Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors
Abstract
:1. Introduction
2. Materials and Methods
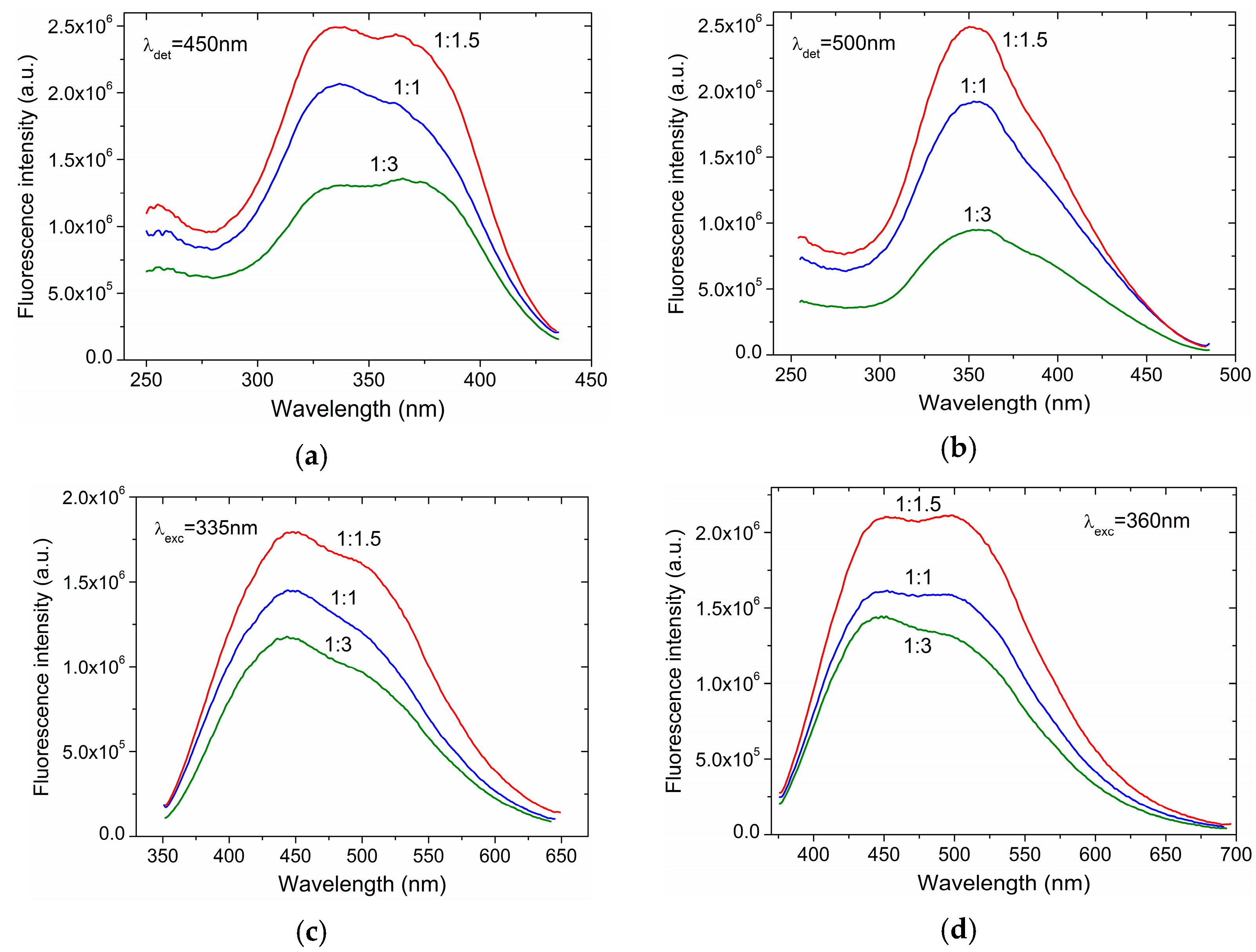
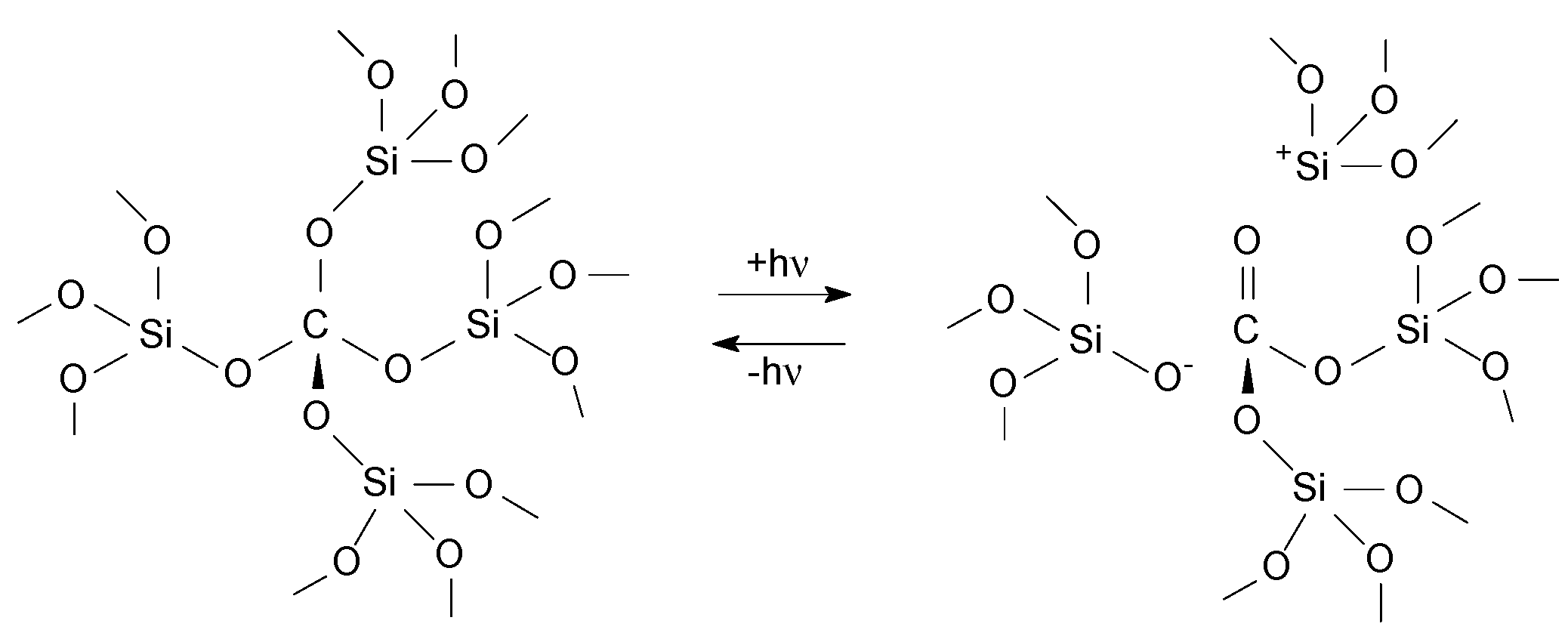
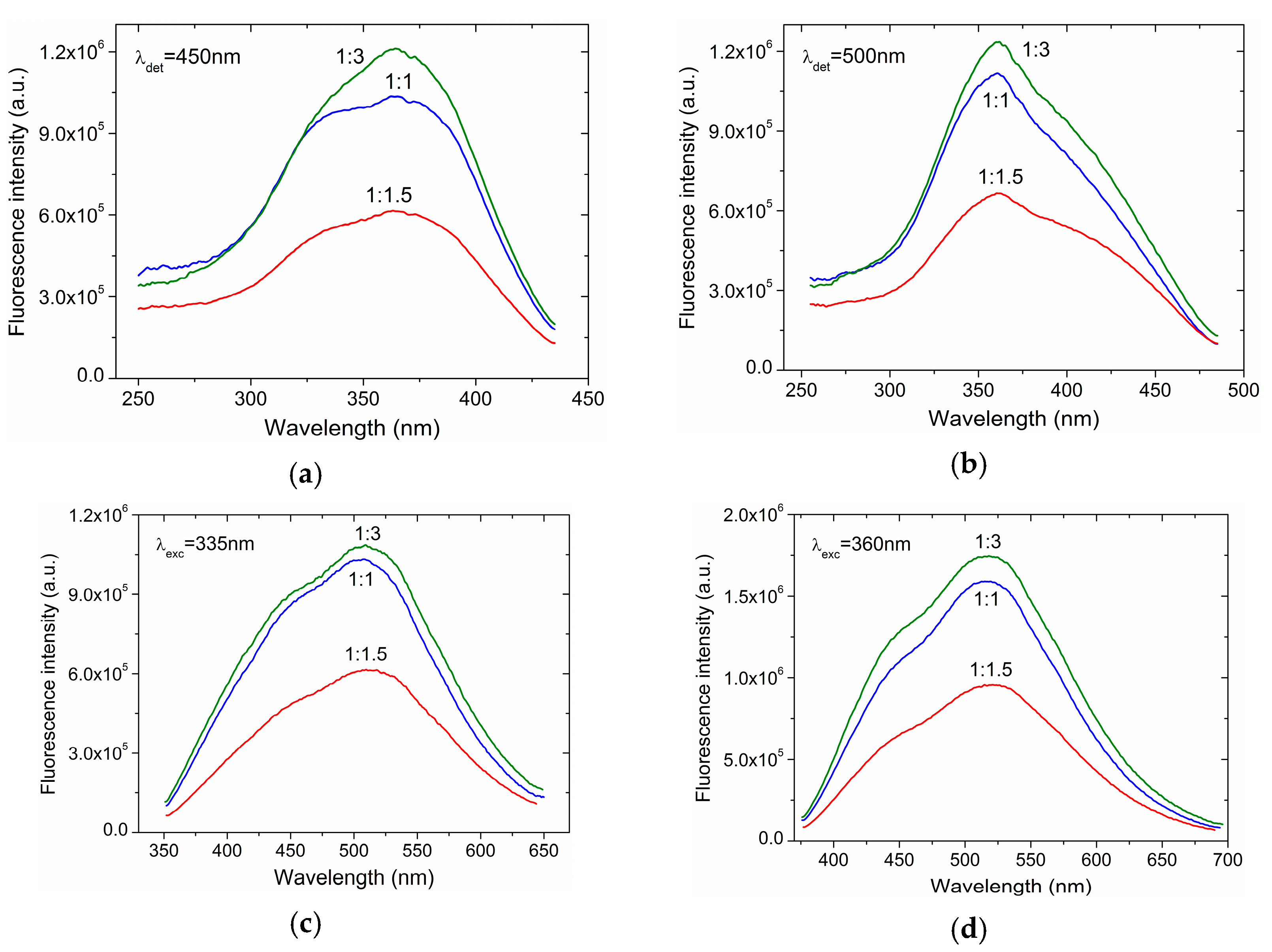
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Z.; Li, Z.; Xu, M.; Ma, Y.; Zhang, J.; Su, Y.; Gao, F.; Wei, H.; Zhang, L. Controllable synthesis of fluorescent carbon dots and their detection application as nanoprobes. Nano-Micro. Lett. 2013, 5, 247–259. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, X.; Hao, H.; Liu, R.; Zhang, D.; Gao, F.; Lu, Q. Green synthesis of fluorescent carbon quantum dots and carbon spheres from pericarp. Sci. China Chem. 2015, 58, 863–870. [Google Scholar] [CrossRef]
- Qian, Z.; Shan, X.; Chai, L.; Ma, J.; Chen, J.; Feng, H. Si-doped carbon quantum dots: A facile and general preparation strategy, bioimaging application, and multifunctional sensor. ACS Appl. Mater. Interfaces 2014, 6, 6797–6805. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, P.; Wang, X.; Cao, L.; Sahu, S.; Liu, J.-H.; Wang, P.; Korch, K.; Tackett, K.N.; Parenzan, A.; Sun, Y.-P. Toward quantitatively fluorescent carbon-based ”quantum” dots. Nanoscale 2011, 3, 2023–2027. [Google Scholar] [CrossRef]
- Yang, C.; Thomsen, R.P.; Ogaki, R.; Kjems, J.; Teo, B.M. Ultrastable green fluorescence carbon dots with high quantum yield for bioimaging and use as theranostic carriers. J. Mater. Chem. B 2015, 3, 4577–4584. [Google Scholar] [CrossRef]
- Cao, L.; Yang, S.-T.; Wang, X.; Luo, P.G.; Liu, J.-H.; Sahu, S.; Liu, Y.; Sun, Y.-P. Competitive performance of carbon “quantum” dots in optical bioimaging. Theranostics 2012, 2, 295–301. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2014, 4, 4976. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Li, S.; Wang, X.; Hu, K.; Wang, L.; Liang, X.; Wu, Y. Multifunctional carbon dots with high quantum yield for imaging and gene delivery. Carbon 2014, 67, 508–513. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Wu, L.; Wang, J.; Ren, J.; Qu, X. Non-enzymatic-browning-reaction: A versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci. Rep. 2014, 4, 3564. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.; Li, D.; Tan, H.; Zhao, Z.; Xied, Z.; Sun, Z. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Green, W.H.; Le, K.P.; Grey, J.; Au, T.T.; Sailor, M.J. White phosphors from a silicate-carboxylate sol-gel precursor that lack metal activator ions. Science 1997, 276, 1826–1828. [Google Scholar] [CrossRef]
- Singh, R.K.; Patel, K.D.; Mahapatra, C.; Kang, M.S.; Kim, H.-W. C-Dot generated bioactive organosilica nanospheres in theranostics: multicolor luminescent and photothermal properties combined with drug delivery capacity. ACS Appl. Mater. Interfaces 2016, 8, 24433–24444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, C.; Lu, Y.; Wu, F.; Ye, G.; Wei, G.; Sun, T.; Chen, J. Visualization of adsorption: Luminescent mesoporous silica-carbon dots composite for rapid and selective removal of U(VI) and in situ monitoring the adsorption behavior. ACS Appl. Mater. Interfaces 2017, 9, 7392–7398. [Google Scholar] [CrossRef]
- Xiang, G.; Ren, Y.; Zhang, H.; Fan, H.; Jiang, X.; He, L.; Zhao, W. Carbon dots based dual-emission silica nanoparticles as ratiometric fluorescent probe for chromium speciation analysis in water samples. Can. J. Chem. 2018, 96, 72–77. [Google Scholar] [CrossRef]
- Suzuki, K.; Malfatti, L.; Takahashi, M.; Carboni, D.; Messina, F.; Tokudome, Y.; Takemoto, M.; Innocenzi, P. Design of carbon dots photoluminescence through organo-functional silane grafting for solid-state emitting devices. Sci. Rep. 2017, 7, 5469. [Google Scholar] [CrossRef]
- Nelson, D.K.; Razbirin, B.S.; Starukhin, A.N.; Eurov, D.A.; Kurdyukov, D.A.; Stovpiaga, E.Yu.; Golubev, V.G. Photoluminescence of carbon dots from mesoporous silica. Opt. Mater. 2016, 59, 28–33. [Google Scholar] [CrossRef]
- Tian, Y.; Ran, Z.; Yang, W. Carbon dot-silica composite nanoparticle: An excitation-independent fluorescence material with tunable fluorescence. RSC Adv. 2017, 7, 43839–43844. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, Zh.; Zhang, X.; Chen, Y. Facile synthesis of blue-emitting carbon dots@mesoporous silica composite spheres. Solid State Sci. 2018, 76, 100–104. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Wang, Y.; Yang, Y.; Liu, X. Efficient resistance against solid-state quenching of carbon dots towards white light emitting diodes by physical embedding into silica. Carbon 2018, 126, 426–436. [Google Scholar] [CrossRef]
- Kuzema, P.O.; Bolbukh, Yu.M.; Tertykh, V.A. Luminescent materials based on organic salts pyrolyzed at the silica surface. Him. Fiz. Tehnol. Poverhni 2018, 9, 404–410. [Google Scholar] [CrossRef]
- Timchenko, V.; Novozhilov, A.; Slepysheva, O. Kinetics of thermal decomposition of thiourea. Russ. J. Gen. Chem. 2004, 74, 1046–1050. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, W.; Gao, H.; Wang, Y.; Guo, H.; Guo, S.; Tang, Z.; Zhang, J. Melem: An efficient metal-free luminescence material. J. Mater. Chem. C 2017, 5, 10746–10753. [Google Scholar] [CrossRef]


| CD Size, nm | Synthetic Technique | PL QY, % | Reference |
|---|---|---|---|
| 2–4 | Hydrothermal treatment of orange pericarp | up to 9 | [3] |
| 5–9 | Solvothermal reaction between SiCl4 and hydroquinone (CDs, doped with Si atoms) | 19 | [4] |
| 5 | CDs modification with oligo(ethylene glycol) diamine (with subsequent doping with ZnS, ZnO or TiO2) | 20 (up to 78) | [5] |
| 2–4 | Heating of oligoethylenimine–β-cyclodextrin in phosphoric acid | 30 | [6] |
| <10 | Thermal treatment of O,O′-bis(3-aminopropyl) polyethylene glycol (with further doping with ZnS) | 40 (60) | [7] |
| 5 | Hydrothermal method using urea and citric acid | 45 | [8] |
| 3–4 | Hydrothermal oxidation of branched polyethylenimine | 55 | [9] |
| 2–5 | Heating of glucose with amino acids in alkaline medium | up to 69 | [10] |
| 5–9 | Hydrothermal synthesis using citric acid and l-cysteine | 73 | [11] |
| 2–3 | Hydrothermal synthesis using citric acid and urea (or thiourea) | 78 (71) | [12] |
| CD Size, nm | Synthetic Technique | PL QY, % | Reference |
|---|---|---|---|
| - | Thermal treatment of sol-gel glasses from tetraalkoxysilane or 3-aminopropyltriethoxysilane (APTES) and various carboxylic acids | up to 45 | [13] |
| 1.3–1.7 | Heat treatment of sol-gel materials from tetraethoxysilane (TEOS) and APTES in the presence of hexadecyltrimethylammonium bromide | up to 60 | [14] |
| 8 | Sol-gel synthesis from organoalkoxysilanes in the presence of CDs obtained via the hydrothermal synthesis using citric acid and ethylenediamine | 9–14 | [17] |
| 4.5 | TEOS hydrolysis in the presence of glucose and 1,2-ethylenediamine | up to 13 | [19] |
| - | Sol-gel synthesis from TEOS in the presence of CDs obtained via the hydrothermal synthesis using citric acid and ethylenediamine | 21.5 | [20] |
| 4–9 | N-(3-(trimethoxysilyl) propyl) ethylenediamine hydrolysis in the presence of CDs obtained by solvothermal treatment of p-phenylenediamine | 41.72 | [21] |
| - | Pyrolysis of citric acid (thio)ureates at the fumed silica surface | 7–11 | [22] |
| Sample | Ur(ThUr):CA Molar Ratio | V(ES-40), mL | V(H2O), mL | m(CA), mg | m(Ur), mg | m(ThUr), mg | V(HCl), mL |
|---|---|---|---|---|---|---|---|
| 1 | 1:1 | 25 | 5 | 83 | 26 | 0.3 | |
| 2 | 1.5:1 | 25 | 5 | 82 | 38 | 0.3 | |
| 3 | 3:1 | 25 | 5 | 64.5 | 60.5 | 0.3 | |
| 4 | 1:1 | 25 | 5 | 83 | 33.3 | 0.3 | |
| 5 | 1.5:1 | 25 | 5 | 83 | 50 | 0.3 | |
| 6 | 3:1 | 25 | 5 | 64.5 | 77.7 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzema, P.; Bolbukh, Y.; Lipke, A.; Majdan, M.; Tertykh, V. Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors. Colloids Interfaces 2019, 3, 11. https://doi.org/10.3390/colloids3010011
Kuzema P, Bolbukh Y, Lipke A, Majdan M, Tertykh V. Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors. Colloids and Interfaces. 2019; 3(1):11. https://doi.org/10.3390/colloids3010011
Chicago/Turabian StyleKuzema, Pavlo, Yulia Bolbukh, Agnieszka Lipke, Marek Majdan, and Valentyn Tertykh. 2019. "Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors" Colloids and Interfaces 3, no. 1: 11. https://doi.org/10.3390/colloids3010011
APA StyleKuzema, P., Bolbukh, Y., Lipke, A., Majdan, M., & Tertykh, V. (2019). Luminescent Sol-Gel Glasses from Silicate–Citrate–(Thio)Ureate Precursors. Colloids and Interfaces, 3(1), 11. https://doi.org/10.3390/colloids3010011





