The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Device Fabrication Process
2.3. Sample Characterization
3. Results
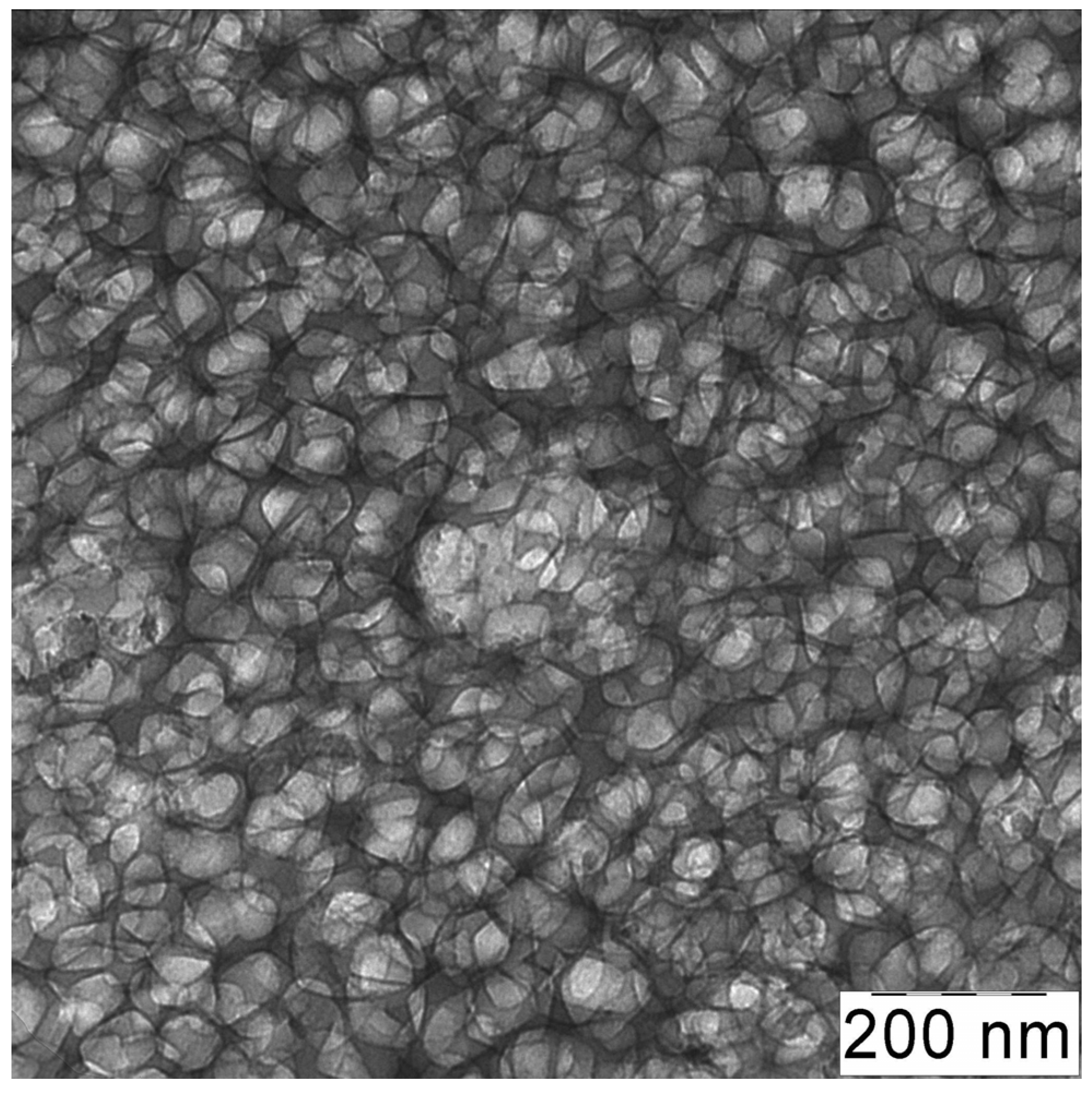
3.1. TEM
3.2. FTIR Spectroscopy
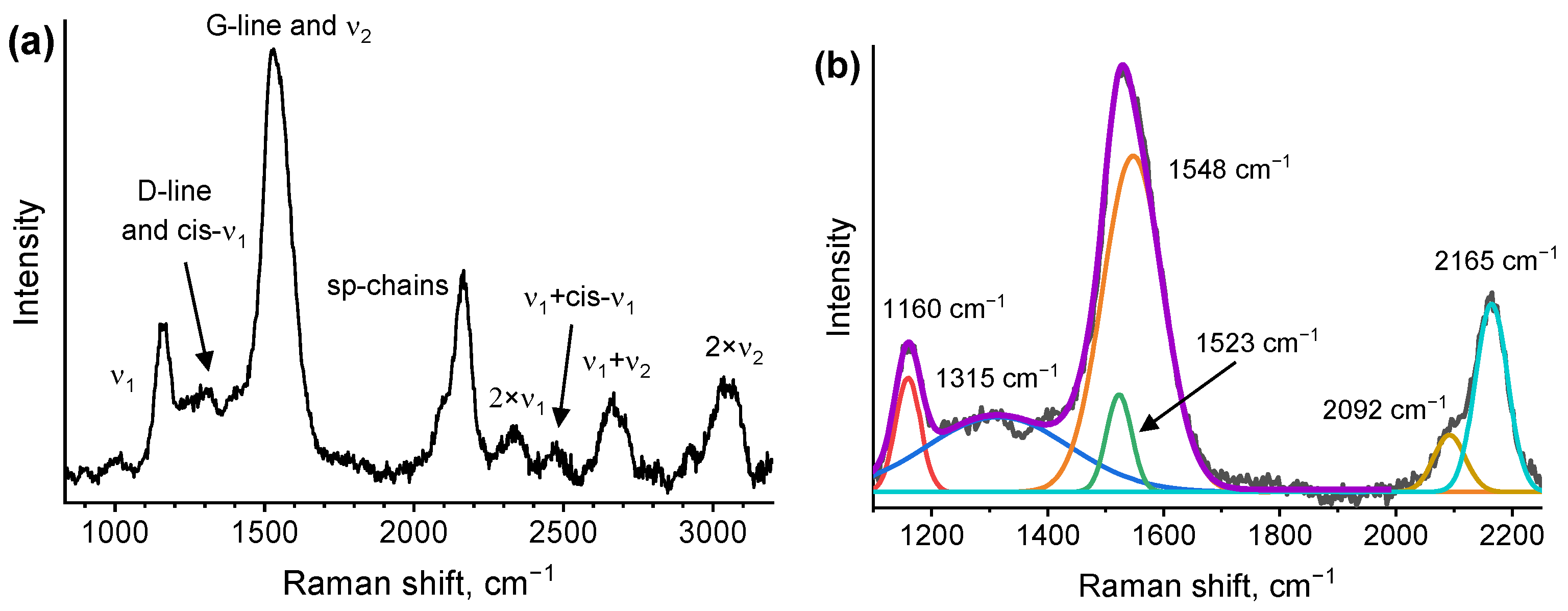
3.3. Raman Spectroscopy
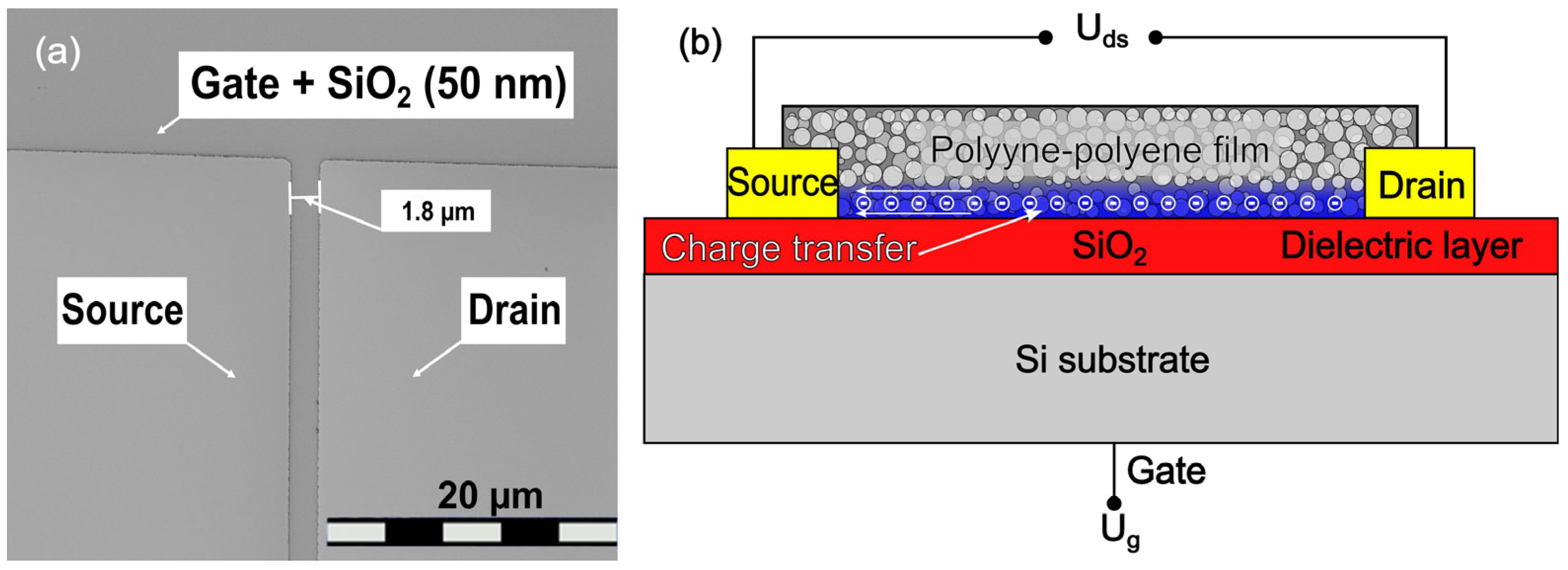
3.4. The Properties of the Field-Effect Transistor with a Polyyne–Polyene Gate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Ouyang, B.; Guo, X.; Guo, Y.; Liu, Y. Advances in Flexible Organic Field-Effect Transistors and Their Applications for Flexible Electronics. Npj. Flex. Electron. 2022, 6, 1–19. [Google Scholar] [CrossRef]
- Efremenko, Y.; Mirsky, V.M. Poly-3-Thienylboronic Acid: A Chemosensitive Derivative of Polythiophene. J. Solid. State Electrochem. 2020, 24, 3105–3111. [Google Scholar] [CrossRef]
- Aliqué, M.; Simão, C.D.; Murillo, G.; Moya, A. Fully-Printed Piezoelectric Devices for Flexible Electronics Applications. Adv. Mater. Technol. 2021, 6, 2001020. [Google Scholar] [CrossRef]
- Bolognesi, M.; Prosa, M.; Seri, M. 9-Biocompatible and Biodegradable Organic Electronic Materials. In Sustainable Strategies in Organic Electronics; Marrocchi, A., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Delhi, India, 2022; pp. 297–338. ISBN 978-0-12-823147-0. [Google Scholar]
- Xu, Y.; Liu, W.; Huang, Y.; Jin, C.; Zhou, B.; Sun, J.; Yang, J. Recent Advances in Flexible Organic Synaptic Transistors. Adv. Electron. Mater. 2021, 7, 2100336. [Google Scholar] [CrossRef]
- Iqbal, H.F.; Ai, Q.; Thorley, K.J.; Chen, H.; McCulloch, I.; Risko, C.; Anthony, J.E.; Jurchescu, O.D. Suppressing Bias Stress Degradation in High Performance Solution Processed Organic Transistors Operating in Air. Nat. Commun. 2021, 12, 2352. [Google Scholar] [CrossRef]
- Han, H.; Kim, C. Unexpected Benefits of Contact Resistance in 3D Organic Complementary Inverters. Adv. Electron. Mater. 2020, 6, 1900879. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with Finite Length: The One-Dimensional Sp Carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [CrossRef]
- Eaton, A.L.; Fielder, M.; Nair, A.K. Mechanical and Thermal Properties of Carbon-Based Low-Dimensional Materials. MRS Bull. 2022, 47, 1001–1010. [Google Scholar] [CrossRef]
- Wang, M.; Lin, S. Ballistic Thermal Transport in Carbyne and Cumulene with Micron-Scale Spectral Acoustic Phonon Mean Free Path. Sci. Rep. 2015, 5, 18122. [Google Scholar] [CrossRef]
- Zanolli, Z.; Onida, G.; Charlier, J.-C. Quantum Spin Transport in Carbon Chains. ACS Nano 2010, 4, 5174–5180. [Google Scholar] [CrossRef]
- La Torre, A.; Botello-Mendez, A.; Baaziz, W.; Charlier, J.-C.; Banhart, F. Strain-Induced Metal–Semiconductor Transition Observed in Atomic Carbon Chains. Nat. Commun. 2015, 6, 6636. [Google Scholar] [CrossRef] [PubMed]
- Mota, E.A.V.; da Silva Paula, M.V.; da Silva, C.A.B., Jr.; Del Nero, J. Tuning Transport Properties in Carbyne-DNA Fragments-Carbyne Devices. Mater. Lett. 2023, 336, 133925. [Google Scholar] [CrossRef]
- Yang, G. Synthesis, Properties, and Applications of Carbyne Nanocrystals. Mater. Sci. Eng. R Rep. 2022, 151, 100692. [Google Scholar] [CrossRef]
- Scaccabarozzi, A.D.; Milani, A.; Peggiani, S.; Pecorario, S.; Sun, B.; Tykwinski, R.R.; Caironi, M.; Casari, C.S. A Field-Effect Transistor Based on Cumulenic Sp-Carbon Atomic Wires. J. Phys. Chem. Lett. 2020, 11, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Pecorario, S.; Scaccabarozzi, A.D.; Fazzi, D.; Gutiérrez-Fernández, E.; Vurro, V.; Maserati, L.; Jiang, M.; Losi, T.; Sun, B.; Tykwinski, R.R.; et al. Stable and Solution-Processable Cumulenic Sp-Carbon Wires: A New Paradigm for Organic Electronics. Adv. Mater. 2022, 34, 2110468. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-Y. Recent Advances in Luminescent Transition Metal Polyyne Polymers. J. Inorg. Organomet. Polym. Mater. 2005, 15, 197–219. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Hukka, T.I.; Pakkanen, T.A.; D’Evelyn, M.P. Chemisorption of Fluorine, Chlorine, HF, and HCl on the Diamond (100)2x1 Surface: An Ab Initio Study. J. Phys. Chem. 1995, 99, 4710–4719. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Chu, M.; Cao, G.; Yang, Y. An Activation-Free Method for Preparing Microporous Carbon by the Pyrolysis of Poly(Vinylidene Fluoride). Carbon 2010, 48, 2812–2814. [Google Scholar] [CrossRef]
- Evsyukov, S.E. Chemical Dehydrohalogenation of Polymers. In Carbyne and Carbynoid Structures; Heimann, R.B., Evsyukov, S.E., Kavan, L., Eds.; Physics and Chemistry of Materials with Low-Dimensional Structures; Springer: Dordrecht, The Netherlands, 1999; Volume 21, pp. 55–74. ISBN 978-94-010-5993-0. [Google Scholar]
- Streletskiy, O.; Zavidovskiy, I.; Yakubovsky, D.; Doroshina, N.; Syuy, A.; Lebedinskij, Y.; Markeev, A.; Arsenin, A.; Volkov, V.; Novikov, S. Tailoring of the Distribution of SERS-Active Silver Nanoparticles by Post-Deposition Low-Energy Ion Beam Irradiation. Materials 2022, 15, 7721. [Google Scholar] [CrossRef]
- Chang, Y.; Pang, Y.; Dang, Q.; Kumar, A.; Zhang, G.; Chang, Z.; Sun, X. Converting Polyvinyl Chloride Plastic Wastes to Carbonaceous Materials via Room-Temperature Dehalogenation for High-Performance Supercapacitor. ACS Appl. Energy Mater. 2018, 1, 10. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, G.; Han, B.; Li, H.; Hu, C.; Pang, Y.; Chang, Z.; Sun, X. Polymer Dehalogenation-Enabled Fast Fabrication of N,S-Codoped Carbon Materials for Superior Supercapacitor and Deionization Applications. Available online: https://pubs.acs.org/doi/pdf/10.1021/acsami.7b08181 (accessed on 7 February 2023).
- Kim, J.-I.; Park, S.-J. A Study of Ion Charge Transfer on Electrochemical Behaviors of Poly(Vinylidene Fluoride)-Derived Carbon Electrodes. J. Anal. Appl. Pyrolysis 2012, 98, 22–28. [Google Scholar] [CrossRef]
- Coleman, M.M.; Wu, M.S.; Harrison, I.R.; Painter, P.C. Vibrational Spectra and Conformation of Poly(Vinylidene Chloride). J. Macromol. Sci. 1978, 15, 463–480. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Imai, S.; Noritsune, M.; Okuwaki, A. Dechlorination of Poly(Vinylidene Chloride) in NaOH/Ethylene Glycol as a Function of NaOH Concentration, Temperature, and Solvent. Polym. Degrad. Stab. 2008, 93, 1979–1984. [Google Scholar] [CrossRef]
- Phan, S.; Padilla-Gamiño, J.L.; Luscombe, C.K. The Effect of Weathering Environments on Microplastic Chemical Identification with Raman and IR Spectroscopy: Part I. Polyethylene and Polypropylene. Polym. Test. 2022, 116, 107752. [Google Scholar] [CrossRef]
- Konikkara, N.; Kennedy, L.J.; Vijaya, J.J. Preparation and Characterization of Hierarchical Porous Carbons Derived from Solid Leather Waste for Supercapacitor Applications. J. Hazard. Mater. 2016, 318, 173–185. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhu, W.X.; Liao, D.W.; Ng, C.F.; Au, C.T. In Situ FTIR Studies of NO Reduction over Carbon Nanotubes (CNTs) and 1wt.% Pd/CNTs. Catal. Today 2004, 93–95, 711–714. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Pamuła, E.; Błażewicz, M.; Paluszkiewicz, C.; Dobrzyński, P. FTIR Study of Degradation Products of Aliphatic Polyesters–Carbon Fibres Composites. J. Mol. Struct. 2001, 596, 69–75. [Google Scholar] [CrossRef]
- Burket, C.L.; Rajagopalan, R.; Marencic, A.P.; Dronvajjala, K.; Foley, H.C. Genesis of Porosity in Polyfurfuryl Alcohol Derived Nanoporous Carbon. Carbon 2006, 44, 2957–2963. [Google Scholar] [CrossRef]
- Karimov, O.K.; Teptereva, G.A.; Chetvertneva, I.A.; Movsumzade, E.M.; Karimov, E.K. The Structure of Lignosulfonates for Production of Carbon Catalyst Support. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 022086. [Google Scholar] [CrossRef]
- Kaur, G.A.; Sharma, V.; Gupta, N.; Shandilya, M.; Rai, R. Structural and Optical Amendment of PVDF into CQDs through High Temperature Calcination Process. Mater. Lett. 2021, 304, 130616. [Google Scholar] [CrossRef]
- Venkatesh, R.; Karthi, N.; Kawin, N.; Prakash, T.; Kannan, C.R.; Karthigairajan, M.; Bobe, K. Synthesis and Adsorbent Performance of Modified Biochar with Ag/MgO Nanocomposites for Heat Storage Application. Adsorpt. Sci. Technol. 2022, 2022, e7423102. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Khairanov, R.K.; Zlobina, N.A.; Pesin, L.A. Modification of the IR Spectra Shape in the 2000–2300 Cm–1 Absorption Band upon the Aging of a Chemically Dehydrofluorinated Poly(Vinylidene Fluoride) Film. J. Surf. Investig. 2020, 14, 1144–1151. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Nishchak, O.Y.; Zavidovskiy, I.A.; Maslakov, K.I.; Pavlikov, A.V. Sp-Based Thin Films Synthesized by Magnetron Sputtering of Dehydrohalogenated Polyvinylidenchloride. Thin Solid Film. 2021, 739, 138993. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nishchak, O.Y.; Khaidarov, A.A.; Savchenko, N.F.; Pavlikov, A.V. Low-Threshold Field Emission Cathode Based on Heat-Treated Dehydrofluorinated Polyvinylidene Fluoride. J. Exp. Theor. Phys. 2022, 135, 844–852. [Google Scholar] [CrossRef]
- Lucotti, A.; Tommasini, M.; Fazzi, D.; Del Zoppo, M.; Chalifoux, W.A.; Ferguson, M.J.; Zerbi, G.; Tykwinski, R.R. Evidence for Solution-State Nonlinearity of Sp-Carbon Chains Based on IR and Raman Spectroscopy: Violation of Mutual Exclusion. J. Am. Chem. Soc. 2009, 131, 4239–4244. [Google Scholar] [CrossRef]
- Leong, T.X.; Collins, B.K.; Dey Baksi, S.; Mackin, R.T.; Sribnyi, A.; Burin, A.L.; Gladysz, J.A.; Rubtsov, I.V. Tracking Energy Transfer across a Platinum Center. J. Phys. Chem. A 2022, 126, 4915–4930. [Google Scholar] [CrossRef]
- Begni, F.; Paul, G.; Lasseuguette, E.; Mangano, E.; Bisio, C.; Ferrari, M.-C.; Gatti, G. Synthetic Saponite Clays as Additives for Reducing Aging Effects in PIM1 Membranes. ACS Appl. Polym. Mater. 2020, 2, 3481–3490. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Alexander, C.M.O.; Cody, G.D. Comparison of FT-IR Spectra of Bulk and Acid Insoluble Organic Matter in Chondritic Meteorites: An Implication for Missing Carbon during Demineralization. Meteorit. Planet. Sci. 2019, 54, 1632–1641. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Y.; Li, Q.; Yan, W.; Zhang, X.; Ouyang, X.; Ouyang, X.; Chen, L.; Liao, B. A Study of Al2O3/MgO Composite Films Deposited by FCVA for Thin-Film Encapsulation. Materials 2023, 16, 1955. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Everall, N.; Littlejohn, D.; Nordon, A.; Bloomfield, M. Dependence of Signal on Depth in Transmission Raman Spectroscopy. Appl. Spectrosc. 2011, 65, 724–733. [Google Scholar] [CrossRef]
- Abdu, Y.A. Raman Micro-Spectroscopy of Nanodiamonds from the Kapoeta Meteorite. Diam. Relat. Mater. 2021, 118, 108536. [Google Scholar] [CrossRef]
- Maia, L.F.; De Oliveira, V.E.; Edwards, H.G.M.; De Oliveira, L.F.C. The Diversity of Linear Conjugated Polyenes and Colours in Nature: Raman Spectroscopy as a Diagnostic Tool. ChemPhysChem 2021, 22, 231–249. [Google Scholar] [CrossRef]
- Streletskiy, O.; Perevedentseva, E.; Zavidovskiy, I.; Karmenyan, A.; Sychev, V.; Sadykova, V.; Kuvarina, A.; Cheng, C.-L. Amorphous Carbon Films with Embedded Well-Dispersed Nanodiamonds: Plasmon-Enhanced Analysis and Possible Antimicrobial Applications. Magnetochemistry 2022, 8, 171. [Google Scholar] [CrossRef]
- Lichtmann, L.S.; Imhoff, E.A.; Sarhangi, A.; Fitchen, D.B. Resonance Raman Spectra of cis (CH)x and (CD)x. J. Chem. Phys. 1984, 81, 168–184. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial Properties of Modified A–C and Ta–C Coatings: The Effects of the Sp2/Sp3 Ratio, Oxidation, Nitridation, and Silver Incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Schaffer, H.E.; Chance, R.R.; Silbey, R.J.; Knoll, K.; Schrock, R.R. Conjugation Length Dependence of Raman Scattering in a Series of Linear Polyenes: Implications for Polyacetylene. J. Chem. Phys. 1991, 94, 4161–4170. [Google Scholar] [CrossRef]
- Casari, C.S.; Li Bassi, A.; Baserga, A.; Ravagnan, L.; Piseri, P.; Lenardi, C.; Tommasini, M.; Milani, A.; Fazzi, D.; Bottani, C.E.; et al. Low-Frequency Modes in the Raman Spectrum of $sp\text{\ensuremath{-}}s{p}^{2}$ Nanostructured Carbon. Phys. Rev. B 2008, 77, 195444. [Google Scholar] [CrossRef]
- Cataldo, F. The Role of Raman Spectroscopy in the Research on Sp-Hybridized Carbon Chains: Carbynoid Structures Polyynes and Metal Polyynides: Raman Spectra of Sp-Hybridized Carbon Chains. J. Raman Spectrosc. 2008, 39, 169–176. [Google Scholar] [CrossRef]
- Demir, A.; Bağci, S.; San, S.E.; Doğruyol, Z. Pentacene-Based Organic Thin Film Transistor with SiO2 Gate Dielectric. Surf. Rev. Lett. 2015, 22, 1550038. [Google Scholar] [CrossRef]
- Xu, G.; Bao, Z.; Groves, J.T. Langmuir−Blodgett Films of Regioregular Poly(3-Hexylthiophene) as Field-Effect Transistors. Langmuir 2000, 16, 1834–1841. [Google Scholar] [CrossRef]
- Garnier, F.; Hajlaoui, R.; El Kassmi, A.; Horowitz, G.; Laigre, L.; Porzio, W.; Armanini, M.; Provasoli, F. Dihexylquaterthiophene, A Two-Dimensional Liquid Crystal-like Organic Semiconductor with High Transport Properties. Chem. Mater. 1998, 10, 3334–3339. [Google Scholar] [CrossRef]
- Demir, A.; Atahan, A.; Bağcı, S.; Aslan, M.; Saif Islam, M. Organic/Inorganic Interfaced Field-Effect Transistor Properties with a Novel Organic Semiconducting Material. Philos. Mag. 2016, 96, 274–285. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Afzali-Ardakani, A.; Furman, B.; Kymissis, J.; Purushothaman, S. Trans-Trans-2,5-Bis-[2-5-(2,2′-Bithienyl)Ethenyl]Thiophene: Synthesis, Characterization, Thin Film Deposition and Fabrication of Organic Field-Effect Transistors. Synth. Met. 1997, 89, 193–197. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic Field-Effect Transistor-Based Flexible Sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, S.; Cho, K.; Harkin, D.; Hwang, W.-T.; Yoo, D.; Kim, J.-K.; Lee, W.; Song, Y.; Ahn, H.; et al. Enhanced Charge Injection Properties of Organic Field-Effect Transistor by Molecular Implantation Doping. Adv. Mater. 2019, 31, 1806697. [Google Scholar] [CrossRef]
- Bao, Z.; Lovinger, A.J. Soluble Regioregular Polythiophene Derivatives as Semiconducting Materials for Field-Effect Transistors. Chem. Mater. 1999, 11, 2607–2612. [Google Scholar] [CrossRef]
- Burch, R.R.; Dong, Y.-H.; Fincher, C.; Goldfinger, M.; Rouviere, P.E. Electrical Properties of Polyunsaturated Natural Products: Field Effect Mobility of Carotenoid Polyenes. Synth. Met. 2004, 146, 43–46. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Khaidarov, A.A.; Pavlikov, A.V.; Minnebaev, K.F. The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination. J. Compos. Sci. 2023, 7, 264. https://doi.org/10.3390/jcs7070264
Streletskiy OA, Zavidovskiy IA, Nuriahmetov IF, Khaidarov AA, Pavlikov AV, Minnebaev KF. The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination. Journal of Composites Science. 2023; 7(7):264. https://doi.org/10.3390/jcs7070264
Chicago/Turabian StyleStreletskiy, Oleg A., Ilya A. Zavidovskiy, Islam F. Nuriahmetov, Abdusame A. Khaidarov, Alexander V. Pavlikov, and Kashif F. Minnebaev. 2023. "The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination" Journal of Composites Science 7, no. 7: 264. https://doi.org/10.3390/jcs7070264
APA StyleStreletskiy, O. A., Zavidovskiy, I. A., Nuriahmetov, I. F., Khaidarov, A. A., Pavlikov, A. V., & Minnebaev, K. F. (2023). The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination. Journal of Composites Science, 7(7), 264. https://doi.org/10.3390/jcs7070264






