Adsorption Experiments
3.2.1. Adsorption Isotherms
The adsorption isotherm is used to describe the distribution of the adsorbate between the aqueous and solid phase in point of adsorption process equilibrium. On the basis of the shape of the isotherm, the nature of the studied process can be determined [55].
The experimental isotherms were compared to the Langmuir and Freundlich isotherm model [56,57].
The Langmuir isotherm can be presented in the form:
Equation (2) in linear form:
where Ce is the equilibrium concentration of dye aqueous solution (mg dm−3); qe is the amount of dye adsorbed by elementary mass of adsorbent (mg g−1); qm is the maximum adsorption capacity (mg g−1); and KL is the adsorption equilibrium constant (dm3 g−1).
In order to check the validity of the model fit to the Langmuir adsorption isotherm, the obtained graph was plotted Ce/qe vs. Ce (Figure 4a–d). The calculated isotherm parameters are shown in Table 4.
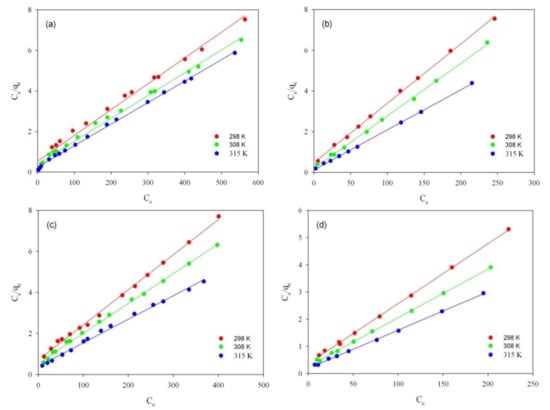
Figure 4.
Langmuir isotherm plots for adsorption of RB on ST-A (a) and CWZ-22 (c), and adsorption of AY17 on ST-A (b) and CWZ-22 (d).
The Freundlich isotherm can be presented in the form:
Equation (4) in linear form:
where KF is the Freundlich isotherm constant (mg1−1/n (dm3)1/n g−1), describing relative intensity; and 1/n is adsorption ability.
In order to check the validity of the model fit to the Freundlich adsorption isotherm obtained, a graph of logqe vs. logCe was plotted (data not shown). The correlation coefficients and isotherm constants were calculated and presented in Table 4.
The experimental adsorption isotherms of investigated dyes on ordered mesoporous carbon and commercial carbon CWZ-22, at temperature 298, 308, and 315 K are presented in Figure 5.
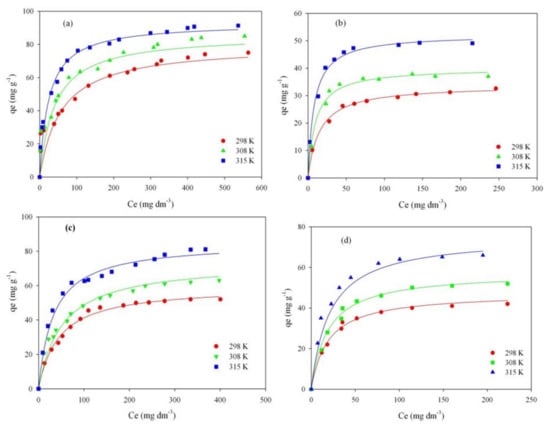
Figure 5.
Adsorption isotherms of RB on ST-A (a) and CWZ-22 (c), and AY17 on ST-A (b) and CWZ-22 (d).
Analyzing data presented in Table 4, it can be observed that adsorption of dyes (RB and AY17) on studied carbons (ST-A and CWZ-22) proceeded in compliance with Langmuir isotherm model (R2 ≥ 0.988). The adsorption capacity (qm) of the AY17 on carbon ST-A at 298, 308, and 315 K is 35, 40, and 53 mg/g, respectively. On the other hand, the adsorption values of AY17 on the CWZ-22 commercial carbon compared are, successively, 46, 56, and 71 mg/g. When comparing the obtained results, it can be concluded that the adsorption capacity of commercial carbon is higher than the synthesized carbon ST-A at all temperatures. In the case of RB, the situation is the opposite. Higher adsorption values were obtained for this dye on synthesized carbon ST-A. The values from the lowest to the highest temperature were, respectively, 83, 91, and 100 mg/g. Adsorption of RB on commercial carbon reached significantly lower values of 58, 69, and 87 mg/g.
Table 5 compares the maximum adsorption capacities of the studied carbons with different carbon adsorbents reported [1,2,3,4,5,11,58,59,60,61,62,63,64,65] for the removal of RB and AY17. The maximum adsorption capacity (qm) of RB and AY on the analyzed materials was moderate compared to other described adsorbents. The OMC materials obtained in works [3,65] have much better adsorption properties relative to RB than the adsorbent ST-A obtained in this work. This may be the result of a different synthesis method caused by the introduction of tetraethyl orthosilicate (TEOS) into the reaction system at the synthesis stage. The removal of silica with HF or NaOH results in materials with significantly higher specific surface area, more developed pore volume and, thus, better adsorption properties obtained in case of RB.

Table 5.
Comparison of the qm of RB and AY17 on different absorbents.
3.2.2. Adsorption Kinetics
The solute uptake rate can be described by adsorption kinetics, which are very important for designing suitable technologies of adsorption [66].
Pseudo-first-order kinetic model [67], pseudo-second-order kinetic model [68], and intraparticle diffusion model [69] were investigated for the adsorption of RB and AY17 on studied carbon materials (ST-A and CWZ-22).
The pseudo-first-order equation is expressed as:
where k1 is the pseudo-first-order rate constants (min−1); t is time of contact between the adsorbent and adsorbate (min); qe is the adsorption value after the equilibrium stabilization (mg g−1); and qt is the adsorption value in given time t (mg g−1).
Equation (6) in linear form:
The rate constants, k1, were calculated from the linear plots of ln(qe − qt) versus t (data not shown), data are presented in Table 5.
The pseudo-second-order equation is given as follows:
Equation (8) can be transformed into linear form:
where k2 is the pseudo-second-order rate constants (g mg−1·min−1).
The rate constants, k2, were calculated from the linear plots of t/qt versus t (Figure 6a–d), and the data are shown in Table 6.
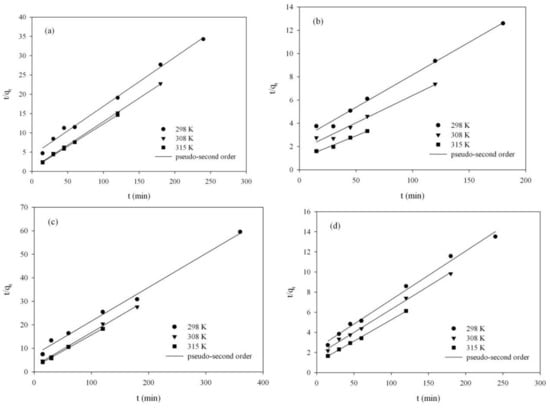
Figure 6.
Pseudo-second-order kinetic model for RB adsorption onto ST-A (a) and CWZ-22 (c), and for AY17 adsorption onto ST-A (b) and CWZ-22 (d).

Table 6.
Kinetic parameters of RB and AY17 adsorption on the carbon studied.
The adsorption results (Table 6) confirmed that the adsorption kinetics for RB and AY17 on studied carbon materials can be described by pseudo-second-order kinetic equation.
To investigate the adsorption mechanism of studied compounds on used adsorbents, the Weber–Morris diffusion model was applied. The diffusion model is expressed by the following form:
where kid is the intraparticle diffusion rate constant (mg g−1 min−1/2) and c is the intercept which represents the thickness of the boundary layer (mg g−1).
The relationship shown in Figure 7a–d allows us to identify the adsorption mechanism. The multilinear plot (solid line on the plot) indicates that in the adsorption process, several steps take part, not just intraparticle diffusion. First, the steep section corresponds to adsorption on the external surface of the adsorbent grain or an immediate adsorption. The second linear part of plot corresponds to a gradual phase, mild adsorption, where intraparticle diffusion is a controlling step of the whole adsorption. The third step corresponds to the stabilization of the adsorption process. If adsorption would only occur due to intraparticle diffusion, then the dependency qt vs. t1/2 would be rectilinear in the whole range; in addition, the curve would pass through the origin of the graph [3,13,62]. The data (Figure 7a–d) indicate that intraparticle diffusion is involved in the adsorption process, but it is not a stage controlling the speed of the entire process.
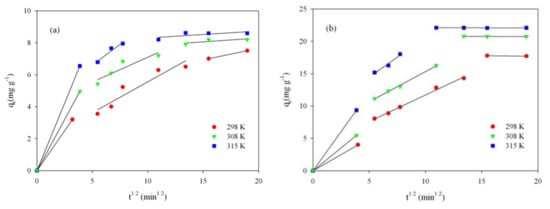
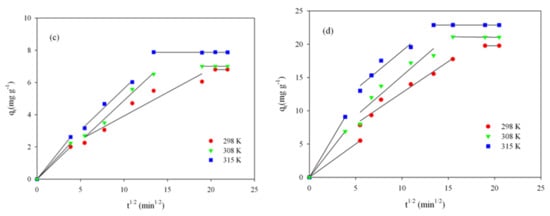
Figure 7.
Intraparticle diffusion model for RB adsorption onto ST-A (a) and CWZ-22 (c), and for AY17 adsorption onto ST-A (b) and CWZ-22 (d).
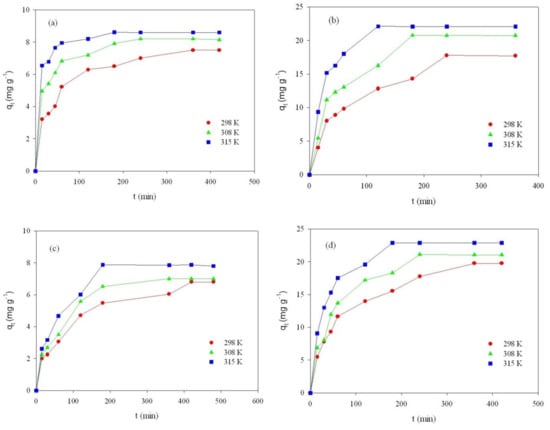
Figure 8.
Effect of contact time on adsorption of RB on ST-A (a) and CWZ-22 (c), and adsorption of AY17 on ST-A (b) and CWZ-22 (d) at 298, 308, and 315 K.
Analyzing the results, it was observed that the adsorption rate increases with increasing temperature for all studied cases (Figure 8a–d).
3.2.3. Thermodynamic Parameters
The standard free energy change (∆G), enthalpy change (∆H), and entropy change (∆S) were calculated applying the following Equations (11)–(13), to investigate the dye adsorptions (RB and AY17) on ST-A and CWZ-22, thermodynamically [2,3].
where R is the universal gas constant (8.314 J/mol K), T is the absolute temperature in Kelvin, and KL is the equilibrium constant obtained from Langmuir equation.
The ∆H and ∆S values were found from the slope and intercept of ln KL versus 1/T plots (Figure 9). Table 7 lists the thermodynamic parameters values obtained at different temperatures for RB and AY17 adsorption on ST-A and CWZ 22.
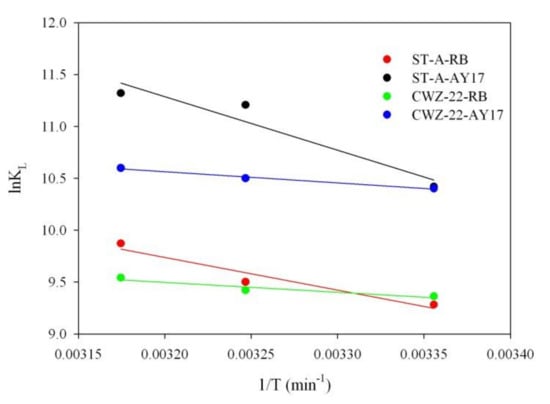
Figure 9.
The van’t Hoff plots of lnKL vs. 1/T for ST-A (RB, AY17) and CWZ-22 (RB, AY17).

Table 7.
Thermodynamic parameters for RB and AY17 adsorption on ST-A and CWZ-22.
Analyzing the determined thermodynamic parameters (Table 7) and negative ∆G values, it can be stated that the adsorption process of studied dyes is spontaneous in nature. The ∆H values (less than 40 kJ/mol) are positive, indicating that the adsorption process is endothermic. The positive values of ∆S show that an increase occurs in the randomness in the system at the solid/solution interface during the adsorption process.
4. Conclusions
Ordered mesoporous carbon and commercial activated carbon were applied in adsorption studies of rhodamine B and acid yellow 17. The adsorption results confirmed that the adsorption kinetics for RB and AY17 on studied carbon materials can be described by pseudo-second-order kinetic equation. The adsorption process of RB and AY17 on investigated carbon adsorbents proceeds in compliance with the Langmuir adsorption model. The commercial carbon had a higher adsorption capacity of AY17 than the synthesized carbon ST-A at all temperatures. In the case of RB, the situation is the opposite. Higher adsorption values were obtained for this dye on ST-A synthesized carbon. The negative value of ∆G and the positive value of ∆H indicate the spontaneous and endothermic nature of adsorption, respectively. The positive value of ∆S demonstrates that the randomness increased during the adsorption process. These results exhibited that ST-A and CWZ-22 carbon materials were effective adsorbents for dye removal from aqueous solutions.
Author Contributions
K.J. designed the conception, performed the experiments, made all calculations and plots, and wrote the manuscript; N.R. helped during the experiment; D.W. reviewed the results and edited the manuscript before the submission.
Funding
There were no sources of financing.
Acknowledgments
This work was supported by Ministry of Science and Higher Education, Poland (research project BS 612 490).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated ricehusk-based activated carbon. Colloids and Surfaces A: Physicochem. Eng. Aspects 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Geçgel, Ü.; Üner, O.; Gökara, G.; Bayrak, Y. Adsorption of cationic dyes on activated carbon obtained from waste Elaeagnus stone. Adsorpt. Sci. Technol 2016, 34, 512–525. [Google Scholar]
- Tang, L.; Cai, Y.; Yang, G.; Liu, Y.; Zeng, G.; Zhou, Y.; Li, S.; Wang, J.; Zhang, S.; Fang, Y.; et al. Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl. Surf. Sci. 2014, 314, 746–753. [Google Scholar] [CrossRef]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of Acid Yellow 36. Dyes and Pigments 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Lacerda, V.S.; López-Sotelo, B.; Correa-Guimarȃes, A.; Hernández-Navarro, S.; Sánchez-Báscones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Martín-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manage. 2015, 155, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Suhas, Application of low-cost adsorbents for dye removal—A review. J. Environ. Manage. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review. J. Hazard. Mater. 2009, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inyinbor, A.A.; Adekol, F.A.; Olatunji, G.A. Adsorption of Rhodamine B Dye from Aqueous Solution on Irvingia gabonensis Biomass: Kinetics and Thermodynamics Studies. S. Afr. J. Chem. 2015, 68, 115–125. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Wang, Y.; Gou, X.; Duan, Y. Adsorption and removal of rhodamine B from aqueous solution by tannic acid functionalized graphene. Colloids Surf. A: Physicochem. Eng. Aspects 2015, 477, 35–41. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, K.S.R.; Chen, R.; Peng, Y. Biosorption of acid yellow 17 from agueous solution by non-living aerobic qranular slvolge. Hazard J. Matter. 2010, 174, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Luo, Y. The adsorption mechanism of anionic and cationic dyes by Jerusalem artichoke stalk-based mesoporous activated carbon. J. Environ. Chem. Eng. 2014, 2, 220–229. [Google Scholar] [CrossRef]
- Hadi, M.; Samarghandi, M.R.; McKay, G. Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: Study of residual errors. Chem. Eng. J. 2010, 160, 408–416. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, Ch.; Yue, Q.; Li, Y.; Li, Ch. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Mayohegzi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manage. 2012, 102, 148–164. [Google Scholar]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics and thermodynamic studies. J. Anal. Appl. Pyrol. 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Kaouah, F.; Boumaza, S.; Berrama, T.; Trari, M.; Bendjama, Z. Preparation and characterization of activated carbon from wild olive cores (oleaster) by H3PO4 for the removal of Basic Red 46. J. Clean. Prod. 2013, 54, 296–306. [Google Scholar] [CrossRef]
- Fernandeza, M.E.; Nunella, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crops Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Li, W.; Wang, P.; Su, Ch. Adsorption of acid and basic dyes by sludge-based activated carbon: Isotherm and kinetic studies. J. Cent. South Univ. 2015, 22, 103–113. [Google Scholar] [CrossRef]
- Ghasemian, E.; Palizban, Z. Comparisons of azo dye adsorption onto activated carbon and silicon carbide nanoparticles loaded on activated carbon. Int. J. Environ. Sci. Technol. 2016, 13, 501–512. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; Lazaridis, N.K. Multi-parametric adsorption effects of the reactive dye removal with commercial activated carbons. J. Mol. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Ahmed, M.J. Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: Review. J. Environ. Chem. Eng. 2016, 4, 89–99. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Bazarafshan, A.A. Sonochemical assisted hydrothermal synthesis of ZnO: Cr nanoparticles loaded activated carbon for simultaneous ultrasound-assisted adsorption of ternary toxic organic dye: Derivative spectrophotometric. optimization. kinetic and isotherm study. Ultraso. Sonochem. 2016, 32, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arabian J. Chem. 2017, 10, 1445–1455. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Selvaraju, N. Sequestration of dye from textile industry wastewater using agricultural waste products as adsorbents. J. Environ. Chem. Eng. 2013, 1, 629–641. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Chiu, W.-T.; Wang, C.-C. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Biores. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, H.; Sharma, M.; Sahore, V. A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ. Monit. Assessment 2011, 183, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ji, T.; Mu, L.; Shi, Y.; Wang, H.; Zhu, J. Pore size dependent molecular adsorption of cationic dye in biomass derived hierarchically porous carbon. J. Environ. Manage. 2017, 196, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Maingi, F.M.; Mbuvi, H.M.; Ng’ang’a, M.M.; Mwangi, H. Adsorption Kinetics and Isotherms of Methylene Blue by Geopolymers Derived from Common Clay and Rice Husk Ash. Phys. Chem. 2017, 7, 87–97. [Google Scholar]
- Ma, L.; Jiang, C.; Lin, Z.; Zou, Z. Microwave Hydrothermal Treated Grape Peel as an Efficient Biosorbent for Methylene Blue Removal. Int. J. Environ. Res. Public Health 2018, 15, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nano tubes. Inter. J. Water Res. Environ. Eng. 2010, 2, 016–028. [Google Scholar]
- Zhao, D.; Zhang, W.; Chen, C.; Wang, X. Adsorption of methyl orange dye onto multiwalled carbon nanotubes. Procedia Environ. Sci. 2013, 18, 890–895. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Batonneau-Gener, I.; Pouilloux, Y.; Hamad, H. Removal of methylene blue by mesoporous CMK-3: Kinetics, isotherms and thermodynamics. J. Mol. Liq. 2016, 223, 763–770. [Google Scholar] [CrossRef]
- Dang, P.T.; Nguyen, H.T.H.; Dao, C.D.; Le, G.H.; Nguyen, Q.K.; Nguyen, K.T.; Tran, H.T.K.; Nguyen, T.V.; Vu, T.A. Ordered Mesoporous Carbons as Novel and Efficient Adsorbent for Dye Remova from Aqueous Solution. Adv. Mater. Sci. Eng. 2016, 1–9. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Yao, J.; Zhang, L.; Liu, X. Adsorption of methylene blue on mesoporous carbons prepared using acid- and alkaline-treated zeolite X as the template. Colloids and Surfaces A: Physicochem. Eng. Aspects 2009, 333, 115–119. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Adsorption of crystal violet dye from aqueous solution using mesoporous materials synthesized at room temperature. Adsorption 2009, 15, 390–405. [Google Scholar] [CrossRef]
- Peng, X.; Huang, D.; Odoom-Wubah, T.; Fu, D.; Huang, J.; Qin, Q. Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: Equilibrium. thermodynamic and kinetics. J. Colloid Interf. Sci. 2014, 430, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Guo, Z.; Ling, H.; Huang, Z.; Tang, D. Effect of pore structure on the adsorption of aqueous dyes to ordered mesoporous carbons. Micro. Meso. Mater. 2016, 227, 104–111. [Google Scholar] [CrossRef]
- Liu, N.; Yin, L.; Zhang, L.; Wang, C.; Lun, N.; Qi, Y.; Wang, C. Ferromagnetic Ni decorated ordered mesoporous carbons as magnetically separable adsorbents for methyl orang. Mater. Chem. and Phys. 2011, 131, 52–59. [Google Scholar] [CrossRef]
- Gościańska, J.; Marciniak, M.; Pietrzak, R. Mesoporous carbons modified with lanthanum (III) chloride for methyl orange adsorption. Chem. Eng. J. 2014, 247, 258–264. [Google Scholar] [CrossRef]
- Choma, J.; Jedynak, K.; Górka, J.; Jaroniec, M. Morphology and Adsorption Properties of Mesoporous Carbons with Silver Nanoparticles. Ochr. Sr. 2011, 33, 3–8. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Jaroniec, M.; Kaneko, K. Physicochemical foundations for characterization of adsorbents by using high-resolution comparative plots. Langmuir 1997, 13, 6589–6596. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M. New methods enabling description of the porous structure of active carbons on the basis of adsorption data. Ochr. Sr. 1999, 21, 13–17. [Google Scholar]
- Kruk, M.; Jaroniec, M.; Gadkaree, K.P. Nitrogen adsorption studies of novel synthetic active carbons. J. Col. Inter. Sci. 1997, 192, 250–256. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distribution in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Lim, C.K.; Bay, H.H.; Noeh, C.H.; Aris, A.; Majid, Z.A.; Ibrahim, Z. Application of zeolite-activated carbon macrocomposite for the adsorption of Acid Orange 7: Isotherm. kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2013, 20, 7243–7255. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Choma, J. Characterization of nanoporous active carbons by using gas adsorption isotherms. Wegiel aktywny w ochr. sr. i przemysle. 2006, 9–19. [Google Scholar]
- Baccar, R.; Sarra, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Langmuir, I. Chemical reactions at low pressures. J. Am. Chem. Soc. 1915, 27, 1139–1143. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die Adsorption in Losungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Ilayaraja, M.; Krishnan, N.P.; Kannan, R.S. Adsorption of Rhodamine-B and Congo red dye from Aqueous Solution using Activated Carbon: Kinetics, Isotherms, and Thermodynamics. J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 79–89. [Google Scholar]
- Ramuthai, S.; Nandhakumar, V.; Thiruchelvi, M.; Arivoli, S.; Vijayakumaran, V. Rhodamine, B. Adsorption-Kinetic, Mechanistic and Thermodynamic Studies. E-J. Chem. 2009, 6. [Google Scholar] [CrossRef]
- Santhi, M.; Kumar, P.E. Adsorption of Rhodamine B from an Aqueous Solution: Kinetic, Equilibrium and Thermodynamic Studies. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 497–510. [Google Scholar]
- Wang, L.; Zhang, J.; Zhao, R.; Li, C.; Li, Y.; Zhang, C. Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: Equilibrium, kinetic and thermodynamic studies. Desalination 2010, 254, 68–74. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhu, T. Application of activated carbon derived from scrap tires for adsorption of Rhodamine, B. J. Environ. Sci. 2010, 22, 1273–1280. [Google Scholar] [CrossRef]
- Njaku, V.O.; Foo, K.Y.; Asif, M.; Hameed, B.H. Preparation of activated carbons from rambutan (Nephelium Lappaceum) peel by microware- induced KOH activation for Acid Yellow 17 dye adsorption. Chem. Eng. J. 2014, 250, 198–204. [Google Scholar] [CrossRef]
- Ashraf, A.; Bindary, E.L.; Mostafa, A.H.; Mostafa, A.D.; Ahmed, M.E. Adsorption of acid yellow 99 by polyacrylonitrille activated carbon composite: Kinetics, thermodynamics and isotherm studies. J. Molecul. Liq. 2014, 197, 236–242. [Google Scholar]
- Tripathi, P.K.; Liu, M.; Gan, L.; Qian, J.; Xu, Z.; Zhu, D.; Rao, N.N. High surface area ordered mesoporous carbon for high-level removal of rhodamine B. J. Mater. Sci. 2013, 48, 8003–8013. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungl. Sven. Veten. Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).