Sustainable Zinc-Ion Battery Separators Based on Silica and Cellulose Fibers Derived from Coffee Parchment Waste
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Extraction of Cellulose Fibers from CP Waste
2.2.1. Dewaxing
2.2.2. Alkali Treatment
2.2.3. Bleaching
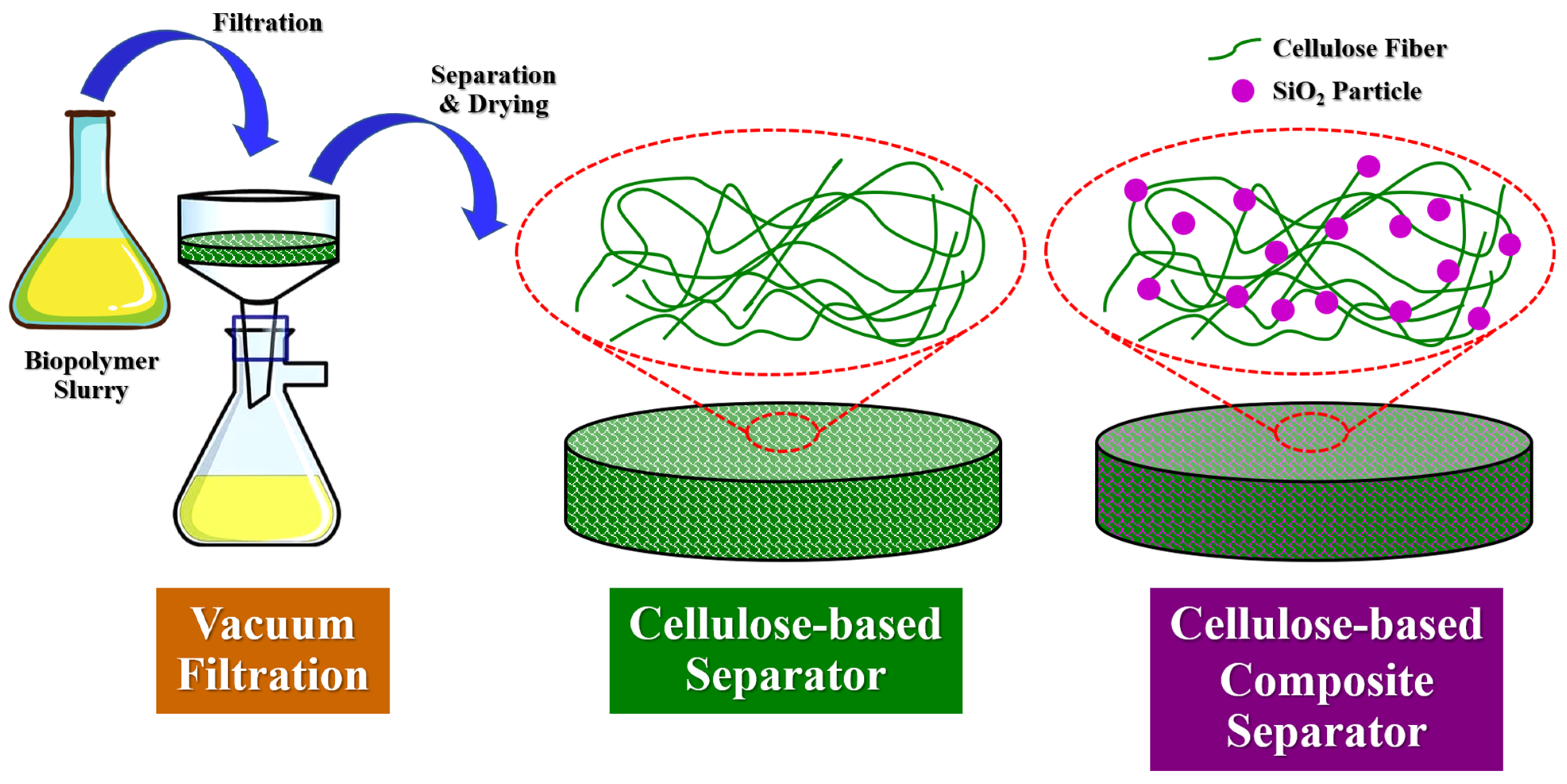
2.3. Preparation of Sustainable Separators from Cellulose Fibers and Silica Particles
2.4. Characterization Methods of Samples
2.4.1. Extracted Cellulose Fibers
2.4.2. Cellulose-Based Composite Separators
2.5. Development and Assembly of Batteries
2.5.1. Zn//Zn Symmetric Cells
2.5.2. ZIBs
2.6. Electrochemical Evaluation
3. Results and Discussion
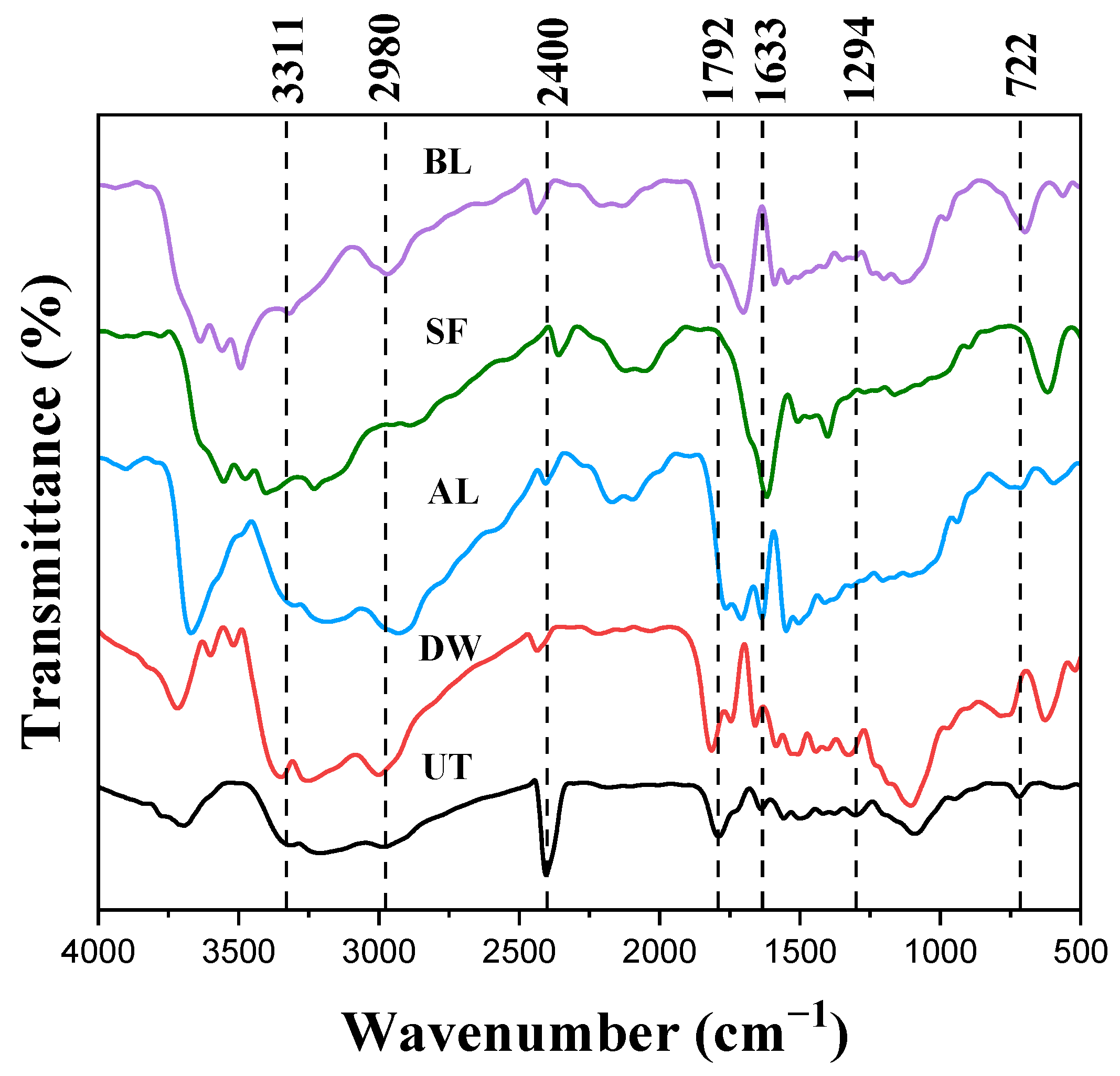
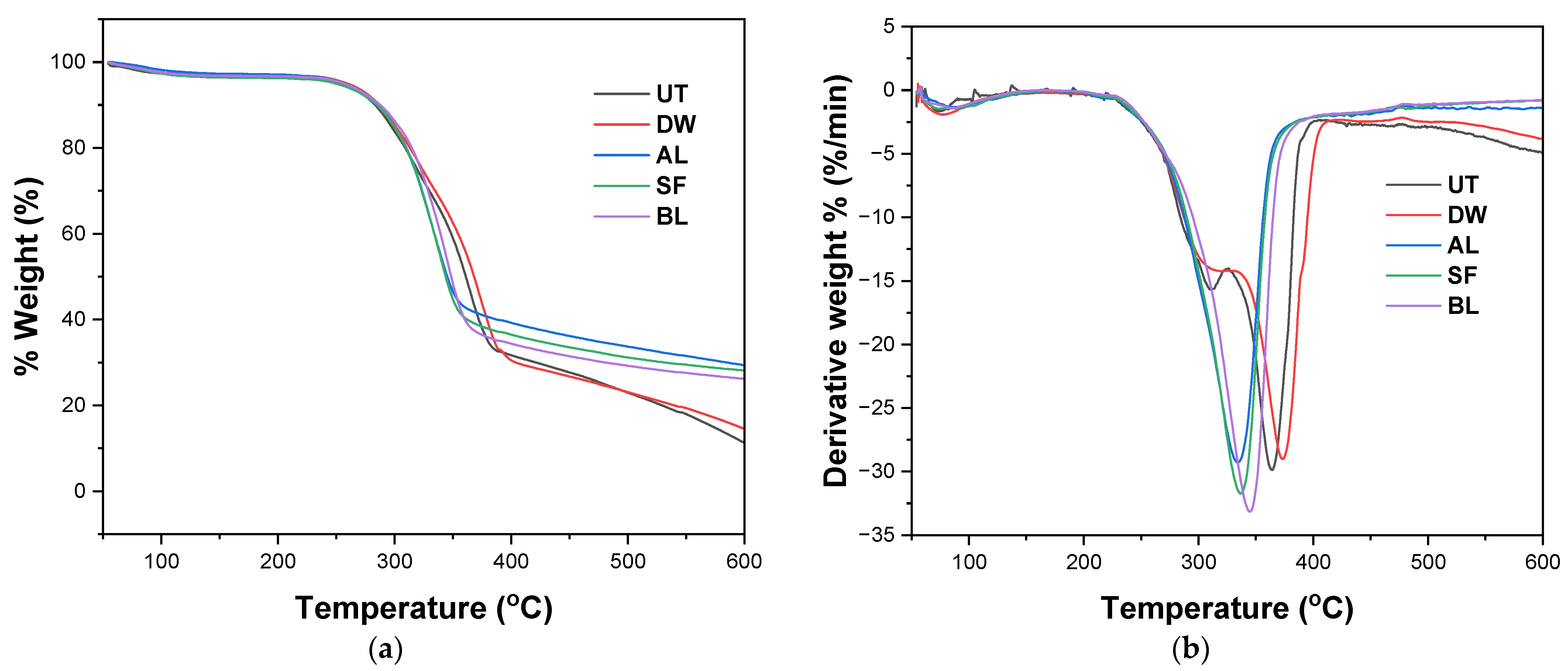
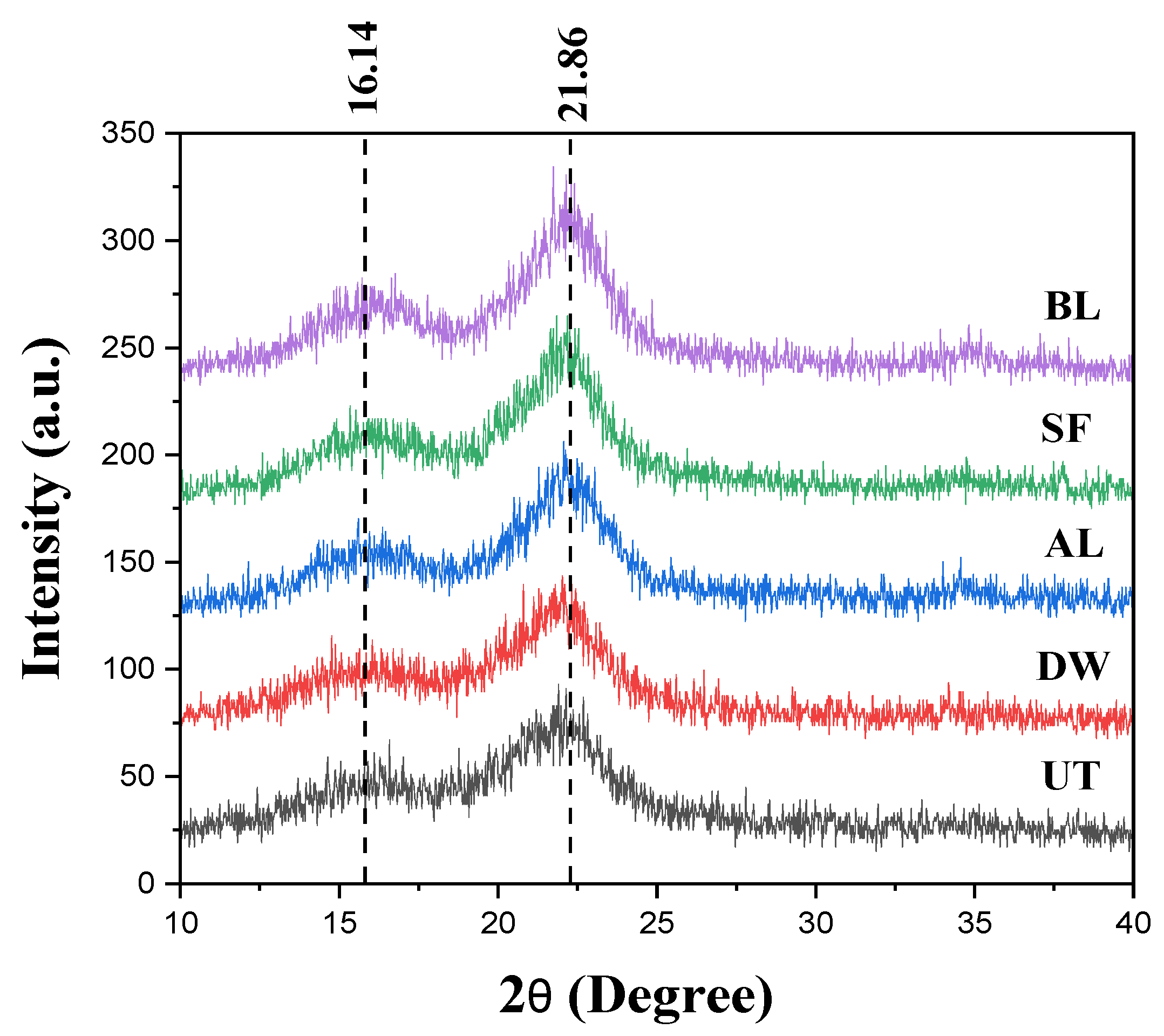
3.1. Characterization of Extracted Cellulose Fibers
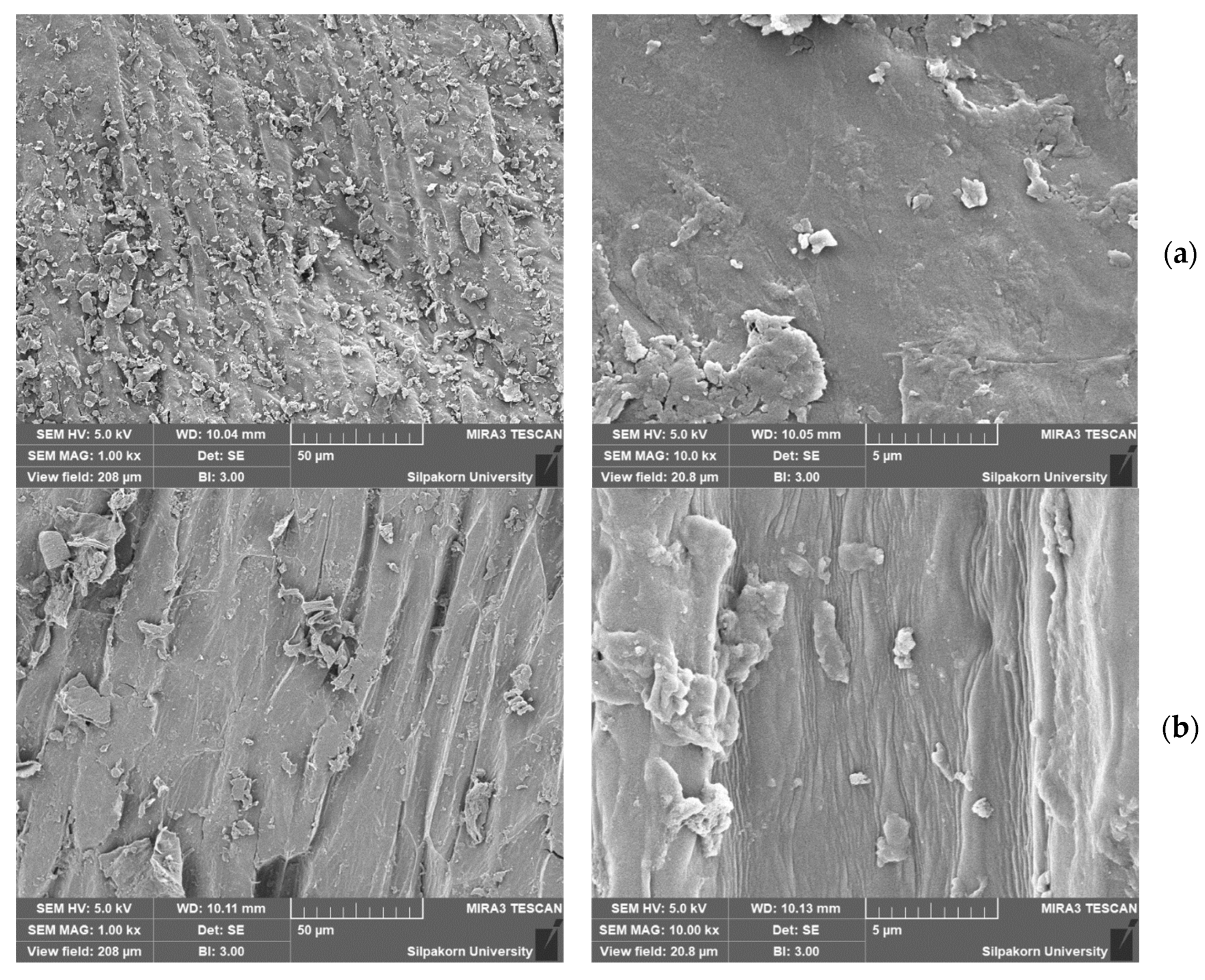
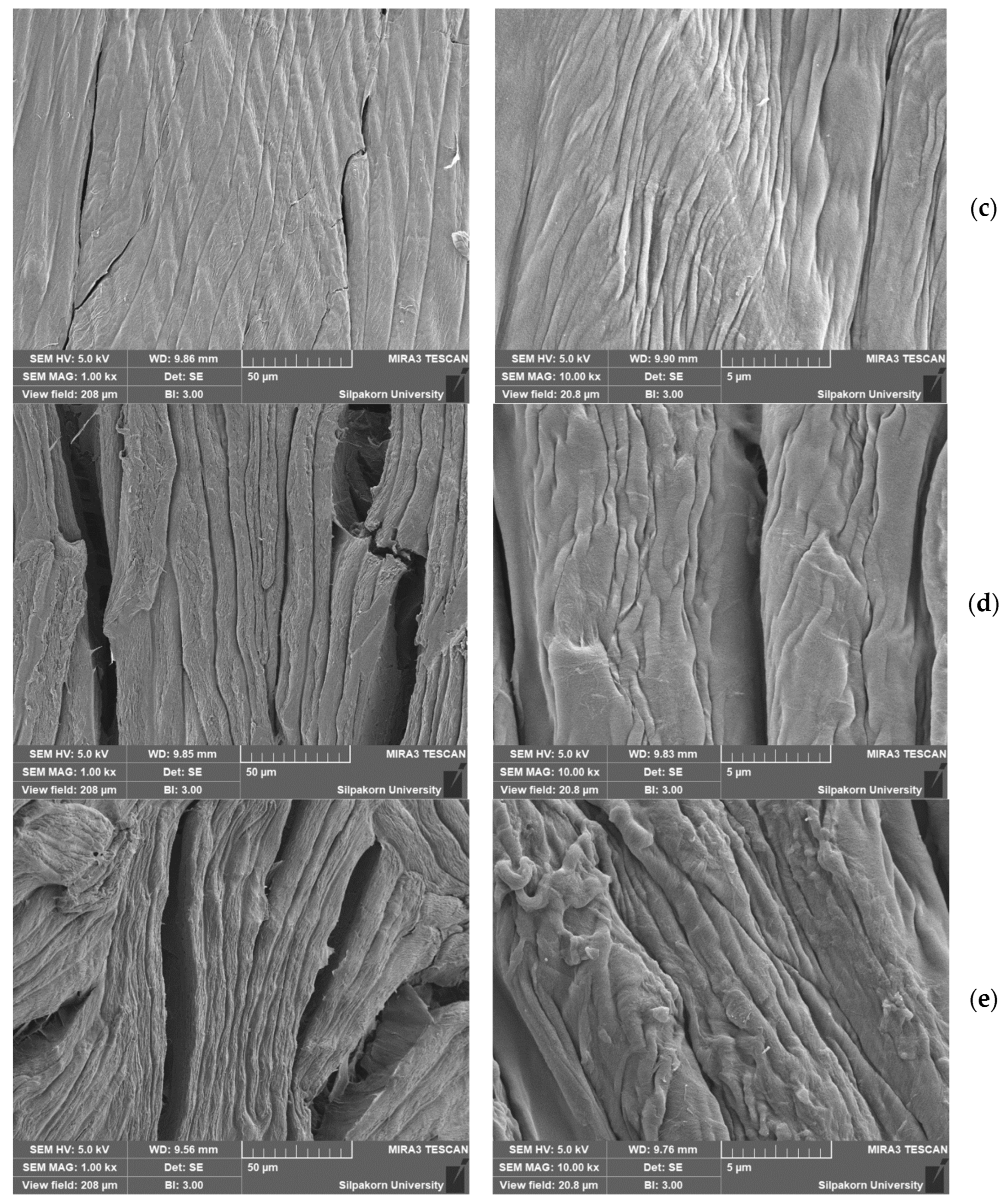
3.2. Characterization of Cellulose-Based Composite Separators
3.3. Electrochemical Testing of Zn//Zn Symmetric Cells
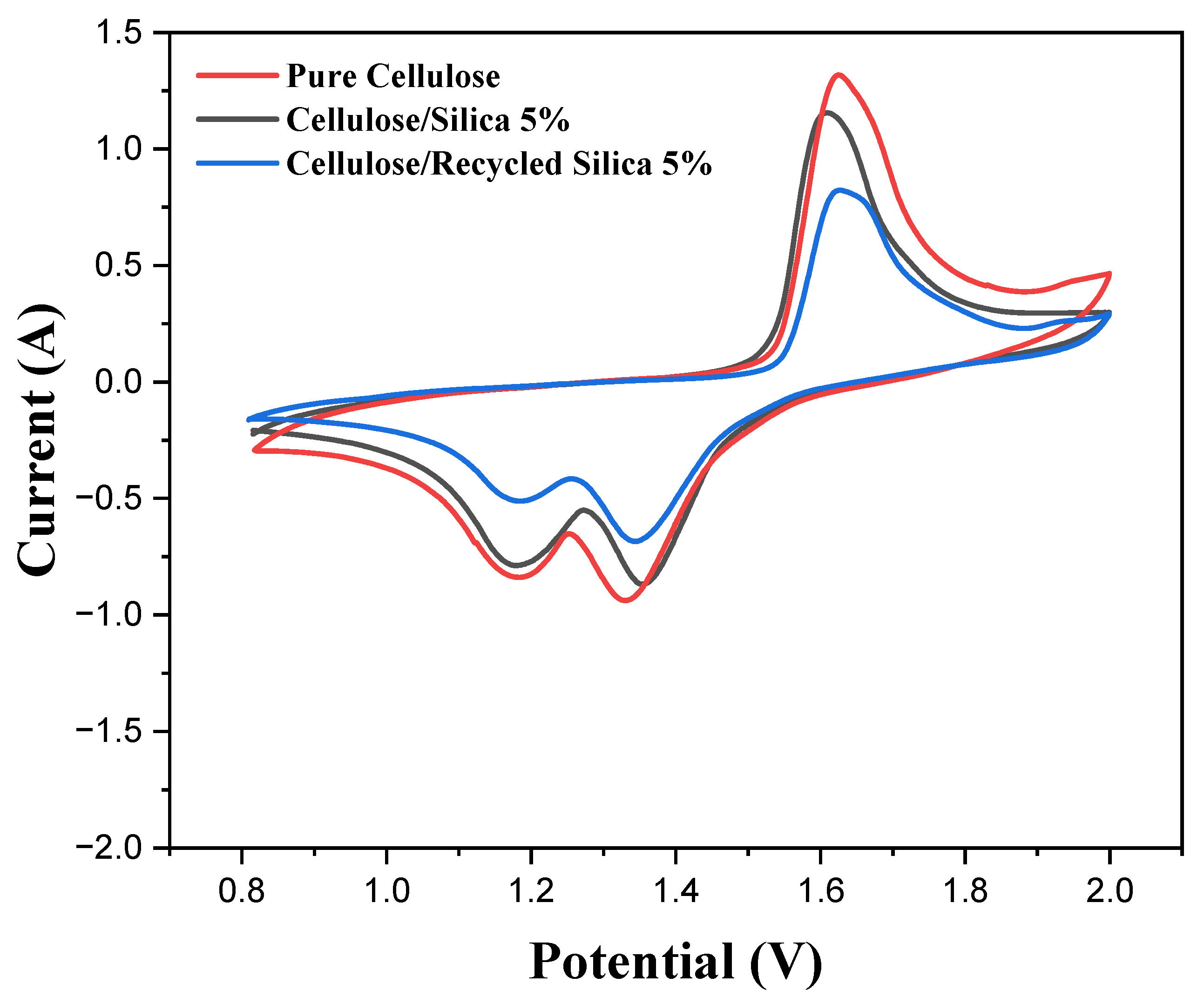
3.4. Electrochemical Testing of ZIBs
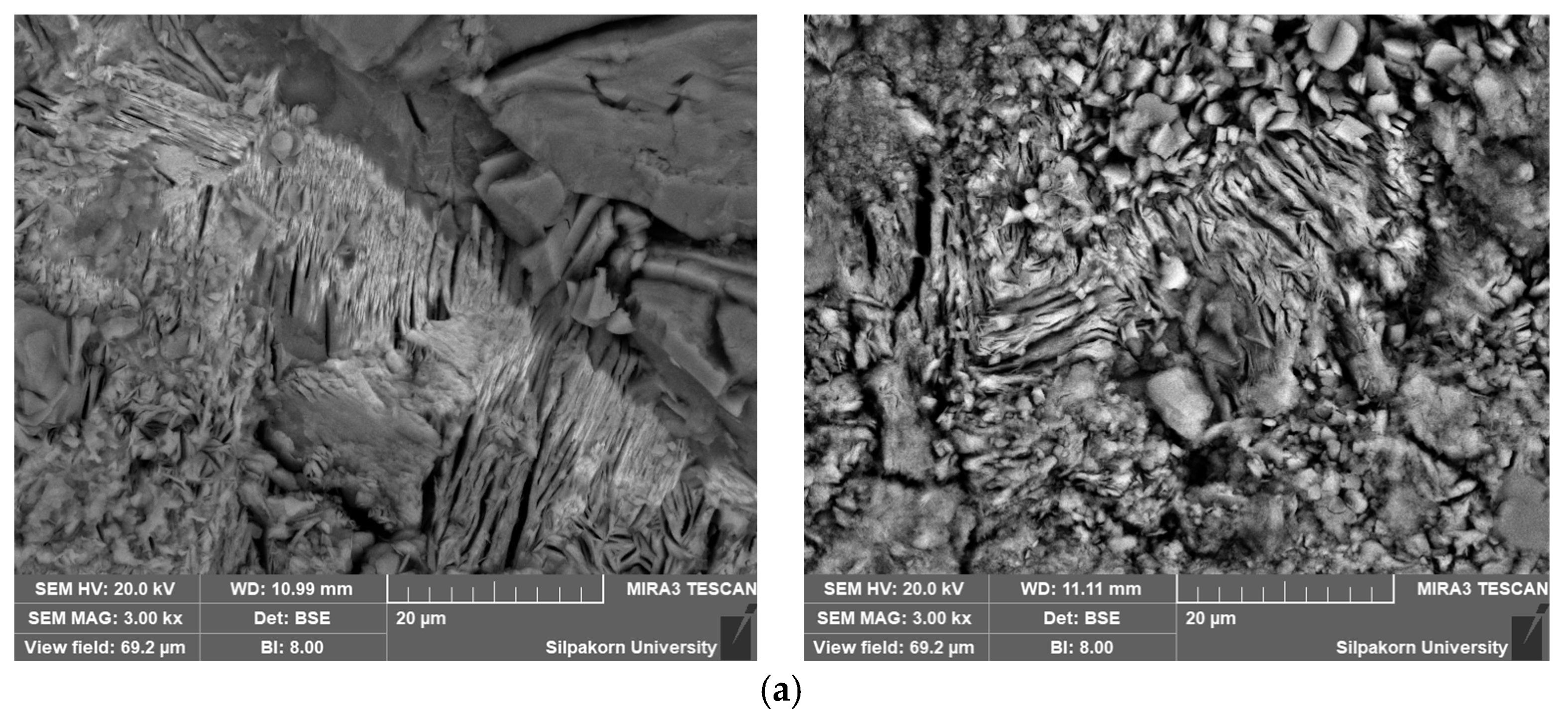
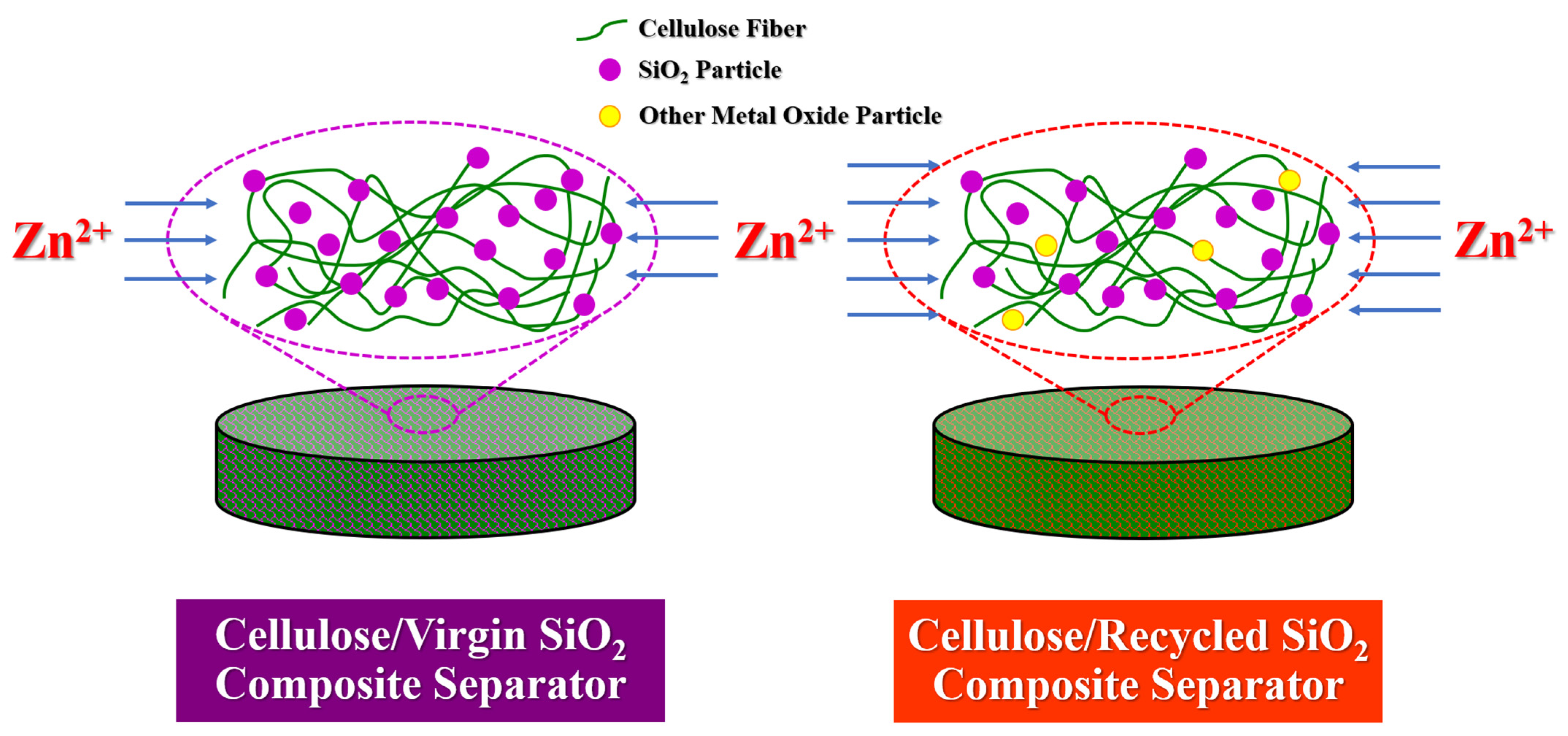
3.5. Integration of Ceramic Particles in Sustainable Separator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZIB | Zinc-ion battery |
| LIB | Lithium-ion battery |
| PE | Polyethylene |
| PP | Polypropylene |
| PAM | Polyacrylamide |
| PVDF | Polyvinylidene fluoride |
| PEO | Poly(ethylene oxide) |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropene) |
| EC | Ethylene carbonate |
| DMC | Dimethyl carbonate |
| NMP | N-methyl-2-pyrrolidone |
| BMImTFSI | 1-Butyl-3-methylimidazolium bis(trifluoromethyl sulfonyl)imide |
| CB | Carbon black |
| CP | Coffee parchment |
| CSC | Coffee silverskin cellulose |
| AR | Analytical reagent |
| UT | Untreated |
| DW | Dewaxed |
| BL | Bleached |
| AL | Alkali-treated |
| SF | Sulfuric acid-treated |
| EV | Electric vehicle |
| CEI | Cathode-electrolyte interface |
| AC | Alternating current |
| LOI | Limiting oxygen index |
| WCA | Water contact angle |
| FT-IR | Fourier transform infrared |
| ATR | Attenuated total reflectance |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDX | Energy dispersive X-ray spectroscopy |
| XRF | X-ray fluorescence |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammetry |
| GCD | Galvanostatic charge–discharge |
References
- Lu, W.; Si, Y.; Zhao, C.; Chen, T.; Li, C.; Zhang, C.; Wang, K. Biomass-derived carbono applications in the field of supercapacitores: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]
- Buasri, A.; Chinaphong, T.; Muangkum, P.; Telakul, A.; Loryuenyong, V. Green synthesis of reduced graphene oxide using pomelo peel and its application in electrochromic device. AIP Conf. Proc. 2021, 2397, 070006. [Google Scholar]
- Lingappan, N.; Lee, W.; Passerini, S.; Pecht, M. A comprehensive review of separator membranes in lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 187, 113726. [Google Scholar] [CrossRef]
- Sitole, S.; Bilibana, M.P.; Ross, N. Electrochemical performance of ZnCo2O4: Versatility in applications. J. Compos. Sci. 2025, 9, 105. [Google Scholar] [CrossRef]
- Panloetparnich, W.; Loryuenyong, V.; Buasri, A. The preparation of composites between polyaniline-silver (PANI-Ag) via interfacial polymerization. IOP Conf. Ser. Mater. Sci. Eng. 2020, 965, 012015. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Usha, Z.R.; Wan, C.; Hassaan, M.M.E.; Chen, X.; Li, L. Recent progress of composite polyethylene separators for lithium/sodium batteries. J. Power Sources 2023, 564, 232853. [Google Scholar] [CrossRef]
- Buasri, A.; Ananganjanakit, T.; Peangkom, N.; Khantasema, P.; Pleeram, K.; Lakaeo, A.; Arthnukarn, J.; Loryuenyong, V. A facile route for the synthesis of reduced graphene oxide (RGO) by DVD laser scribing and its applications in the environment-friendly electrochromic devices (ECD). J. Optoelectron. Adv. Mater. 2017, 19, 492–500. [Google Scholar]
- Lin, J.-Y.; Wu, B.-D.; Hung, F.-Y. A study on the charging–discharging mechanism of all solid-state aluminum–carbon composite secondary batteries. J. Compos. Sci. 2025, 9, 166. [Google Scholar] [CrossRef]
- Pandurangan, P. Recent progression and opportunities of polysaccharide assisted bio-electrolyte membranes for rechargeable charge storage and conversion devices. Electrochem 2023, 4, 212–238. [Google Scholar] [CrossRef]
- Turossi, T.C.; Júnior, H.L.O.; Monticeli, F.M.; Dias, O.T.; Zattera, A.J. Cellulose-derived battery separators: A minireview on advances towards environmental sustainability. Polymers 2025, 17, 456. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Yu, L.; Gu, J.; Pan, C.; Zhang, J.; Wei, Z.; Zhao, Y. Recent developments of composite separators based on high-performance fibers for lithium batteries. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107132. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved strategies for separators in zinc-ion batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite separators for robust high rate lithium ion batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Wu, S.; Fu, G.; Lv, W.; Wei, J.; Chen, W.; Yi, H.; Gu, M.; Bai, X.; Zhu, L.; Tan, C.; et al. A single-step hydrothermal route to 3D hierarchical Cu2O/CuO/rGO nanosheets as high-performance anode of lithium-ion batteries. Small 2018, 14, 1702667. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Lin, J.; Yang, Y.; Chen, Y.; Zeng, G. Recent advances in cellulose-based separators for zinc ion batteries: A review. Int. J. Biol. Macromol. 2025, 306, 141326. [Google Scholar] [CrossRef]
- Dong, N.; Zhang, F.; Pan, H. Towards the practical application of Zn metal anodes for mild aqueous rechargeable Zn batteries. Chem. Sci. 2022, 13, 8243–8252. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Yang, X.; Liu, J.; Liu, X.-X.; Sun, X. High-voltage manganese oxide cathode with two-electron transfer enabled by a phosphate proton reservoir for aqueous zinc batteries. ACS Energy Lett. 2022, 7, 1814–1819. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Yang, D.; Tan, H.; Rui, X.; Yu, Y. Electrode materials for rechargeable zinc-ion and zinc-air batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 395–427. [Google Scholar] [CrossRef]
- Mageto, T.; Bhoyate, S.D.; Mensah-Darkwa, K.; Kumar, A.; Gupta, R.K. Development of high-performance zinc-ion batteries: Issues, mitigation strategies, and perspectives. J. Energy Storage 2023, 70, 108081. [Google Scholar] [CrossRef]
- Panloetparnich, W.; Loryuenyong, V.; Buasri, A. Facile synthesis of polyaniline-nickel oxide composites via interfacial polymerization. Mater. Today Proc. 2021, 52, 2485–2489. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Nguyen, H.T.; Tran, N.H. Synthesis of ZnO nanorods and its application in zinc-silver secondary batteries. Electrochem 2023, 4, 70–83. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S. Recent advances on materials for lithium-ion batteries. Energies 2021, 14, 3145. [Google Scholar] [CrossRef]
- Serra, J.P.; Uranga, J.; Gonçalves, R.; Carlos, M.; Costa, C.M.; Caba, K.; Guerrero, P.; Lanceros-Mendez, S. Sustainable lithium-ion battery separators based on cellulose and soy protein membranes. Electrochim. Acta 2023, 462, 142746. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, G.; Zhang, X.; Feng, Y.; Li, N.; Shi, J.; Qu, X. In situ interfacial polymerization of lithiophilic COF@PP and POP@PP separators with lower shuttle effect and higher ion transport for high-performance Li–S batteries. Chem. Eng. J. 2022, 442, 136352. [Google Scholar] [CrossRef]
- Casas, X.; Niederberger, M.; Lizundia, E. A sodium-ion battery separator with reversible voltage response based on water-soluble cellulose derivatives. ACS Appl. Mater. Interfaces 2020, 12, 29264–29274. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Zhuang, J.; Yuan, Y.; Wang, W. Cellulose microspheres enhanced polyvinyl alcohol separator for high-performance lithium-ion batteries. Carbohydr. Polym. 2023, 300, 120231. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, H.Q.; Jin, G.; Liu, S.; Wu, J.W.; Wu, H.; Yang, Y.Q.; Wang, Q.; Wang, S. Cellulose-based electrolytes for advanced lithium-ion batteries: Recent advances and future perspectives. ChemNanoMat 2022, 8, e202200142. [Google Scholar] [CrossRef]
- Lunsamrong, S.; Wongnak, N.; Sahavattarnakorn, G.; Buasri, A. Application of rice straw cellulose for oil-water separation. Adv. Sci. Technol. 2024, 150, 31–37. [Google Scholar] [CrossRef]
- Sant’Ana Júnior, D.B.; Kelbert, M.; Hermes de Araújo, P.H.; de Andrade, C.J. Physical pretreatments of lignocellulosic biomass for fermentable sugar production. Sustain. Chem. 2025, 6, 13. [Google Scholar] [CrossRef]
- Islam, M.d.A.; Ong, H.L.; Villagracia, A.R.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.-A. Biomass–derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crops Prod. 2021, 170, 113694. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, L.; Liu, J.; Wu, L. Multi-functionalization of cotton fabrics with excellent flame retardant, antibacterial and superhydrophobic properties. Int. J. Biol. Macromol. 2024, 254, 127889. [Google Scholar] [CrossRef]
- Raafat, L.; Wicklein, B.; Majer, G.; Jahnke, T.; Diem, A.M.; Bill, J.; Burghard, Z. Shape-conformable, eco-friendly cellulose aerogels as high-performance battery separators. ACS Appl. Energy Mater. 2021, 4, 763–774. [Google Scholar] [CrossRef]
- Ren, J.; Guo, J.; Luo, X.; Dai, X.; Zhang, G.; Ping, P.; Kong, D. A sustainable green strategy: Flame-retardant cellulose-based separators for enhancing the safety and cycle stability of lithium-ion batteries. J. Power Sources 2025, 628, 235862. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Ruenroengrit, K.; Kunyuan, J.; Ruttanadech, N.; Kaewtrakulchai, N.; Puengjinda, P.; Chaiammart, N.; Chutipaijit, S.; Buasri, A.; Fuji, M.; Eiad-ua, A.; et al. Coconut residue-derived nanoporous carbon via hydrothermal carbonization for nanoporous carbon-based supercapacitor electrodes. Polymers 2025, 17, 1752. [Google Scholar] [CrossRef]
- Malarat, S.; Khongpun, D.; Limtong, K.; Sinthuwong, N.; Soontornapaluk, P.; Sakdaronnarong, C.; Posoknistakul, P. Preparation of nanocellulose from coffee pulp and its potential as a polymer reinforcement. ACS Omega 2023, 8, 25122–25133. [Google Scholar] [CrossRef]
- Dominici, F.; García, D.G.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-polyethylene-based composites reinforced with alkali and palmitoyl chloride-treated coffee silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef]
- Amensisa, Y.E.; Demsash, H.D.; Tefera, M.E. Extraction and characterization of cellulose from coffee husk and brewery’s spent grain fibers using alkali-hydrogen peroxide treatment method. Adv. Mater. Sci. Eng. 2024, 2024, 5101871. [Google Scholar] [CrossRef]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef] [PubMed]
- Panyamao, P.; Charumanee, S.; Ruangsuriya, J.; Saenjum, C. Efficient isolation of cellulosic fibers from coffee parchment via natural acidic deep eutectic solvent pretreatment for nanocellulose production. ACS Sustain. Chem. Eng. 2023, 11, 13962–13973. [Google Scholar] [CrossRef]
- Campuzano, F.; Escobar, D.M.; Torres L, A.M. Physicochemical characterization of coffee parchment of species Coffea arabica variety Castillo®. Coffee Sci. 2024, 19, e192182. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Chen, T.; Wang, R.; Zhang, Z.; Hua, F.; Yang, R. Ultra-light cellulose nanofibril membrane for lithium-ion batteries. J. Membr. Sci. 2020, 595, 117550. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, X.; He, Z.; Ni, Y. Potential of cellulose-based materials for lithium-ion batteries (LIB) separator membranes. J. Bioresour. Bioprod. 2016, 1, 18–21. [Google Scholar]
- Zeleke, N.M.; Sinha, D.K.; Mengesha, G.A. Chemical composition and extraction of micro crystalline cellulose from outer skin isolated coffee husk. Adv. Mater. Sci. Eng. 2022, 2022, 7163359. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohydr. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Khamsanga, S.; Kheawhom, S.; Qin, J.; Pattananuwat, P. MnCo2O4 spinel microsphere assembled with flake structure as a cathode for high-performance zinc ion battery. J. Energy Storage 2023, 64, 107148. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Khamsawat, J.; Danwong, P.; Buasri, A.; Pattananuwat, P. Application of coffee silverskin cellulose/polyacrylamide gel polymer electrolytes for rechargeable zinc-ion batteries. Sci 2024, 6, 50. [Google Scholar] [CrossRef]
- Silva, I.G.C.B.; Antonio, A.S.; Carvalho, E.M.; Santos, G.R.C.; Pereira, H.M.G.; Junior, V.F.V. Method optimization for the extraction of chlorogenic acids from coffee parchment: An ecofriendly alternative. Food Chem. 2024, 458, 139842. [Google Scholar] [CrossRef]
- Manosong, W.; Nuanla-ong, M.; Udomvech, A. Isolation of fiber-cellulose and characterization from oil palm frond for 3D and 4D printing materials application. ASEAN J. Sci. Technol. Rep. 2024, 27, e253216. [Google Scholar] [CrossRef]
- Bozaci, E.; Altınışık Tağaç, A. Extraction and characterization of new cellulosic fiber from Catalpa bignonioides fruits for potential use in sustainable products. Polymers 2023, 15, 201. [Google Scholar] [CrossRef]
- Kassab, Z.; Kassem, I.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; Achaby, M.E. Tomato plant residue as new renewable source for cellulose production: Extraction of cellulose nanocrystals with different surface functionalities. Cellulose 2020, 27, 4287–4303. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Yahya, E.B.; Abdul Khalil, H.P.S.; Shaah, M.A.; Suriani, A.B.; Mohamed, A.; Alfatah, T.; Abdullah, C.K. Extraction and isolation of cellulose nanofibers from carpet wastes using supercritical carbon dioxide approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019, 92, 1987–1996. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Fonseca, C.S.; Silva Gomes, M.; Rompa, V.D.; Martins, M.A.; Tonoli, G.H.D.; Mendes, L.M. How the surface wettability and modulus of elasticity of the Amazonian paricá nanofibrils films are affected by the chemical changes of the natural fibers. Eur. J. Wood Wood Prod. 2018, 76, 1581–1594. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 2019, 26, 5831–5849. [Google Scholar] [CrossRef]
- Gupta, V.; Ramakanth, D.; Verma, C.; Maji, P.K.; Gaikwad, K.K. Isolation and characterization of cellulose nanocrystals from amla (Phyllanthus emblica) pomace. Biomass Convers. Biorefinery 2023, 13, 15451–15462. [Google Scholar] [CrossRef]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and characterization of carboxymethyl cellulose-based composite films reinforced by cellulose nanocrystals derived from pea hull waste for food packaging applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Meng, X.; Zhao, J.; Miao, M.; Shi, L.; Zhang, S.; Fang, J. Extraction and preparation of cellulose nanocrystals from dealginate kelp residue: Structures and morphological characterization. Cellulose 2015, 22, 1763–1772. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L.; Gauss, C. The effects of alkaline digestion, bleaching and ultrasonication treatment of fibre on 3D printed harakeke fibre reinforced polylactic acid composites. Compos. Part A Appl. Sci. Manuf. 2023, 166, 107384. [Google Scholar] [CrossRef]
- Souza, N.F.; Pinheiro, J.A.; Silva, P.; Morais, J.P.S.; Souza Filho, M.M.; Brígida, A.I.S.; Muniz, C.R.; Freitas Rosa, M. Development of chlorine-free pulping method to extract cellulose nanocrystals from pressed oil palm mesocarp fibers. J. Biobased Mater. Bioenergy 2015, 9, 372–379. [Google Scholar] [CrossRef]
- Zope, G.; Goswami, A.; Kulkarni, S. Isolation and characterization of cellulose nanocrystals produced by acid hydrolysis from banana pseudostem. BioNanoScience 2022, 12, 463–471. [Google Scholar] [CrossRef]
- Kader, A.H.A.; Dacrory, S.; Khattab, T.A.; Kamel, S.; Abou-Yousef, H. Hydrophobic and flame-retardant foam based on cellulose. J. Polym. Environ. 2022, 30, 2366–2377. [Google Scholar] [CrossRef]
- Jo, J.H.; Jo, C.-H.; Qiu, Z.; Yashiro, H.; Shi, L.; Wang, Z.; Yuan, S.; Myung, S.-T. Nature-derived cellulose-based composite separator for sodium-ion batteries. Front. Chem. 2020, 8, 153. [Google Scholar] [CrossRef]
- Durgadevi, N.; Swarnalatha, V. Polythiophene functionalized hydrophobic cellulose kitchen wipe sponge and cellulose fabric for effective oil–water separation. RSC Adv. 2017, 7, 34866. [Google Scholar] [CrossRef]
- Meng, X.; Dong, Y.; Zhao, Y.; Liang, L. Preparation and modification of cellulose sponge and application of oil/water separation. RSC Adv. 2020, 10, 41713. [Google Scholar] [CrossRef]
- Ding, G.; Qin, B.; Liu, Z.; Zhang, J.; Zhang, B.; Hu, P.; Zhang, C.; Xu, G.; Yao, J.; Cui, G. A polyborate coated cellulose composite separator for high performance lithium ion batteries. J. Electrochem. Soc. 2015, 162, A834–A838. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H.; Du, Z.; Liu, Y.; Sun, Q.; Li, H. A high-safety, flame-retardant cellulose-based separator with encapsulation structure for lithium-ion battery. SmartMat 2023, 4, e1182. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Yin, X.; Li, S.; Li, B.; Zhao, N.; Zhu, J.; Dai, L.; Wang, L.; He, Z.; et al. Recent advances in cellulose-based separators for zinc-based batteries: Performances, mechanism and perspectives. Green Energy Environ. 2025, in press. [CrossRef]
- Niu, J.; Cao, J.; Zhang, X.; Zhang, D.; Yang, C.; Lolupiman, K.; Zeng, Z.; Zhang, X.; Qin, J. Titanium nitride–cellulose nanofiber composite separator for Zn anode stability in aqueous batteries. ACS Appl. Energy Mater. 2024, 7, 7496–7504. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, K.; Pei, P.; Wei, M.; Liu, X.; Xiao, Y.; Zhang, P. Zinc dendrite growth and inhibition strategies. Mater. Today Energy 2021, 20, 100692. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Fan, L.; Shuai, Y.; Zhang, N. Ultrathin and super-tough membrane for antidendrite separator in aqueous zinc-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100824. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, L.; Huang, W.; Jia, H.; Zhao, Q.; Wang, Y.; Fei, B.; Pan, F. Simultaneously regulating uniform Zn2+ flux and electron conduction by MOF/rGO interlayers for high-performance Zn anodes. Nano-Micro Lett. 2021, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Xu, F.; Alcoutlabi, M.; Mao, Y.; Lozano, K. Fibrous cellulose membrane mass produced via forcespinning® for lithium-ion battery separators. Cellulose 2015, 22, 1311–1320. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Zhang, X.; Okhawilai, M.; Wang, S.; Han, J.; Qin, J.; Huang, Y. Modulating Zn deposition via ceramic-cellulose separator with interfacial polarization effect for durable zinc anode. Nano Energy 2021, 89, 106322. [Google Scholar] [CrossRef]
- Pavlin, N.; Hribernik, S.; Kapun, G.; Talian, S.D.; Njel, C.; Dedryvère, R.; Dominko, R. The role of cellulose based separator in lithium sulfur batteries. J. Electrochem. Soc. 2019, 166, A5237. [Google Scholar] [CrossRef]
- Yao, M.; Yuan, Z.; Li, S.; He, T.; Wang, R.; Yuan, M.; Niu, Z. Scalable assembly of flexible ultrathin all-in-one zinc-ion batteries with highly stretchable, editable, and customizable functions. Adv. Mater. 2021, 33, 2008140. [Google Scholar] [CrossRef]
- Deng, L.; Sun, K.; Liu, J.; Li, Z.; Cao, J.; Liao, S. High performance aqueous zinc-ion batteries developed by PANI intercalation strategy and separator engineering. Molecules 2024, 29, 3147. [Google Scholar] [CrossRef]
- Qu, H.; Guo, W.; Li, W.; Shao, L.; Chen, Y.; Su, S.; Hang, L.; Jiang, G. Bifunctional high-strength and anti-shuttling separator based on negatively-charged-cellulose-nanofibers for high-energy and stable flexible Zn-I2 batteries. Appl. Surf. Sci. 2025, 686, 162180. [Google Scholar] [CrossRef]
- Zhou, H.; Gu, J.; Wei, Y.; Zhang, W.; Kang, J.; Huang, J.-Q.; Zhang, B.; Hu, C.; Lin, X. Cellulose filter papers derived separator featuring effective ion transferring channels for sodium-ion batteries. J. Power Sources 2023, 558, 232649. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z.-S. Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Yang, P.; Yuan, W.; Chen, J. Tris(pentafluoro)phenylborane electrolyte additive regulates the highly stable and uniform CEI membrane components to improve the high-voltage behaviors of NCM811 lithium-ion batteries. J. Colloid Interface Sci. 2024, 676, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ono, L.K.; Qi, Y. Understanding the active formation of a cathode–electrolyte interphase (CEI) layer with energy level band bending for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 221–231. [Google Scholar] [CrossRef]
- Liu, Y.; He, H.; Gao, A.; Ling, J.; Yi, F.; Hao, J.; Li, Q.; Shu, D. Fundamental study on Zn corrosion and dendrite growth in gel electrolyte towards advanced wearable Zn-ion battery. Chem. Eng. J. 2022, 446, 137021. [Google Scholar] [CrossRef]
- Hu, F.; Li, M.; Gao, G.; Fan, H.; Ma, L. The gel-state electrolytes in zinc-ion batteries. Batteries 2022, 8, 214. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Li, X.; Liao, T.; Zhai, J.; Wang, X.; Huo, K. Biomass-based functional separators for rechargeable batteries. Battery Energy 2024, 3, 20240015. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Juikatu, P.; Sirisukha, P.; Kumleing, U.; Buasri, A. The preparation of luminescent and reversible thermochromic Mn-doped Ca-Zn-Al-O inorganic materials. J. Asian Ceram. Soc. 2022, 10, 597–603. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Charoensuk, J.; Charupongtawitch, R.; Usakulwattana, A.; Buasri, A. Kinetics of photocatalytic degradation of methylene blue by TiO2-graphene nanocomposites. J. Nanosci. Nanotechnol. 2016, 16, 296–302. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. Cellulose: Characteristics and applications for rechargeable batteries. Int. J. Biol. Macromol. 2022, 219, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Aruchamy, K.; Ramasundaram, S.; Divya, S.; Chandran, M.; Yun, K.; Oh, T.H. Gel polymer electrolytes: Advancing solid-state batteries for high-performance applications. Gels 2023, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, L.; Xue, J.; Su, M.; Zhang, F.; Ding, L.; Wang, S.; Wang, H. Nano-alumina@cellulose-coated separators with the reinforced-concrete-like structure for high-safety lithium-ion batteries. Chin. J. Chem. Eng. 2024, 68, 83–93. [Google Scholar] [CrossRef]
- Muench, S.; Burges, R.; Lex-Balducci, A.; Brendel, J.C.; Jäger, M.; Friebe, C.; Wild, A.; Schubert, U.S. Printable ionic liquid-based gel polymer electrolytes for solid state all-organic batteries. Energy Storage Mater. 2020, 25, 750–755. [Google Scholar] [CrossRef]
- Lei, B.; Li, G.-R.; Chen, P.; Gao, X.-P. A Quasi-solid-state solar rechargeable battery with polyethylene oxide gel electrolyte. ACS Appl. Energy Mater. 2019, 2, 1000–1005. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, X.; Fu, C.; Tu, J.; Tan, Y.; Jiao, S. Room temperature solid state dual-ion batteries based on gel electrolytes. J. Mater. Chem. A 2018, 6, 4313–4323. [Google Scholar] [CrossRef]











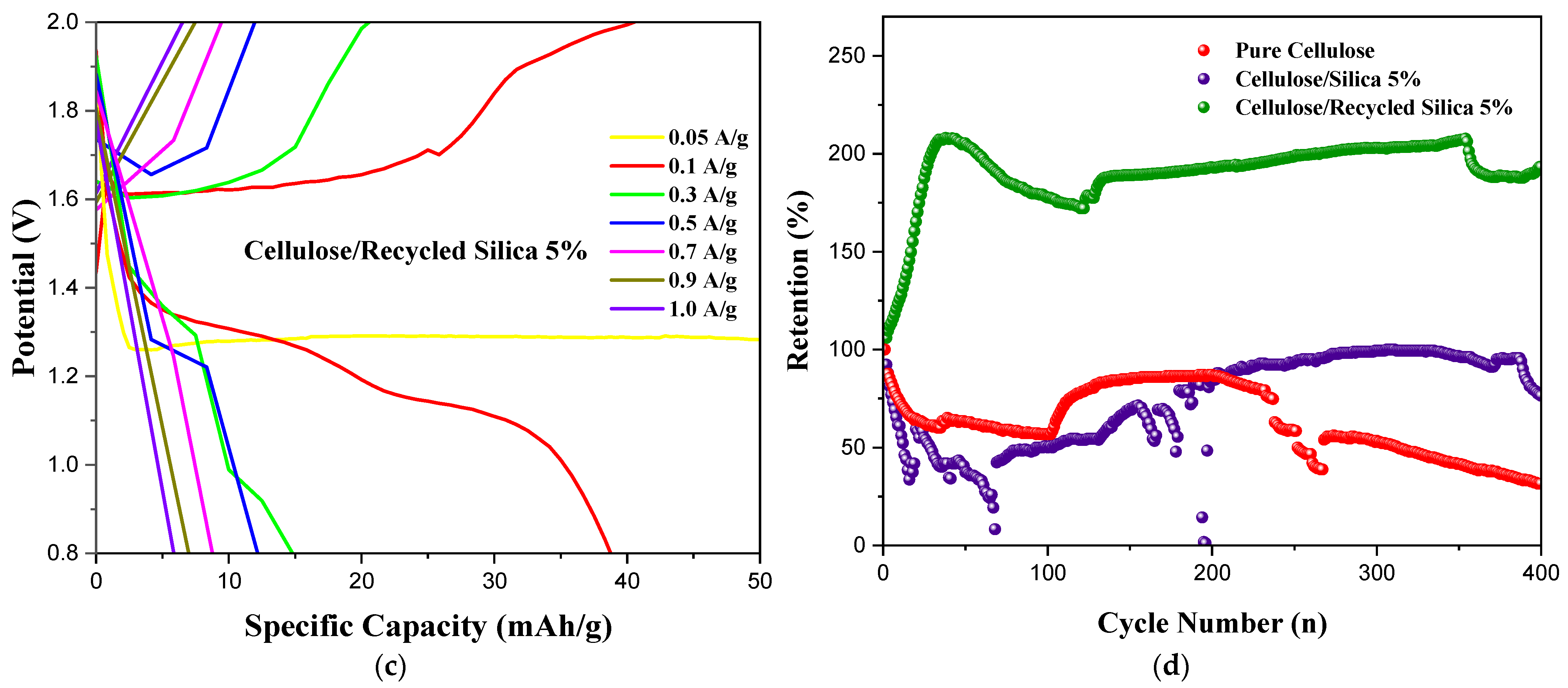
| Polymer Separator | Fabrication Technique | Specific Capacity (mAh/g) | Cyclic Stability | Ionic Conductivity (mS/cm) | Reference |
|---|---|---|---|---|---|
| Cellulose/Recycled SiO2/ZnSO4 | Solution/Vacuum Filtration | 24 | 190% After 400 Cycles | 0.274 | This Work |
| Cellulose/Virgin SiO2/ZnSO4 | Solution/Vacuum Filtration | 13 | 78% After 400 Cycles | 0.195 | This Work |
| Coffee Silverskin Cellulose (CSC)/Polyacrylamide (PAM)/ZnSO4 | In Situ Polymerization | 37 | 50% After 200 Cycles | 9.10 | [53] |
| Cellulose-PE/Al2O3/Lithium Hexafluorophosphate (LiPF6)/Ethylene Carbonate (EC)/Dimethyl Carbonate (DMC) | Slurry Coating | 110 | 100% After 200 Cycles | 0.502 | [97] |
| Methacrylate Polymer/1-Butyl-3-Methylimidazolium bis(Trifluoromethyl Sulfonyl)Imide (BMImTFSI) | In Situ Polymerization | 24 | 77% After 1000 Cycles | 740 | [98] |
| Poly(Ethylene Oxide) (PEO)/Lithium Perchlorate (LiClO4) | Solution Casting | 8 | 86% After 30 Cycles | 0.39 | [99] |
| Poly(Vinylidene Fluoride-co-Hexafluoropropene) (PVDF-HFP)/LiPF6 | Phase Inversion | 80 | 82% After 1000 Cycles | 2.40 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loryuenyong, V.; Plongmai, B.; Pajantorn, N.; Pattananuwat, P.; Buasri, A. Sustainable Zinc-Ion Battery Separators Based on Silica and Cellulose Fibers Derived from Coffee Parchment Waste. J. Compos. Sci. 2025, 9, 452. https://doi.org/10.3390/jcs9080452
Loryuenyong V, Plongmai B, Pajantorn N, Pattananuwat P, Buasri A. Sustainable Zinc-Ion Battery Separators Based on Silica and Cellulose Fibers Derived from Coffee Parchment Waste. Journal of Composites Science. 2025; 9(8):452. https://doi.org/10.3390/jcs9080452
Chicago/Turabian StyleLoryuenyong, Vorrada, Buntita Plongmai, Nitikorn Pajantorn, Prasit Pattananuwat, and Achanai Buasri. 2025. "Sustainable Zinc-Ion Battery Separators Based on Silica and Cellulose Fibers Derived from Coffee Parchment Waste" Journal of Composites Science 9, no. 8: 452. https://doi.org/10.3390/jcs9080452
APA StyleLoryuenyong, V., Plongmai, B., Pajantorn, N., Pattananuwat, P., & Buasri, A. (2025). Sustainable Zinc-Ion Battery Separators Based on Silica and Cellulose Fibers Derived from Coffee Parchment Waste. Journal of Composites Science, 9(8), 452. https://doi.org/10.3390/jcs9080452








