Collagen/Polypyrrole Biomimetic Electroactive Composite Coating with Fiber Network Structure on Titanium Surface for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Col/Ppy Composite Films
2.2. Characterization of the Composite Films
2.3. Cell Culture
2.4. Cell Viability Assay and Alkaline Phosphatase (ALP) Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Materialistic Characterization of the Composite Films
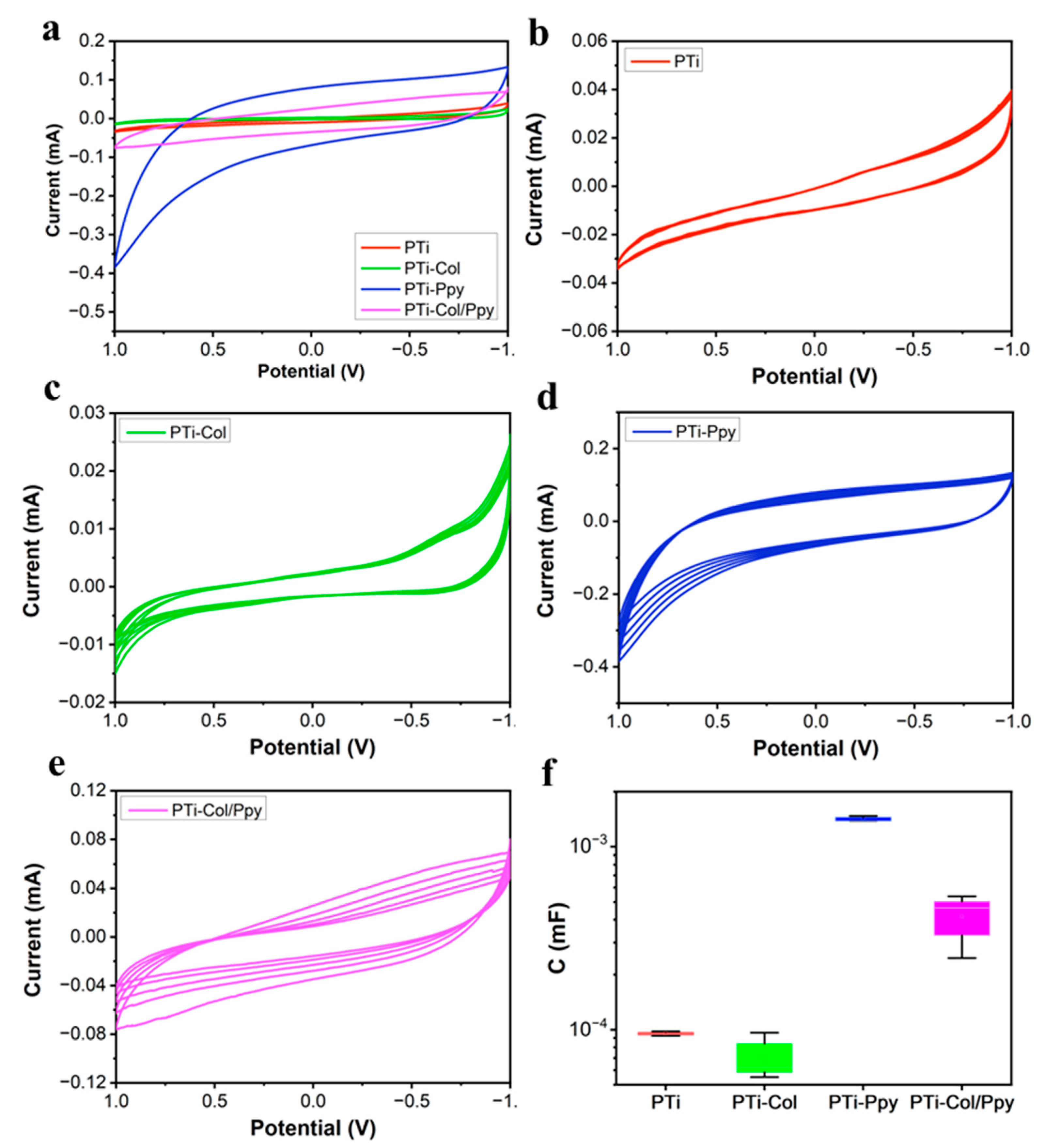
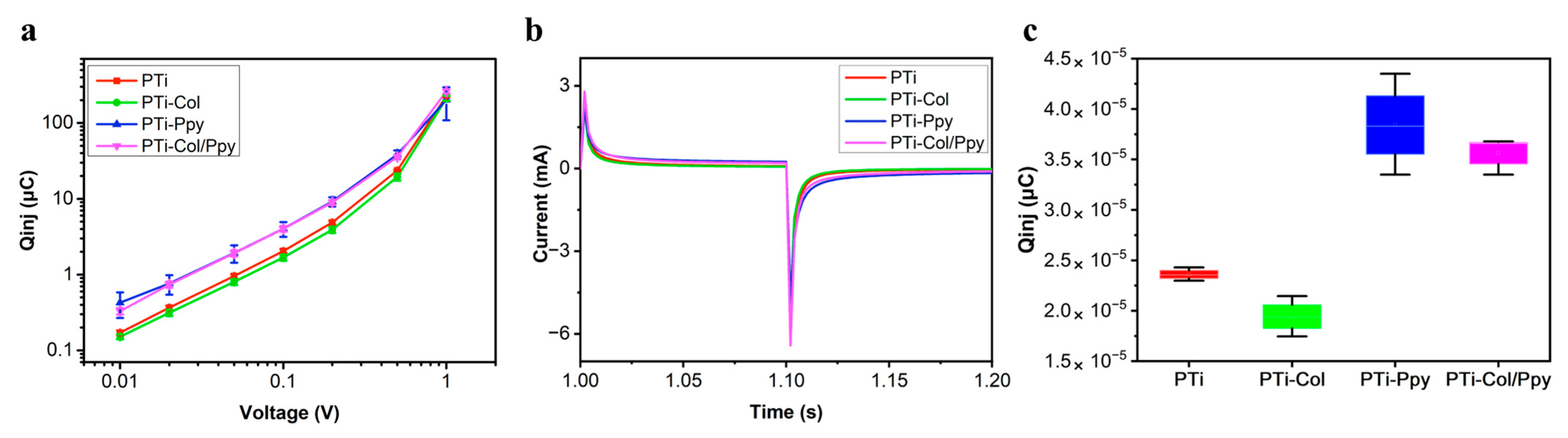
3.2. Electrochemical Performance of the Composite Films
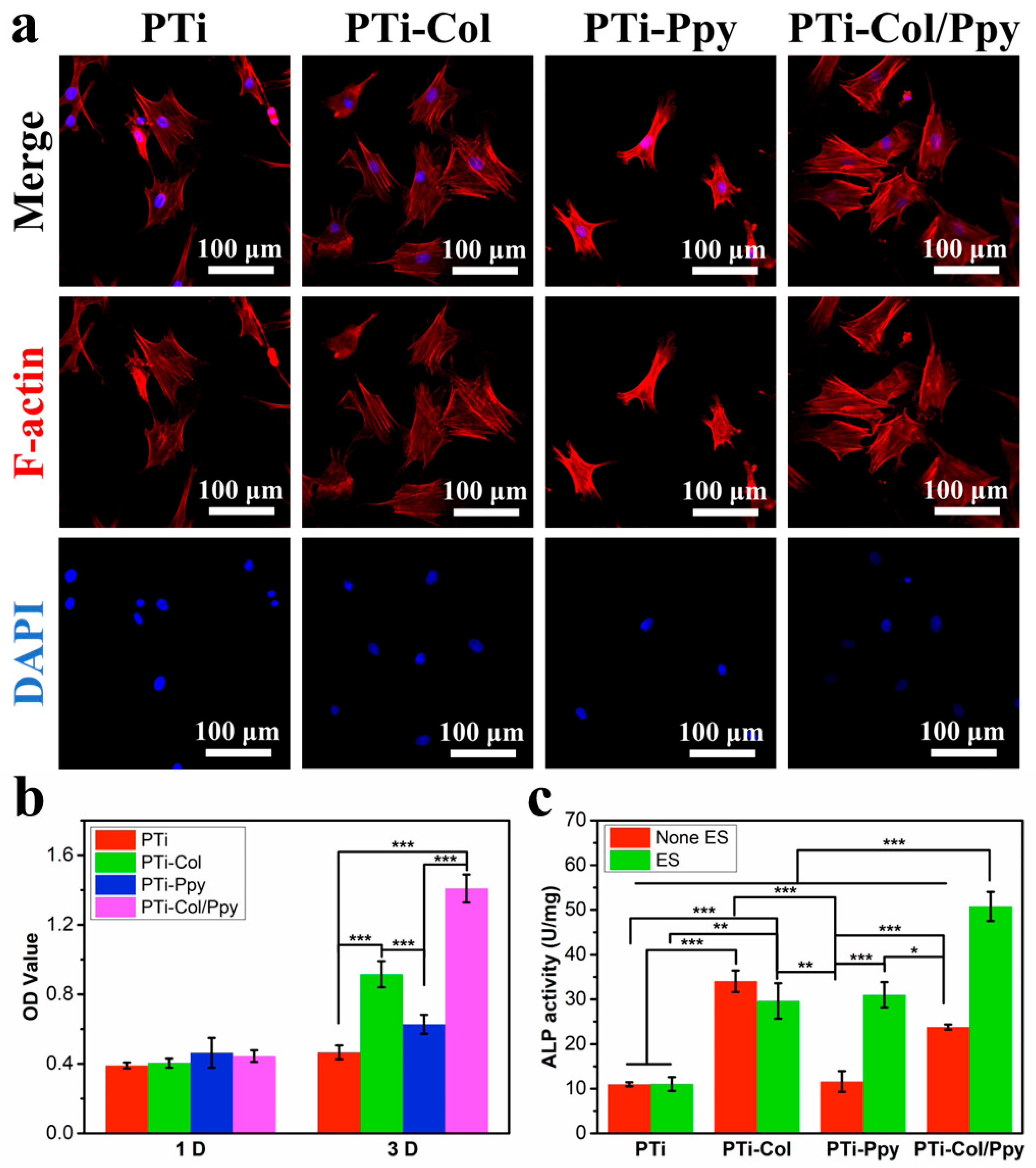
3.3. Biological Effects of the Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharm.-Base 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Tian, Y.; Panayi, A.C.; Mi, B.; Liu, G. Recent Advances in Enhancement Strategies for Osteogenic Differentiation of Mesenchymal Stem Cells in Bone Tissue Engineering. Front. Cell Dev. Biol. 2022, 10, 824812. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic Delivery of Signals for Bone Tissue Engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, Z.; He, X.; Zhu, Y.; Xu, X.; Yang, H.; Mei, G.; Chen, S.; Ma, B.; Zhu, R. Application of Bioactive Materials for Osteogenic Function in Bone Tissue Engineering. Small Methods 2024, 8, 2301283. [Google Scholar] [CrossRef]
- Sivakumar, P.M.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Bone Tissue Engineering: Anionic Polysaccharides as Promising Scaffolds. Carbohydr. Polym. 2022, 283, 119142. [Google Scholar] [CrossRef]
- Cao, Z.; Bian, Y.; Hu, T.; Yang, Y.; Cui, Z.; Wang, T.; Yang, S.; Weng, X.; Liang, R.; Tan, C. Recent Advances in Two-Dimensional Nanomaterials for Bone Tissue Engineering. J. Mater. 2023, 9, 930–958. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 917. [Google Scholar] [CrossRef]
- Aykora, D.; Uzun, M. Bone Tissue Engineering for Osteointegration: Where are we Now? Polym. Bull. 2024, 81, 8595–8605. [Google Scholar] [CrossRef]
- Gholami, K.; Ege, F.; Barzegar, R. Prediction of Composite Mechanical Properties: Integration of Deep Neural Network Methods and Finite Element Analysis. J. Compos. Sci. 2023, 7, 54. [Google Scholar] [CrossRef]
- Cui, J.; Yu, B.; Li, D.; Fu, Z.; Yang, X.; Jiang, L.; Wang, X.; Lin, K. Remodeling Electrophysiological Microenvironment for Promoting Bone Defect Repair Via Electret Hybrid Electrospun Fibrous Mat. Adv. Fiber Mater. 2024, 6, 1855–1873. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, Z.; Wu, F.; Zhu, Z.; Liu, J.; Qi, X.; Li, Q.; Wang, X.; Ma, Q.; Xiang, H. Advances in Self-Powered Biomaterials for Bone Defect Repair. Adv. Compos. Hybrid Mater. 2024, 8, 38. [Google Scholar] [CrossRef]
- Lv, Z.; Ji, Y.; Wen, G.; Liang, X.; Zhang, K.; Zhang, W. Structure-Optimized and Microenvironment-Inspired Nanocomposite Biomaterials in Bone Tissue Engineering. Burn. Trauma 2024, 12, tkae036. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.; He, X.; Fan, S.; Liu, P.; Wu, C.; Cheng, K. Pvtf Nanoparticles/Pla Electroactive Degradable Membrane for Bone Tissue Regeneration. Coatings 2024, 14, 115. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Zhang, S.; Mei, X.; Ying, B.; Li, R.; Qin, Y. Ecm-Inspired 3D Printed Polyetherimide Scaffold with Arg-Gly-Asp Peptides for the Improvement of Bioactivity and Osteogenic Differentiation of Osteoblasts. Mater. Today Commun. 2022, 30, 103166. [Google Scholar] [CrossRef]
- Jin, Z.; Lu, B.; And Xu, Y. Constructing an Electrical Microenvironment Based on Electroactive Polymers in the Field of Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 937–967. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, L.; Wang, Z.; Xu, F.; Gu, G.; Cui, F.; Guo, Z. Recent Progress in the Research of Biomaterials Regulating Cell Behavior. Rsc Adv. 2014, 4, 63807–63816. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular Matrix-Based Materials for Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Oliver-Cervelló, L.; Martin-Gómez, H.; Gonzalez-Garcia, C.; Salmeron-Sanchez, M.; Ginebra, M.; Mas-Moruno, C. Protease-Degradable Hydrogels with Multifunctional Biomimetic Peptides for Bone Tissue Engineering. Front. Bioeng. Biotech. 2023, 11, 1192436. [Google Scholar] [CrossRef]
- Zhou, H.; Li, W.; Pan, L.; Zhu, T.; Zhou, T.; Xiao, E.; Wei, Q. Human Extracellular Matrix (Ecm)-like Collagen and its Bioactivity. Regen. Biomater. 2024, 11, rbae008. [Google Scholar] [CrossRef] [PubMed]
- McColgan-Bannon, K.I.S.; Upson, S.; Gentile, P.; Tausif, M.; Russell, S.; Dalgarno, K.; Ferreira, A.M. Biomimetic Properties of Force-Spun Phbv Membranes Functionalised with Collagen as Substrates for Biomedical Application. Coatings 2019, 9, 350. [Google Scholar] [CrossRef]
- Li, W.; Chi, N.; Clutter, E.D.; Zhu, B.; Wang, R.R. Aligned Collagen-Cnt Nanofibrils and the Modulation Effect on Ovarian Cancer Cells. J. Compos. Sci. 2021, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Lin, Z.; Tao, Y.; Huang, Y.; Xu, T.; Niu, W. Applications of Marine Collagens in Bone Tissue Engineering. Biomed. Mater. 2021, 16, 042007. [Google Scholar] [CrossRef]
- Tanrikulu, I.C.; Dang, L.; Nelavelli, L.; Ellison, A.J.; Olsen, B.D.; Jin, S.; Raines, R.T. Synthetic Collagen Hydrogels through Symmetric Self-Assembly of Small Peptides. Adv. Sci. 2024, 11, 2303228. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Que, R.A.; Crakes, D.R.; Abdulhadi, F.; Niu, C.; Da Silva, N.A.; Wang, S. Tailoring Collagen to Engineer the Cellular Microenvironment. Biotechnol. J. 2018, 13, 1800140. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv. Mater. 2011, 23, H90–H94. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhu, T.; Xu, F.; Wang, Z.; Zheng, Y.; Tang, Q.; Chen, L.; Shen, Y.; Zhu, J. Neural Stem/Progenitor Cells on Collagen with Anchored Basic Fibroblast Growth Factor as Potential Natural Nerve Conduits for Facial Nerve Regeneration. Acta Biomater. 2017, 50, 188–197. [Google Scholar] [CrossRef]
- Mohammad, F.M.B.; Yogeswaran, L. Recent Development in the Fabrication of Collagen Scaffolds for Tissue Engineering Applications: A Review. Curr. Pharm. Biotechnol. 2019, 20, 992–1003. [Google Scholar]
- Bonfrate, V.; Manno, D.; Serra, A.; Salvatore, L.; Sannino, A.; Buccolieri, A.; Serra, T.; Giancane, G. Enhanced Electrical Conductivity of Collagen Films through Long-Range Aligned Iron Oxide Nanoparticles. J. Colloid. Interf. Sci. 2017, 501, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, V.F.; Brown, C.P. Hierarchical Piezoresponse in Collagen. Adv. Mater. Technol. 2022, 7, 2101166. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, 2007429. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I. Electrical Stimulation as a Novel Tool for Regulating Cell Behavior in Tissue Engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J. Research Trends of Piezoelectric Biomaterials in Osteochondral Tissue Engineering. Mater. Today Commun. 2024, 41, 110264. [Google Scholar] [CrossRef]
- Tandon, B.; Magaz, A.; Balint, R.; Blaker, J.J.; Cartmell, S.H. Electroactive Biomaterials: Vehicles for Controlled Delivery of Therapeutic Agents for Drug Delivery and Tissue Regeneration. Adv. Drug Deliv. Rev. 2018, 129, 148–168. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, L.; Liu, Z.; Weng, W.; Cheng, K. Electrical Potential Specified Release of Bsa/Hep/Polypyrrole Composite Film and its Cellular Responses. Acs Appl. Mater. Inter. 2019, 11, 25457–25464. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically Conductive Polydopamine–Polypyrrole as High Performance Biomaterials for Cell Stimulation In Vitro and Electrical Signal Recording In Vivo. Acs Appl. Mater. Inter. 2018, 10, 33032–33042. [Google Scholar] [CrossRef]
- Elyashevich, G.; Rosova, E.; Zoolshoev, Z.; Saprykina, N.; Kuryndin, I. Reversibility of Swelling, Ph Sensitivity, Electroconductivity, and Mechanical Properties of Composites Based on Polyacrylic Acid Hydrogels and Conducting Polymers. J. Compos. Sci. 2023, 7, 261. [Google Scholar] [CrossRef]
- Wu, C.; He, X.; Weng, W.; Zhang, T.; Huang, D.; Cheng, K.; Chen, Z. Electroactive Extracellular Matrix/Polypyrrole Composite Films and their Microenvironmental Effects on Osteogenic Differentiation of Bmscs. Chem. Eng. J. 2022, 443, 136508. [Google Scholar] [CrossRef]
- Kim, D.H.; Richardson-Burns, S.M.; Hendricks, J.L.; Sequera, C.; Martin, D.C. Effect of Immobilized Nerve Growth Factor On Conductive Polymers: Electrical Properties and Cellular Response. Adv. Funct. Mater. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Da Silva, F.A.G.; Alcaraz-Espinoza, J.J.; Da Costa, M.M.; de Oliveira, H.P. Synthesis and Characterization of Highly Conductive Polypyrrole-Coated Electrospun Fibers as Antibacterial Agents. Compos. Part. B Eng. 2017, 129, 143–151. [Google Scholar] [CrossRef]
- Duchet, J.; Legras, R.; Demoustier-Champagne, S. Chemical Synthesis of Polypyrrole: Structure–Properties Relationship. Synth. Met. 1998, 98, 113–122. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, B.; Jian, W.; Santhanam, R. In Situ Cyclic Voltammetry-Surface-Enhanced Raman Spectroscopy: Studies on the Doping–Undoping of Polypyrrole Film. Thin Solid. Film. 2000, 374, 85–91. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hwang, B.J. Interaction of Copper(I)-Polypyrrole Complexes Prepared by Depositing–Dissolving Copper onto and from Polypyrroles. Thin Solid. Film. 1999, 339, 233–239. [Google Scholar] [CrossRef]
- Duan, Y.Y.; Clark, G.M.; Cowan, R.S.C. A Study of Intra-Cochlear Electrodes and Tissue Interface by Electrochemical Impedance Methods in Vivo. Biomaterials 2004, 25, 3813–3828. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.; Maiti, T.K.; et al. Electroconductive Multi-Functional Polypyrrole Composites for Biomedical Applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Xu, Y.; Xiang, S.; Zhang, X.; Zhou, H.; Zhang, H. High-Performance Pseudocapacitive Removal of Cadmium Via Synergistic Valence Conversion in Perovskite-Type Femno3. J. Hazard. Mater. 2022, 439, 129575. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Silke, M.; Qiu, W.; Xu, M.; Borghs, G.; Chen, H. Electrochemical Deposition of Polypyrrole/Graphene Oxide Composite on Microelectrodes Towards Tuning the Electrochemical Properties of Neural Probes. Sens. Actuators B Chem. 2011, 158, 176–184. [Google Scholar] [CrossRef]
- Antony, N.; Mohanty, S.; Nayak, S.K. Electrochemical Inspection of Polypyrrole/Chitosan/Zinc Oxide Hybrid Composites. J. Appl. Polym. Sci. 2020, 137, 49561. [Google Scholar] [CrossRef]
- Lin, W.; He, X.; Guo, X.; Xu, D.; Cheng, K. Biocompatible Pvtf Coatings on Ti with Improved Bonding Strength. Coatings 2023, 13, 1224. [Google Scholar] [CrossRef]
- Yow, S.; Lim, T.H.; Yim, E.K.F.; Lim, C.T.; Leong, K.W. A 3D Electroactive Polypyrrole-Collagen Fibrous Scaffold for Tissue Engineering. Polymers 2011, 3, 527–544. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, Z.; Chen, Z.; Xu, K.; Wu, C.; Duan, X.; Dong, L.; Chen, Z.; Weng, W.; Cheng, K. Accelerated Bone Regeneration on the Metal Surface through Controllable Surface Potential. Acs Appl. Mater. Interfaces 2023, 15, 46493–46503. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, L.; Li, Z.; Gao, F.; Zhang, Q.; Bianco, A.; Liu, H.; Ge, S.; Ma, B. Materials-Mediated in Situ Physical Cues for Bone Regeneration. Adv. Funct. Mater. 2024, 34, 2306534. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Wang, H.; Yang, D.; Liu, C.; Dou, W.; Jiang, X.; Deng, H.; Yang, R. Regulation of Tio2 @Pvdf Piezoelectric Nanofiber Membranes on Osteogenic Differentiation of Mesenchymal Stem Cells. Nano Energy 2023, 115, 108742. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic Field and Nano-Scaffolds with Stem Cells to Enhance Bone Regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]



| Atomic Percent (%) | C | N | O |
|---|---|---|---|
| PTi | --- | --- | --- |
| PTi-Col | 12.9 | 26.5 | 60.6 |
| PTi-Ppy | 33.9 | 23.9 | 42.2 |
| PTi-Col/Ppy | 22.6 | 23.8 | 53.6 |
| I1575/I1500 | (I1045 + I970)/(I1080 + I930) | |
|---|---|---|
| PTi | 1.002 ± 0.004 | --- |
| PTi-Col | 1.003 ± 0.010 | --- |
| PTi-Ppy | 1.086 ± 0.015 | 1.028 ± 0.008 |
| PTi-Col/Ppy | 1.069 ± 0.020 | 1.041 ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Xin, X.; He, X.; Weng, W.; Wu, C.; Cheng, K. Collagen/Polypyrrole Biomimetic Electroactive Composite Coating with Fiber Network Structure on Titanium Surface for Bone Tissue Engineering. J. Compos. Sci. 2025, 9, 325. https://doi.org/10.3390/jcs9070325
Liang Y, Xin X, He X, Weng W, Wu C, Cheng K. Collagen/Polypyrrole Biomimetic Electroactive Composite Coating with Fiber Network Structure on Titanium Surface for Bone Tissue Engineering. Journal of Composites Science. 2025; 9(7):325. https://doi.org/10.3390/jcs9070325
Chicago/Turabian StyleLiang, Yuan, Xin Xin, Xuzhao He, Wenjian Weng, Chengwei Wu, and Kui Cheng. 2025. "Collagen/Polypyrrole Biomimetic Electroactive Composite Coating with Fiber Network Structure on Titanium Surface for Bone Tissue Engineering" Journal of Composites Science 9, no. 7: 325. https://doi.org/10.3390/jcs9070325
APA StyleLiang, Y., Xin, X., He, X., Weng, W., Wu, C., & Cheng, K. (2025). Collagen/Polypyrrole Biomimetic Electroactive Composite Coating with Fiber Network Structure on Titanium Surface for Bone Tissue Engineering. Journal of Composites Science, 9(7), 325. https://doi.org/10.3390/jcs9070325







