Abstract
Regenerative endodontics seeks to restore the vascularized pulp–dentin complex following conventional root canal therapy, yet reliable neovascularization within the constrained root canal remains a key challenge. This study investigates the development of an injectable, dual-curing hydrogel based on methacrylated decellularized amniotic membrane (dAM-MA) and compares its performance to a conventional gelatin methacryloyl (GelMA). The dAM-MA platform was designed for biphasic release, incorporating both free vascular endothelial growth factor (VEGF) for an initial burst and matrix-metalloproteinase-cleavable VEGF conjugates for sustained delivery. The dAM-MA hydrogel achieved shape-fidelity via thermal gelation at 37 °C and possessed tunable stiffness (0.5–7.8 kPa) after visible-light irradiation. While showing high cytocompatibility comparable to GelMA (>125% hDPSC viability), the dAM-MA platform markedly outperformed the control in promoting endothelial tube formation (up to 800 µm total length; 42 branch points at 96 h). The biphasic VEGF release from dAM-MA matched physiological injury kinetics, driving both early chemotaxis and late vessel maturation. These results demonstrate that dAM-MA hydrogels combine native extracellular matrix complexity with practical, dual-curing injectability and programmable VEGF kinetics, offering a promising scaffold for minimally invasive pulp–dentin regeneration.
1. Introduction
The success of conventional endodontic procedures in eliminating infection and preserving tooth structure belies an inherent limitation: the treated tooth remains devitalized, prone to fracture, and unable to mount further biological repair [1]. Regenerative endodontic procedures (REPs) aim to overcome this by reconstituting the physiological pulp–dentin complex, restoring vascularity, innervation, and dentin deposition. Achieving this goal hinges on recreating a microenvironment within the disinfected root canal that supports stem cell recruitment, controlled biomaterial degradation, and neovascularization [2].
Angiogenesis is the sine qua non of pulp regeneration, as nascent vessels supply the oxygen, nutrients, and signaling factors essential for dentinogenesis [3]. In vivo, injured pulp upregulates vascular endothelial growth factor (VEGF) within hours, peaking at 24–48 h at concentrations of 20–50 ng/mL to drive endothelial cell chemotaxis and proliferation [4,5,6]. However, this endogenous surge rapidly subsides, often before vessel maturation is complete, which risks vessel regression and failed regeneration [7,8].
An ideal REP scaffold must therefore emulate this physiological cascade by delivering VEGF in a biphasic profile: an initial burst to recruit endothelial progenitors, followed by a sustained release to support vascular stabilization [4,7,9,10]. Simple diffusion-controlled hydrogels typically release growth factors too quickly, while tethered-only systems lack the crucial early recruiting signal [11,12]. While some biphasic strategies exist, they often rely on complex fabrication or require multiple injections [13].
A closer look at the common hydrogel platforms used in these strategies reveals the fundamental kinetic trade-offs that make achieving an ideal biphasic profile so challenging. For example, ionically-crosslinked alginate exhibits a modest initial burst (~20%) but slow cumulative release (~60% by day 7) in vitro [14], while pure photo-crosslinked gelatin methacryloyl (GelMA) shows a very rapid burst (>80% within 1–2 days) [15]. In contrast, MMP-degradable PEG matrices can minimize passive diffusion (<15% burst) but require proteolytic activity for significant release [16], and simple thermoresponsive collagen gels also show a large initial release (~53% on day 1) [17]. While advanced affinity-based strategies can extend release to several weeks [18] and enzyme-sensitive linkers can provide cell-triggered release [16], none of these single-mode platforms effectively combine a rapid initial dose, sustained long-term support, and tunable mechanics in a clinically practical, injectable format. Such limitations underscore the need for a scaffold that couples on-demand gelation with precise control over both the burst and sustained phases of VEGF release.
Among the materials developed to address these needs, GelMA is often considered a “gold standard” for photocrosslinkable matrices due to its facile fabrication and tunable mechanics [19,20,21]. However, as a single-protein system, it lacks the full complement of bioactive cues found in native tissues [22,23]. Hydrogels derived from decellularized extracellular matrix (dECM) overcome this limitation by retaining the complex biochemical milieu of the source tissue, which is known to enhance cell–matrix interactions and immunomodulation [22,24]. Indeed, recent work has demonstrated that thermoresponsive dECM hydrogels can achieve superior bioactivity compared to GelMA [25].
To translate the biochemical advantages of materials like dECM into a clinically practical format, dual-curing strategies have emerged. These approaches address key challenges by sequentially harnessing two orthogonal crosslinking mechanisms to balance injectability with mechanical robustness. For example, a GelMA system photopolymerized in situ and then enzymatically crosslinked increased its compressive modulus by approximately 3.5-fold without impairing cell viability [26]. Similarly, a hyaluronic acid scaffold combining permanent and dynamic chemical networks yielded an injectable gel that stiffens post-implantation [27]. Quantitative models further show that increasing crosslink density slows VEGF diffusion (reducing burst release and prolonging the release half-life, t50), offering a rational basis for tuning the degree of methacrylation (DoM) to achieve bespoke biphasic kinetics [16,18]. Together, these dual-cure strategies provide a translationally viable route to scaffolds that gel rapidly upon injection, then stiffen and sustain growth-factor release under physiological conditions.
This study directly compares two injectable, dual-curing platforms (methacrylated decellularized amniotic membrane (dAM-MA) and GelMA), each formulated to provide biphasic VEGF release through a combination of free and MMP-cleavable conjugates. The central hypothesis is that while both systems can be tuned to produce a biphasic release profile, the dAM-MA platform will exhibit superior mechanical tunability, degradation control, and in vitro bioactivity due to the inherent complexity of its native ECM components. This paper, therefore, details the fabrication, physicochemical characterization, VEGF kinetics, and comparative in vitro performance of these platforms to establish a clinically relevant scaffold for pulp–dentin regeneration.
2. Materials and Methods
2.1. Materials
Gelatin (Type A, 300 bloom, porcine skin; Sigma-Aldrich, St. Louis, MO, USA), methacrylic anhydride (MA; Sigma-Aldrich, St. Louis, MO, USA), and the visible-light photoinitiator components Eosin Y (TCI America, Portland, OR, USA), triethanolamine (TEOA; Sigma-Aldrich, St. Louis, MO, USA), and N-vinylcaprolactam (VC; Alfa Aesar, Haverhill, MA, USA) were used for hydrogel crosslinking. All other chemicals were purchased from MilliporeSigma (MilliporeSigma, Burlington, MA, USA) and used as received unless otherwise specified. Additional reagents included sterile, decellularized human amniotic membrane sheets (dAM; PuraGraft, Las Vegas, NV, USA), recombinant human VEGF165 (R&D Systems, Minneapolis, MN, USA), and active, recombinant human matrix metalloproteinase-2 (MMP-2; EMD Millipore, Billerica, MA, USA). For in vitro assays, human dental pulp stem cells (hDPSCs; Lonza, Walkersville, MD, USA) and human umbilical vein endothelial cells (HUVECs; Lonza, Walkersville, MD, USA) were cultured in media and supplements purchased from Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Hydrogel Synthesis and Characterization
2.2.1. dAM-MA Synthesis
To create a solubilized precursor, dAM sheets were processed using a method adapted from established protocols for dECM hydrogel fabrication [25,28,29]. Sheets were cut into small fragments, frozen at −80 °C, and lyophilized. The dried fragments were cryo-milled into a fine powder. This powder was digested in a solution of 1 mg/mL pepsin in 0.01 M HCl at a concentration of 10 mg/mL for 48 h at room temperature with constant stirring. The pH of the solubilized dAM solution was then adjusted to 7.4 with the dropwise addition of 1 M NaOH and 10× phosphate buffered saline (PBS).
For functionalization, the dAM solution was cooled to 4 °C. The solution was divided into three batches to investigate the effect of the degree of functionalization. The three formulations, designated dAM-MA-0.5x, dAM-MA-1x, and dAM-MA-2x, were synthesized by adding 0.4, 0.8, and 1.6 mL of MA per gram of dAM protein, respectively, while maintaining the pH at 7.4. The reaction proceeded for 12 h under vigorous stirring [30]. The functionalized dAM-MA solution was dialyzed (12–14 kDa MWCO tubing) against deionized water for 5 days to remove unreacted MA and byproducts, then lyophilized and stored at −80 °C.
2.2.2. GelMA Synthesis
Gelatin methacryloyl (GelMA) was synthesized according to established methods [20]. Briefly, 10% (w/v) porcine gelatin was dissolved in PBS at 60 °C. MA was added dropwise and allowed to react for 3 h. The reaction was quenched by dilution with warm PBS. The GelMA solution was dialyzed against deionized water for 7 days, lyophilized, and stored at −80 °C.
2.2.3. Characterization of Degree of Methacrylation
The degree of methacrylation (DoM) was characterized using two complementary methods. First, 1H nuclear magnetic resonance (NMR) spectroscopy (Bruker Avance III 600 MHz) provided qualitative confirmation of the modification. Analysis of spectra from lyophilized samples dissolved in D2O (10 mg/mL) confirmed the presence of characteristic methacrylate vinyl proton signals (~5.4–5.7 ppm), which provided direct evidence of successful functionalization.
Following this confirmation, the DoM for each dAM-MA batch and the GelMA control was quantified using a fluoraldehyde-based fluorescence assay, which measures the consumption of primary amines. For this assay, a standard curve was generated for each batch using its corresponding unmodified dAM (prepared in a 0.05–2 mg/mL dilution series in PBS). The dAM-MA samples (n = 3) were prepared at 0.5 mg/mL. In an opaque 96-well plate, each sample, standard, or PBS blank (300 µL) was mixed with fluoraldehyde reagent (600 µL; Sigma) for 1 min. Subsequently, 250 µL of the resulting mixture was loaded in triplicate, and fluorescence was measured (Ex: 360 nm, Em: 450 nm). After subtracting the blank’s average fluorescence, the DoM was calculated by comparing sample intensities to the linear calibration curve. The GelMA control was specifically synthesized to match the DoM of the dAM-MA-1x batch.
2.3. Synthesis and Characterization of the MMP-Cleavable VEGF Conjugate
2.3.1. Preparation of Site-Specific VEGF-MMP Peptide Conjugate
A custom peptide linker was synthesized using standard Fmoc (9-fluorenylmethoxycarbonyl) solid-phase peptide synthesis (CSBio, Menlo Park, CA, USA). The resulting peptide had the sequence Maleimide–GCRDGPQG↓IWGQDRCG-NH2, where ↓ indicates the MMP-2 cleavage site [9]. The peptide was purified by reverse-phase high-performance liquid chromatography (HPLC), and its identity was confirmed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.
Recombinant human VEGF was buffer-exchanged into 10 mM HEPES, 150 mM NaCl, 1 mM EDTA, pH 7.0 (HEPES-H) using a 7 kDa desalting column (protein 0.8–1.0 mg⋅mL−1). To expose the single free thiol (Cys116) in the heparin-binding domain without disrupting the intramolecular cystine knot, VEGF was incubated with 0.2 mM TCEP at 4 °C for 15 min. Excess reductant was immediately removed by a second desalting step into chilled HEPES-H. Free-thiol content (≥0.9 mol SH mol−1 protein) was verified with Ellman’s reagent.
To create the conjugate, reduced VEGF (500 µL, 0.5 mg/mL; 21 nmol) was mixed with 1.2 eq Peptide-MAL (25 nmol in ≤5% DMSO) and incubated for 30 min at 4 °C with end-over-end rotation. Unreacted maleimide was quenched with 5 mM L-cysteine for 5 min. The crude reaction was clarified by centrifugation (12,000× g, 5 min, 4 °C).
The resulting VEGF-MMP conjugate was purified by size-exclusion chromatography (Superdex 75 Increase 10/300 GL; ÄKTA Pure) in sterile PBS (0.22 µm filtered, Ca2+/Mg2+-free) at 0.4 mL/min. Fractions corresponding to the VEGF homodimer (38 kDa) were pooled and concentrated to 0.3 mg/mL using a 10 kDa MWCO spin filter (Amicon Ultra). Purified VEGF-MMP conjugate was aliquoted (50 µL) in PBS/0.1% BSA, flash-frozen in liquid N2, and stored at −80 °C. To ensure protein stability, each aliquot was used only once, and repeated freeze–thaw cycles were avoided.
2.3.2. Conjugate Quantification and Bioactivity Verification
Following purification, the final concentration of the VEGF-MMP conjugate was quantified using a human VEGF DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol. Unconjugated VEGF of known concentrations was used to generate the standard curve, ensuring accurate quantification of the specific protein.
To confirm that the conjugation process did not impair biological function, the bioactivity of the conjugated VEGF was assessed using an MTT proliferation assay. HUVECs were seeded in a 96-well plate at a density of 5000 cells/well in their complete growth medium and allowed to attach overnight. The medium was then replaced with EGM-2 basal medium (without growth supplements), and the cells were serum-starved for 4 h. Subsequently, the basal medium was supplemented with one of three conditions: a negative control group with no added growth factor; a positive control group with 50 ng/mL unconjugated VEGF; or the experimental group with an equivalent molar concentration of the purified VEGF-MMP-Cys conjugate.
After 24 h of incubation, cell proliferation was assessed by adding MTT solution (0.5 mg/mL in PBS) to each well and incubating for 4 h. The resulting formazan crystals were dissolved in dimethyl sulfoxide (DMSO), and the absorbance was measured at 570 nm using a microplate reader. This assay confirmed that the conjugation process did not impair the mitogenic activity of VEGF.
2.3.3. Preparation of VEGF-MMP-Functionalised dAM-MA
Lyophilized VEGF-MMP-Cys (0.25 mg, ≈10 nmol) was dissolved in 500 µL of ice-cold reduction buffer (10 mM HEPES, 150 mM NaCl, 1 mM EDTA, pH 7.0) and reduced with 0.2 mM TCEP on ice for 15 min. The TCEP was immediately removed by buffer exchange into degassed coupling buffer (20 mM HEPES, 150 mM NaCl, pH 8.3) using a 7 kDa Zeba spin column. Free thiol content was confirmed to be ≥0.9 mol per mol of VEGF monomer via Ellman’s method.
The polymer backbones (dAM-MA batches and GelMA) were dissolved at 4% w/v in ice-cold, degassed coupling buffer containing 0.02% (v/v) polysorbate-20. The reduced VEGF-MMP-Cys solution was added dropwise to the polymer solution to achieve a 2:1 molar ratio of peptide thiol to methacrylate. The pH was adjusted to 8.3–8.5 with triethylamine, and the mixture was sparged with nitrogen, sealed, and reacted for 2 h at 20 °C on an end-over-end rotator. The reaction was then quenched with 1 mM L-cysteine, and excess low-molecular-weight reagents were removed using a second Zeba column.
The crude conjugate was purified by centrifugal diafiltration (Amicon Ultra-15, 100 kDa MWCO) using three concentration/dilution cycles against sterile PBS containing 0.3 M NaCl. The final purified conjugate was recovered, snap-frozen in liquid nitrogen, lyophilized, and stored desiccated at −80 °C in the dark.
The conjugation efficiency was determined by quantifying the amount of unreacted VEGF-MMP-Cys conjugate. To do this, the pooled filtrates from the purification step were collected and the VEGF content was measured using a human VEGF DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA). The amount of covalently bound VEGF was then calculated by subtracting the unreacted amount from the initial total added to the reaction.
2.4. Fabrication of Biphasic Release Hydrogels
Biphasic release hydrogels were prepared using a visible-light photoinitiating system. First, a photoinitiator cocktail was prepared by dissolving Eosin Y (0.1 mM), triethanolamine (TEOA; 0.75% w/v), and N-vinylcaprolactam (VC; 0.5% w/v) in 1× PBS. All precursor solutions were then formulated in 1 mL of this cocktail to achieve a final polymer concentration of 50 mg/mL and a total VEGF payload of 100 ng per hydrogel.
Three distinct loading conditions were prepared. The first was a biphasic formulation, where polymer blends were designed to deliver 50 ng of covalently bound VEGF. Specifically, these blends combined base polymer with tethered precursor in the following amounts: 47.40 mg and 2.60 mg for dAM-MA-0.5x; 48.73 mg and 1.27 mg for dAM-MA-1x; 49.21 mg and 0.79 mg for dAM-MA-2x; and 48.90 mg and 1.10 mg for GelMA. After polymer dissolution, free recombinant VEGF was added to a final concentration of 50 ng/mL.
The second condition was a bound-only formulation, where blends were prepared so that the tethered macromer alone delivered the entire 100 ng of VEGF. These compositions were: 44.90 mg of base polymer plus 5.10 mg of tethered precursor for dAM-MA-0.5x; 47.46 mg plus 2.54 mg for dAM-MA-1x; 48.42 mg plus 1.58 mg for dAM-MA-2x; and 47.80 mg plus 2.20 mg for GelMA.
Finally, a control hydrogel was prepared with only free VEGF by dissolving 50 mg of an unmodified dAM batch in the photoinitiator cocktail and adding 100 ng/mL of free recombinant VEGF.
The resulting light-curable (LC) formulations were drawn into 1 mL Luer-lock syringes and stored at 4 °C (for a maximum of one week) until use. For injection, a 22-gauge angled cannula was used, and in situ crosslinking was achieved by illuminating with green LEDs (520 nm, 30° beam angle, 100 µW/cm2) for a maximum of 4 min to form the crosslinked biphasic hydrogel.
2.5. Physicochemical Characterization
2.5.1. Rheological Analysis
All rheological measurements were performed on a TA Instruments DHR-2 rheometer with a 20 mm parallel-plate geometry, a 500 µm gap, and a 0.1 N normal-force preload. A Peltier stage maintained temperatures at 10, 25, and 37 °C (±0.1 °C).
The viscosity of the pre-gel solutions was measured as a function of shear rate (0.01–1000 s−1) at each temperature, with each condition run in triplicate (n = 3) and averaged. Photogelation kinetics were monitored by a time-sweep test (after a 60 s equilibration at 25 °C), recording the storage (G′) and loss (G′′) moduli at a constant strain (1%) and frequency (1 Hz) during 240 s of light exposure [30]. The final mechanical stiffness of the cured hydrogels was determined by a frequency sweep from 0.1 to 10 Hz at 1% strain, also in triplicate (n = 3).
2.5.2. Microstructure and Swelling
The internal microstructure of lyophilized hydrogel samples was imaged using a scanning electron microscope (SEM, JEOL, Tokyo, Japan).
To determine the equilibrium swelling ratio, the initial weight of lyophilized hydrogels () was recorded. Samples were then immersed in PBS at 37 °C for 24 h to reach a fully hydrated state; at this point, their wet weight () were recorded once mass stabilized (no change over two consecutive 1 h measurements, typically by 24 h). The equilibrium swelling ratio was calculated as follows [31]:
2.5.3. Enzymatic and Hydrolytic Degradation
Lyophilized hydrogels (n = 3 per formulation) were pre-weighed () and incubated at 37 °C with gentle orbital agitation (50 rpm) in either PBS containing 10 U/mL of active MMP-2 for enzymatic degradation or in pure PBS for hydrolytic degradation controls [30]. At predetermined time points (1, 2, 3, 5, 10, and 14 days), each sample was removed, gently rinsed twice with deionized water to remove residual enzyme or buffer components, lyophilized, and then weighed (). The incubation medium was refreshed at each time point. Percentage mass loss was calculated relative to the initial dry weight using the equation [31]:
2.6. Comparative VEGF Release Kinetics
Immediately following polymerization (Section 2.4), each 100 µL hydrogel construct (biphasic-loaded, bound-only, or free-VEGF control) was immersed in 1 mL of release medium (PBS supplemented with 10 U/mL recombinant human MMP-2) within a 1.5 mL microcentrifuge tube [6]. The tubes were maintained at 37 °C with gentle orbital agitation (50 rpm). At a series of predetermined time points (12, 24, 48, 72, 96, 120, 144, 168, 240, and 336 h), the entire release medium was collected for analysis and stored at −20 °C. Each hydrogel was then immediately replenished with 1 mL of fresh release medium.
The VEGF concentration in each collected fraction was quantified in triplicate using a Human VEGF DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions, with calibration against unconjugated VEGF standards. Cumulative release profiles were generated by summing the measurements from each time point and expressing the result as a percentage of the total 100 ng payload.
2.7. In Vitro Biological Assays
2.7.1. Cell Culture
hDPSCs were cultured in α-MEM supplemented with 15% fetal bovine serum (FBS) and 1% penicillin–streptomycin. HUVECs were cultured in EGM-2 BulletKit medium (Lonza). All cells were maintained at 37 °C in a humidified incubator with 5% CO2.
2.7.2. Cytocompatibility Assay
For cytocompatibility studies, hDPSCs were encapsulated in each hydrogel formulation (dAM-MA-0.5x, -1x, -2x, and GelMA) at a density of 2 × 106 cells/mL. The cell-laden precursors were photocured as 50 µL discs in a 96-well plate (n = 3 per condition).
Parallel 2D controls were generated by seeding the same density of hDPSCs directly into empty 96-well plates. After 48 h, viability was assessed qualitatively by LIVE/DEAD™ staining (Thermo Fisher) with fluorescence images captured from three random fields per well using a fluorescence microscope (Nikon Eclipse Ti). Quantitative viability was measured by MTT assay: hydrogels were incubated with 0.5 mg/mL MTT for 4 h at 37 °C, crystals dissolved in DMSO, and absorbance read at 570 nm (n = 3); values are reported as % of 2D control [6].
2.7.3. Tube Formation Assay
HUVECs (2 × 104 cells/well) were seeded onto Matrigel®-coated 96-well plates and allowed to adhere for 30 min at 37 °C. The standard growth medium was then replaced with various conditioned media, collected at 12- and 96-h time points from different hydrogel release studies.
The treatment groups included several controls: a negative control consisting of base medium without added VEGF; a positive control with fresh medium supplemented with 50 ng/mL of free recombinant VEGF; and a material control using conditioned media from a non-methacrylated, thermo-responsive dAM hydrogel containing only physically entrapped VEGF. The primary experimental groups used conditioned media from the dAM-MA-2x hydrogels, testing both the bound-only and the biphasic formulations.
After a 6-h incubation at 37 °C in 5% CO2, tube networks were imaged in three randomly selected fields per well using an inverted fluorescence microscope (EVOS FL Auto) at 10× magnification. Total tube length and branch point count were quantified using the Angiogenesis Analyzer plugin for ImageJ (version 1.54) [32].
2.8. Statistical Analysis
All quantitative data are presented as mean ± standard deviation (SD) for at least three independent experiments (n = 3). Prior to hypothesis testing, data distributions were assessed for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test. Comparisons between two groups were performed using two-tailed unpaired Student’s t-tests, while multiple group comparisons were conducted by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. In cases where data violated normality or equal variance assumptions, nonparametric alternatives (Mann–Whitney U test for two groups; Kruskal–Wallis test with Dunn’s post hoc correction for multiple groups) were applied. All statistical analyses were conducted in R [33].
3. Results
3.1. Characterization of Methacrylated Polymers
The dual-curing synthesis and VEGF-conjugation strategy are illustrated in Figure 1. Lyophilized dAM was cryo-milled, pepsin-digested, and then reacted with methacrylic anhydride at 0.5×, 1×, or 2× stoichiometry to yield the dAM-MA precursors. This process was highly efficient, with material recoveries of 0.82 ± 0.05 g/g (for 0.5×), 0.85 ± 0.04 g/g (for 1×), and 0.88 ± 0.03 g/g (for 2×) relative to the starting dAM weight (n = 3).
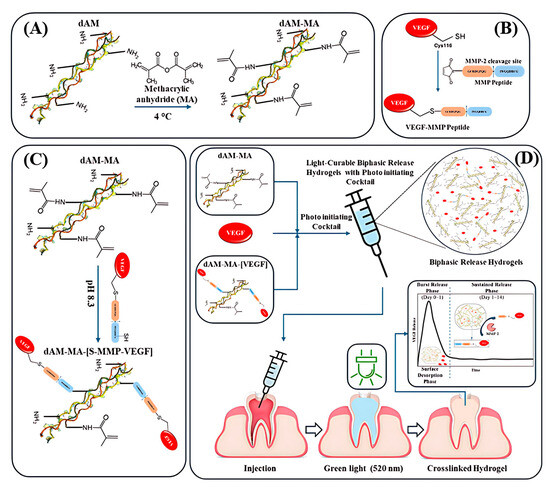
Figure 1.
Schematic workflow: (A) Decellularized amniotic membrane (dAM) is methacrylated to produce photocrosslinkable dAM-MA. (B) MMP-cleavable peptide is conjugated to VEGF (VEGF-MMP). (C) VEGF-MMP is tethered to dAM-MA. (D) Free and tethered VEGF plus photoinitiator form an injectable hydrogel that delivers an initial VEGF burst followed by sustained, enzyme-triggered release.
Successful methacrylation was confirmed by the appearance of vinyl proton peaks at 5.4–5.7 ppm in 1H NMR spectra (Figure 2A). Subsequently, fluoraldehyde assays quantified the degree of methacrylation (DoM) as 27.4 ± 3.2% (0.5×), 51.6 ± 6.3% (1×), and 74.6 ± 4.8% (2×) (n = 3). All pairwise comparisons between the dAM-MA groups were statistically significant (ANOVA/Tukey’s; 0.5× vs. 1×, p < 0.001; 1× vs. 2× and 0.5× vs. 2×, p < 0.0001).
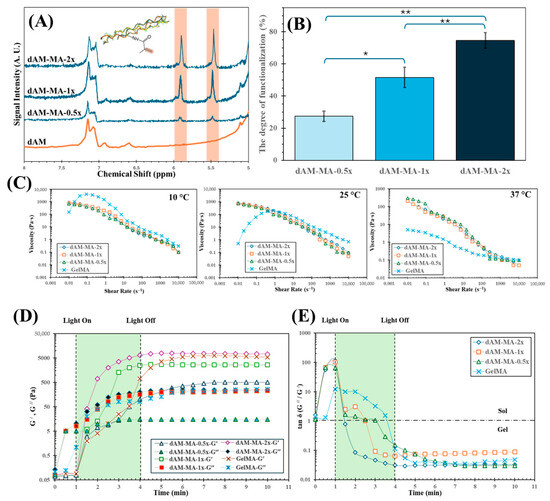
Figure 2.
Physicochemical characterization of dAM-MA hydrogels. (A) ^1H NMR spectra showing methacrylate vinyl peaks (5.4–5.7 ppm). (B) Degree of methacrylation (DoM) by fluoraldehyde assay (mean ± SD; n = 3; * p < 0.001; ** p < 0.0001). (C) Shear-rate-dependent viscosities at 10, 25, and 37 °C for dAM-MA formulations and GelMA. Photogelation kinetics under 520 nm light (D) storage (G′, solid lines) and loss (G′′, dashed lines) moduli (E) tan δ (G′′/G′).
All dAM-MA precursors underwent thermal gelation at 37 °C, forming self-supporting physical gels within 5 min as determined by a tube-inversion test. The precursors exhibited shear-thinning behavior at 37 °C (Figure 2C), with viscosities at a shear rate of 1 s−1 measured at 20 mPa·s (0.5×), 14 mPa·s (1×), 15 mPa·s (2×), and 1 mPa·s (GelMA). Comparable trends at 37 °C, 25 °C, and 10 °C are detailed in Table 1.

Table 1.
Viscosities (mean ± SD, n = 3) at 1 s−1 for dAM-MA (0.5×, 1×, 2×) and GelMA at 10 °C, 25 °C, and 37 °C.
Upon green-light irradiation (520 nm, 24 mJ/cm2), all precursors underwent rapid photopolymerization, as shown by time-sweep rheometry (Figure 2D,E). The storage and loss moduli crossover points (G′/G′′) were reached at approximately 1.5 min (2×), 2.5 min (1×), 3.5 min (0.5×), and 4.0 min (GelMA). After 4 min of illumination, the plateau storage moduli (G′) were 7.8 ± 0.3 kPa (2×), 2.7 ± 0.2 kPa (1×), 0.5 ± 0.05 kPa (0.5×), and 6.3 ± 0.4 kPa (GelMA) (n = 3). Relative to the initial thermal gel baseline (G′ ≈ 0.1 kPa), photopolymerization increased the modulus by ≥50-fold for the 2× formulation (ANOVA/Tukey’s, p < 0.01).
3.2. VEGF–Peptide Conjugate
Reactive thiol groups on reduced VEGF were coupled to the MMP-cleavable peptide via maleimide chemistry. The amount of unbound VEGF in the purification filtrate was quantified using a Human VEGF DuoSet ELISA kit (R&D Systems; range 15–1000 pg·mL−1), which yielded an average conjugation efficiency of 87.3 ± 4.3% (mean ± SD, n = 3). Subsequent analysis by reverse-phase HPLC (Phenomenex Luna C18, 5 µm, 250 × 4.6 mm; 5–60% ACN over 30 min at 1 mL/min; 214 nm) confirmed that the final VEGF–MMP conjugate possessed >96% purity.
The bioactivity of the conjugate was assessed on HUVECs that were seeded at 5 × 103 cells/well in 96-well plates and serum-starved for 4 h. The cells were then treated for 24 h with 50 ng/mL of either unconjugated VEGF or the VEGF–MMP conjugate (n = 3). Proliferation was quantified using an MTT assay (0.5 mg/mL, 4 h) and expressed relative to an untreated control group (defined as 100 ± 8%). Unconjugated VEGF increased cell viability to 150 ± 10% (p = 0.0002 vs. control), while the conjugate increased viability to 140 ± 8% (p = 0.0004 vs. control). There was no significant difference between the two VEGF forms (p = 0.12; one-way ANOVA/Tukey’s). Representative Calcein-AM fluorescence images (Figure 3B) further corroborate the comparable bioactivity.
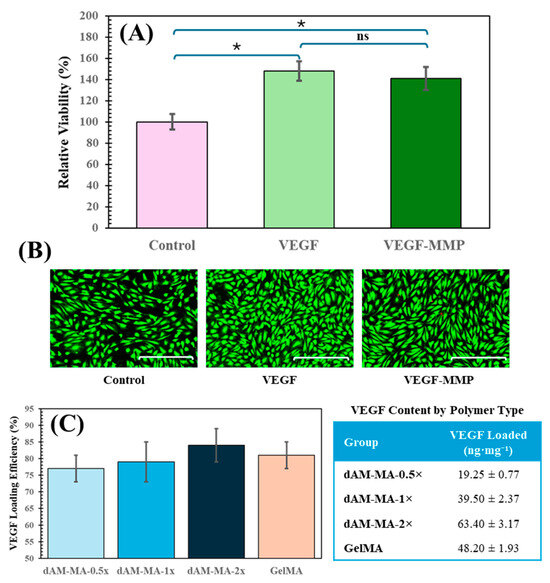
Figure 3.
(A) HUVECs proliferation (MTT) with 50 ng/mL VEGF or VEGF-MMP (mean ± SD; * p < 0.001; ns = not significant). (B) Calcein-AM images of live HUVECs (scale bar = 200 µm). (C) VEGF loading efficiencies (%) and retained content (ng mg−1) in each hydrogel (mean ± SD; ns = not significant).
The VEGF-peptide conjugate was reacted with the various hydrogel precursors at a 2:1 molar ratio of peptide thiol to methacrylate. ELISA was then used to quantify the unbound VEGF, determining the amount of successfully retained conjugate. All formulations demonstrated high and relatively consistent conjugation efficiencies: 77% for 0.5x, 79% for 1x, 84% for 2x, and 81% for GelMA.
However, because the number of available methacrylate sites increases with DoM, the absolute amount of VEGF tethered per hydrogel mass differed substantially. The dAM-MA-0.5x batch retained only 19.3 ± 0.8 ng/mg, whereas the dAM-MA-1x and -2x batches retained 39.5 ± 2.4 ng/mg and 63.4 ± 3.2 ng/mg, respectively (GelMA: 48.2 ± 1.9 ng/mg; mean ± SD, n = 3). In other words, a modest 9% increase in conjugation efficiency from the 0.5x to the 2x formulation translated into a more than threefold higher VEGF load per milligram of polymer. This tunability (combining high reaction efficiency with DoM-dependent loading capacity) enables precise control over the total growth factor dose delivered by each hydrogel formulation (Figure 3C).
3.3. Hydrogel Physicochemical Properties & Release
Hydrogel degradation was assessed under both enzymatic and hydrolytic conditions. Under enzymatic challenge with MMP-2 (Figure 4A), the unmodified dAM degraded rapidly, losing 25 ± 5% of its mass by Day 1 and fully degrading by Day 3. In contrast, functionalized hydrogels showed increased stability that correlated with a higher DoM. The dAM-MA-2x formulation was the most stable, losing only 20 ± 3% mass by Day 1 and retaining 15% of its mass at Day 14 (p < 0.01 vs. 0.5x at Day 1). The dAM-MA-1x and dAM-MA-0.5x hydrogels exhibited intermediate degradation profiles. GelMA demonstrated stability comparable to that of the dAM-MA-1x batch.
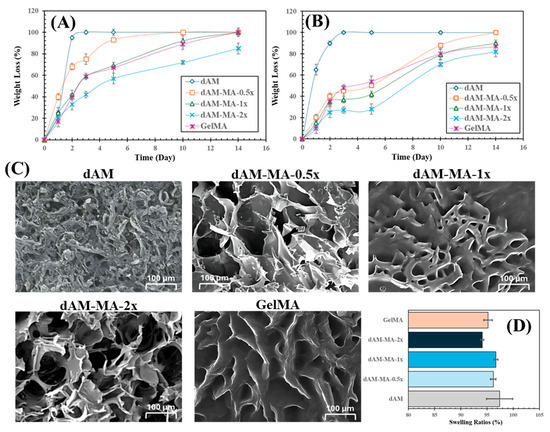
Figure 4.
Hydrogel degradation, morphology and swelling. (A) Enzymatic mass loss over 14 days in 10 U/mL MMP-2. (B) Hydrolytic mass loss in PBS. dAM-MA stability increases with DoM. (C) SEM of lyophilized hydrogels (scale bars = 100 µm). (D) Equilibrium swelling ratios at 24 h (mean ± SD).
In PBS alone (Figure 4B), dAM also hydrolyzed quickly, degrading completely by Day 3. Methacrylation significantly improved hydrolytic stability. The dAM-MA-2x formulation was the most robust, retaining over 70% of its mass through Day 10.
SEM micrographs revealed that methacrylation altered the hydrogel architecture (Figure 4C). Unmodified dAM displayed a dense, fibrous mesh, while methacrylation resulted in more defined, open-pore networks. Pore size was inversely related to the DoM: dAM-MA-0.5x had large, irregular pores (~150–200 µm), which became smaller and more uniform with increasing functionalization, with dAM-MA-2x forming the smallest pores (~80–100 µm). GelMA exhibited a honeycomb-like morphology with pores of ~100–120 µm. Despite these structural differences, all hydrogels were highly hydrophilic. Equilibrium swelling ratios at 24 h were consistently high across all groups, ranging from 94.1 ± 0.3% to 97.4 ± 2.5% (Figure 4D).
The hydrogel formulation and DoM significantly modulated the release kinetics of VEGF (Figure 5A–E). In the biphasic-loaded hydrogels, increasing the DoM reduced the initial burst release and prolonged the overall release profile. The burst release (at 12 h) was highest from unmodified dAM (56.0 ± 2.1%) and lowest from dAM-MA-2x (32.2 ± 1.2%). Consequently, the time to 50% cumulative release (t50) was shortest for dAM (10.7 ± 0.9 h) and longest for dAM-MA-2x (37.2 ± 3.1 h).
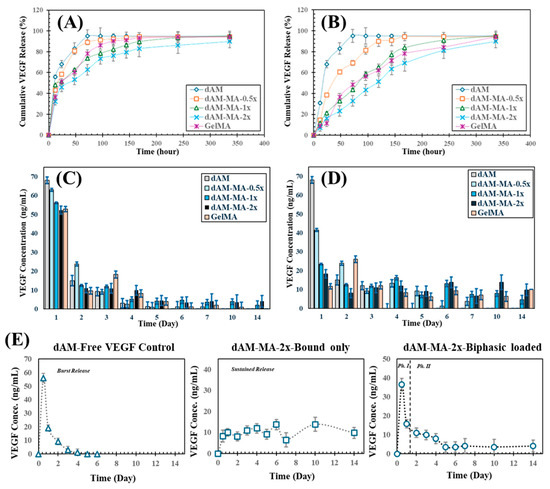
Figure 5.
VEGF release kinetics. (A,B) Cumulative release profiles for biphasic-loaded (A) and bound-only (B) hydrogels (mean ± SD). (C,D) Corresponding daily release rates. (E) Deconstruction of biphasic release: free-VEGF burst (left), tethered-VEGF sustained release (middle), and combined biphasic profile (right).
This DoM-dependent control over release was even more pronounced in the bound-only hydrogels (Figure 5E). The dAM-MA-2x formulation provided the most sustained release, releasing only 7.3 ± 0.8% of its VEGF payload by 12 h, with a t50 of 116.1 ± 5.5 h. In contrast, the bound-only GelMA control released 9.3 ± 0.9% in the burst phase and had a t50 of 77.1 ± 4.2 h.
These findings demonstrate that creating a more densely crosslinked network, particularly in the dAM-MA-2x formulation, effectively impedes both VEGF diffusion and enzymatic degradation, thereby achieving prolonged and tunable bioactive release.
To rigorously quantify this programmable release and establish a predictive framework, we fitted each cumulative release curve to a biphasic exponential model:
where Fb is the burst fraction, kb is the burst-phase rate constant, and ks is the sustained-phase rate constant. This model was chosen because it provides a minimal yet mechanistically interpretable framework to describe two distinct release regimes (an early burst followed by a slower, degradation-controlled phase) without overfitting [34,35]. All fits exhibited excellent agreement with the experimental data (; Table S1), confirming the model’s ability to capture the release behaviors.
Regression of these extracted parameters against the degree of methacrylation (DoM) yielded robust quadratic “programmability” curves (Table S2; Figures S1 and S2). The burst fraction (Fb) followed a parabolic trend, suggesting an optimal crosslinking window for maximizing early VEGF availability [34]:
The sustained-phase rate constant (ks) exhibited a complementary U-shaped dependence, reflecting the competing effects of diffusion restriction and network erosion on late-stage release [35,36]:
To elucidate the mechanistic basis for these trends, gel degradation was analyzed under identical conditions. Mass-loss profiles were well-described by a first-order decay model:
The resulting degradation rate constants (kd) decreased linearly with DoM (Table S1), consistent with more densely crosslinked networks resisting degradation [34]. A strong positive correlation between slowed degradation (a lower kd) and a reduced sustained-release rate (a lower ks) is presented in Figure S3, confirming that gels that erode more slowly also release their tethered VEGF more gradually. This relationship is described by the following:
These combined data establish a clear, quantitative framework linking DoM, network stability, and protein release kinetics, substantiating the claim of truly programmable delivery.
3.4. In Vitro Biological Evaluation
Human dental pulp stem cells (hDPSCs) were encapsulated in each hydrogel formulation, and their viability was compared to that of 2D. MTT assay was used to assess viability at 24 and 48 h (mean ± SD, n = 3; one-way ANOVA with Tukey’s post hoc test). At 24 h, all hydrogel groups supported high viability (98–102%) relative to the 2D control (100 ± 4%), with no significant differences observed between any groups. By 48 h, however, viability had significantly increased in all hydrogel groups, reaching 125 ± 6% for dAM-MA-0.5x, 130 ± 5% for dAM-MA-1x, 145 ± 7% for dAM-MA-2x, and 128 ± 6% for GelMA, all of which were significantly higher than the 2D control (p < 0.001). Qualitative LIVE/DEAD™ staining (Calcein-AM/EthD-1) confirmed high viability and uniform cell distribution within all hydrogel constructs (Figure 6B; scale bars = 100 µm).
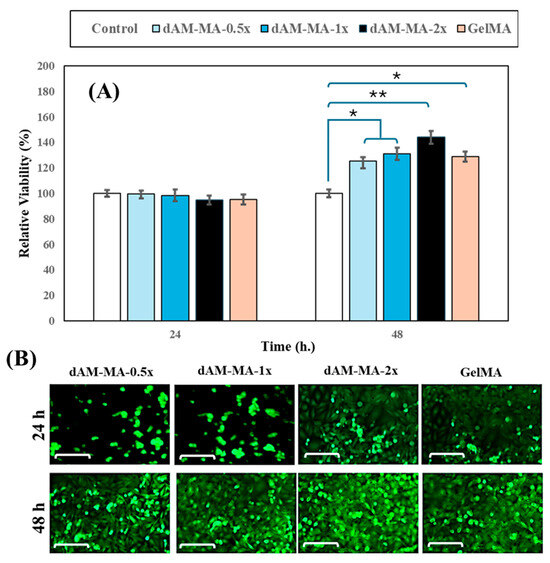
Figure 6.
Cytocompatibility of dAM-MA and GelMA hydrogels. (A) hDPSC viability at 24 and 48 h relative to 2D control (mean ± SD; * p < 0.001, ** p < 0.0001). (B) Representative Calcein-AM images at 24 and 48 h (scale = 100 µm).
The bioactivity of the released VEGF was assessed by treating HUVECs plated on Matrigel with conditioned media collected at 12 h (“early release”) and 96 h (“late release”) from the various hydrogel formulations. Negative controls received basal medium only, while positive controls received fresh medium containing 50 ng/mL free VEGF. Tube formation was quantified by total tube length and the number of branch points per field (mean ± SD, n = 3; one-way ANOVA/Tukey’s).
At the 12-h time point, conditioned media from all VEGF-releasing hydrogels induced significant tube formation compared to the negative control (120 ± 15 µm; p < 0.001). The dAM-VEGF control induced a tube length of 800 ± 50 µm, while the biphasic and bound-only formulations induced 680 ± 45 µm and 550 ± 40 µm, respectively. The positive control reached 900 ± 65 µm. By 96 h, the biphasic formulation maintained robust networks (780 ± 60 µm), proving significantly more effective than both the bound-only formulation (700 ± 55 µm; p < 0.01) and the dAM-VEGF control, whose network had regressed (200 ± 20 µm; p < 0.001).
Branch point analysis mirrored these trends. At 12 h, the biphasic (38 ± 3 points) and bound-only (30 ± 4 points) groups were significantly higher than the negative control (5 ± 1 point; p < 0.001). At 96 h, the biphasic group (42 ± 4 points) maintained a more complex network than both the bound-only group (35 ± 3 points; p < 0.01) and the dAM-VEGF control (15 ± 2 points; p < 0.001). Representative fluorescence images further illustrate these differences in network morphology (Figure 7A,C; Calcein-AM; scale bars = 100 µm).
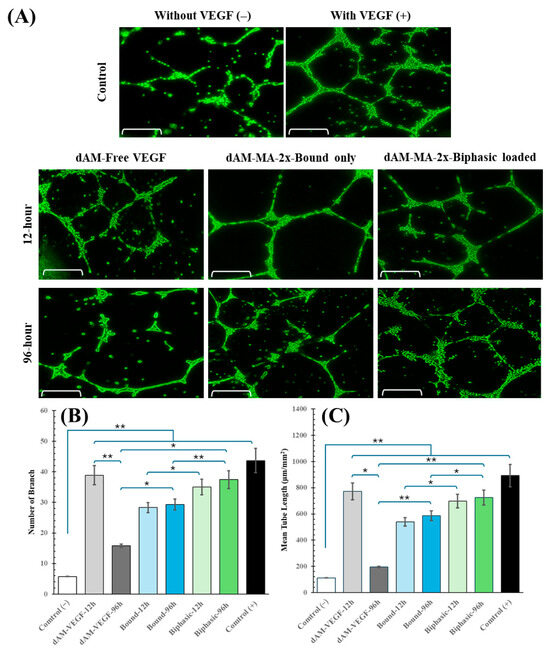
Figure 7.
Angiogenic response to released VEGF. (A) Representative HUVEC tube networks after 12 h and 96 h conditioned media (scale = 100 µm). (B,C) Quantification of total tube length (B) and branch points (C) (mean ± SD; * p < 0.001, ** p < 0.0001).
Together, these data confirm that all hydrogels are highly cytocompatible and demonstrate that the biphasic formulations are superior, delivering early-phase VEGF to trigger rapid angiogenesis and late-phase VEGF to sustain the resulting endothelial networks. This dual-action release profile meets key requirements for regenerating a functional pulp–dentin complex.
4. Discussion
4.1. Summary of Key Findings
This work describes the engineering of an injectable, dual-curing hydrogel based on decellularized amniotic membrane methacryloyl (dAM-MA), which was designed for the tunable, biphasic release of Vascular Endothelial Growth Factor (VEGF). The platform demonstrated excellent cytocompatibility with human dental pulp stem cells (hDPSCs), achieving over 125% viability relative to 2D controls after 48 h (Section 3.4). This finding is consistent with prior reports showing that the Eosin Y-based photoinitiation system used here supports robust cell survival [19,21]. Rheological characterization confirmed rapid thermal gelation at 37 °C within 5 min and DoM-dependent storage moduli ranging from 0.5 to 7.8 kPa upon exposure to green light (Section 3.1), in line with analyses of thermo-responsive ECM hydrogels [19,21,30].
Degradation studies demonstrated that increasing methacrylation markedly slows enzymatic and hydrolytic breakdown: the dAM-MA-2x formulation retained 85% of its mass after 14 days in MMP-2, whereas unmodified dAM was fully degraded by Day 3 (Section 3.3), echoing trends observed in hybrid ECM-based scaffolds [22,23,24]. VEGF release assays revealed a pronounced initial burst (32–56% in 12 h) followed by sustained delivery (up to 95% by 96 h), with t50 values extending from 10.7 h (dAM) to 37.2 h (dAM-MA-2x) (Section 3.3), demonstrating physiological mimicry of growth-factor presentation [4,5]. Conditioned media from biphasic dAM-MA hydrogels elicited rapid and sustained endothelial tube formation on Matrigel equivalent to free VEGF controls (Section 3.4), confirming functional angiogenic signaling [11].
Overall, these results validate the design paradigm: decellularized ECM-based, dual-curing hydrogels afford precise control over mechanics, degradation, and VEGF kinetics, forming a promising injectable scaffold for regenerative endodontics [3,10,37,38,39,40].
4.2. The dECM Advantage
Decellularized amniotic membrane (dAM) offers a particularly potent scaffold for regenerative endodontics by preserving the intricate biochemical and biophysical cues of the native extracellular matrix (ECM) [10,37]. These are features unattainable with single-component hydrogels. First, proteomic analyses reveal that dAM retains a spectrum of collagens (I, III, IV, VI, VII), laminin, fibronectin, elastin, and glycosaminoglycans, including heparan sulfate and hyaluronic acid [41]. These molecules collectively orchestrate cell adhesion, migration, proliferation, and differentiation [37,38,42,43]. In particular, heparan sulfate proteoglycans are known to sequester endogenous and exogenous growth factors (e.g., VEGF), protecting them from proteolytic degradation and facilitating sustained receptor engagement on endothelial and progenitor cells [44].
Second, dAM presents matrix-bound bioactive peptides (so-called matricryptins) that are exposed upon partial degradation and subsequently regulate angiogenesis and immunomodulation. For instance, collagen IV fragments released during matrix remodeling stimulate endothelial tubulogenesis, while laminin-derived peptides enhance cell survival [45]. These dynamic feedback loops between cells and the ECM are absent in GelMA, which, despite including RGD motifs, lacks the structural diversity and reservoir function of a native ECM [21].
Third, immunogenicity concerns are mitigated by rigorous decellularization, which removes over 90% of cellular DNA and major histocompatibility antigens. This results in minimal inflammatory infiltration and a predominantly M2-type (pro-regenerative) macrophage polarization upon implantation [10]. Such an anti-inflammatory milieu supports tissue repair, whereas poorly degraded GelMA constructs can provoke prolonged foreign-body responses [21].
Functionally, the in vitro comparisons in this study demonstrate that biphasic dAM-MA hydrogels outperform GelMA in supporting endothelial network formation (Section 3.4). The fibrous topology of dAM (with pore sizes tunable by methacrylation from 80 to 200 µm) likely provides not only space for cell infiltration but also directional guidance for angiogenic sprouting. In contrast, GelMA’s more uniform honeycomb-like pores may offer a less instructive architecture.
The comparative analysis in this study further highlights the distinct advantages of the dAM-MA platform over conventional GelMA. While both materials were highly cytocompatible, the dAM-MA system demonstrated superior functional properties rooted in its native ECM composition. Its dual-curing nature provided immediate stability at 37 °C via thermo-gelation (a feature absent in GelMA) before photocrosslinking achieved a robust, tunable modulus. This, combined with pronounced shear-thinning behavior, ensures predictable delivery and retention in complex root canal geometries, overcoming the risk of leakage associated with low-viscosity GelMA. Functionally, despite equivalent VEGF loading strategies, the preserved ECM components and instructive microarchitecture of dAM-MA synergistically enhanced endothelial tube formation well beyond that of the GelMA hydrogels. Furthermore, dAM-MA offers intrinsic immunomodulatory properties not present in the more bio-inert GelMA. In summary, by combining the practical tunability of a methacrylated polymer with the potent bioactivity of a native dECM, the dAM-MA hydrogel represents a more advanced and functionally superior scaffold for regenerative endodontics.
4.3. Linking Material Properties to Biological Outcomes
Importantly, the highest-stiffness hydrogel (dAM-MA-2x; G′ ≈ 7.8 kPa) supported the most extensive angiogenesis. It yielded a total tube length of 780 ± 60 µm at 96 h, which was 20% more than the GelMA control (6.3 kPa; 700 ± 55 µm) and 35% more than the softest hydrogel, dAM-MA-0.5x (0.5 kPa; 580 ± 50 µm) (Section 3.4). This demonstrates a direct, monotonic relationship between matrix rigidity and endothelial network formation, consistent with mechanotransduction models in which increased stiffness enhances YAP/TAZ-mediated pro-angiogenic signaling [46,47].
The degree of methacrylation directly influenced the hydrogel’s pore architecture, establishing a key control over both cell infiltration and release kinetics. The large, interconnected pores (150–200 µm) in the low-DoM dAM-MA-0.5x hydrogel correlated with a significant initial VEGF burst (56% at 12 h), which drove early tube formation. In contrast, the small pores (80–100 µm) of the high-DoM dAM-MA-2x formulation slowed VEGF diffusion (burst = 32%; t50 = 37.2 h) and sustained the angiogenic cues, resulting in a higher branch-point density at later time points. These observations align with established theories of diffusion–degradation coupling in hydrogel networks [12].
Finally, the DoM-dependent degradation rates further extended the duration of bioactivity. Whereas unmodified dAM degraded completely by Day 3, the dAM-MA-2x hydrogel retained 85% of its mass through Day 14, maintaining the availability of tethered VEGF as the matrix remodeled. This prolonged presentation of the growth factor underpins the sustained endothelial activation observed, as shown by the 42 ± 4 branch points at 96 h, compared to 15 ± 2 for faster-degrading formulations.
Clinically, these findings suggest that tuning the DoM can be used to tailor hydrogel behavior to match patient-specific needs: softer, fast-releasing gels could be utilized for rapid revascularization in acute injuries, while stiffer, slow-releasing matrices could provide prolonged support for large defects [48].
4.4. Advantages of Tunable Biphasic VEGF Delivery
Regenerative endodontics seeks to reestablish a functional pulp–dentin complex by guiding progenitor cells and blood vessels into the disinfected root canal space—a milieu characterized by narrow branching channels and limited oxygen diffusion [1]. In vivo, injured pulp tissue upregulates VEGF within hours, peaking between 24 and 48 h at local concentrations estimated between 20 and 50 ng/mL, which optimally drive endothelial cell chemotaxis and proliferation [3]. However, endogenous VEGF levels decline rapidly thereafter, jeopardizing vessel maturation and stability [2].
The injectable dAM-MA hydrogel developed in this study addresses these temporal challenges by delivering VEGF in a two-phase profile. A burst phase, which releases 32–56% of the 100-nanogram payload within the first 12 h, achieves peak concentrations that match physiologic injury responses, as reflected by the extensive HUVEC tube outgrowth observed in vitro. This rapid surge promotes endothelial progenitor recruitment from the periapical tissues, which is critical in the absence of a native vascular network. As the hydrogel degrades in response to MMP-2 (present at 1–10 ng/mL in healing tissues), the sustained phase (12–336 h) releases the remainder of the VEGF payload gradually (t50 up to 37 h in dAM-MA-2x), maintaining concentrations above the 5–10 ng/mL threshold required for lumen formation and stabilization of neovessels [43].
This design overcomes key limitations of existing delivery platforms. Simple diffusion-controlled matrices often release growth factors too rapidly, causing premature signal dissipation, while tethered-only systems can lack the initial recruiting signal needed to delay the onset of angiogenesis [49]. By combining free and MMP-cleavable tethered VEGF within a single formulation, the hydrogels described here ensure both timely progenitor cell recruitment and sustained signaling as tissue remodeling proceeds.
Moreover, the degree of methacrylation serves as a tunable “dial” to match patient-specific biological conditions. In clinical scenarios where inflammation and MMP levels may vary, such as in immature versus mature root canals, the burst/sustained ratio and release half-life (t50) can be tailored by adjusting the DoM. This ensures robust angiogenic cues can be provided under diverse healing environments.
Finally, achieving this biphasic profile with a single-injection, dual-curing system simplifies the clinical workflow and reduces risks associated with multiple interventions, a critical advantage in the confined root canal environment where repeated access is impractical. This clinically practical approach is underpinned by clear quantitative “design rules” for programmable delivery, established by fitting release profiles to a biphasic exponential model. This analysis yielded quadratic functions that link the DoM to both the burst fraction and the sustained-phase release rate, enabling the prediction of VEGF dosing at clinically relevant time points. Mechanistically, this control is rooted in network stability; complementary degradation studies confirmed that slower polymer erosion (a lower kd) directly correlates with a more prolonged sustained release rate (ks) [35]. This dual control over diffusion and degradation extends prior programmable hydrogel systems and offers a modular platform for other growth factors. Future work should validate these findings in vivo and explore composite scaffolds with independently tunable release clocks. By aligning these predictable material properties, biological performance, and a simplified clinical workflow, the dAM-MA platform holds strong promise for advancing pulp–dentin regeneration.
4.5. Clinical and Translational Implications
The injectable, dual-curing dAM-MA hydrogel is well-suited for minimally invasive endodontic protocols by enabling delivery through fine-gauge cannulae, rapid thermo-gelation to prevent backflow, and on-demand, light-activated stiffening to withstand masticatory forces [1]. Its biphasic VEGF release avoids the need for repeat administrations (a critical advantage in the confined root canal environment) by providing an early surge that recruits endothelial progenitors and a prolonged phase that supports vessel maturation [3]. The incorporation of decellularized ECM not only enhances cytocompatibility and angiogenesis but also delivers endogenous anti-inflammatory cytokines (e.g., IL-10, TGF-β), promoting an M2 macrophage-driven healing response with minimal fibrosis in vivo [10,37].
From a regulatory standpoint, the use of lyophilized dAM-MA precursors and FDA-recognized photoinitiators (Eosin Y/TEOA/VC) offers a clear path toward scalable manufacturing and compliance with tissue-engineered medical product guidelines [21]. Future translation will require confirmation of VEGF kinetics and immune responses in ex vivo tooth-slice and large-animal root-canal models, with endpoints including micro-CT angiography of pulp vascularization, and dentin bridge formation.
By aligning material properties, biological performance, and clinical workflow, the dAM-MA platform holds strong promise for advancing pulp–dentin regeneration toward routine endodontic practice.
4.6. Limitations and Future Directions
Although the in vitro data from this study confirm tunable mechanics and biphasic VEGF release, injectability and photo-crosslinking performance have not yet been demonstrated in anatomically relevant root-canal geometries. Likewise, HUVEC tube-formation assays do not fully replicate the complex host response, neovascularization, or degradation kinetics seen in vivo. Finally, the immunogenicity and long-term behavior of dAM-MA (especially regarding potential inflammatory or off-target angiogenic effects) remain uncharacterized. Future work will include ex vivo tooth-slice models and small-animal REP studies to bridge these translational gaps.
5. Conclusions
This study has demonstrated that a dual-curing hydrogel derived from decellularized amniotic membrane methacryloyl (dAM-MA) offers an injectable, biomimetic scaffold capable of precise mechanical tuning, controlled degradation, and programmable biphasic VEGF release. Thermo-responsive gelation ensures immediate in situ retention, while visible-light photopolymerization delivers stiffness tailored to root canal anatomy. The preserved complexity of dAM’s native ECM (including collagens, glycosaminoglycans, and matricryptic peptides) synergizes with VEGF to drive robust endothelial network formation and stem cell viability, outperforming single-component GelMA. By mimicking physiological growth-factor kinetics through an early burst and a sustained release phase, this platform reconciles the need for both rapid progenitor recruitment and long-term vascular maturation from a single injection. Collectively, these findings position dAM-MA hydrogels as a promising scaffold for regenerative endodontics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9080424/s1, Table S1. Biphasic-model fit and degradation parameters for hydrogels with varying degrees of methacrylation (DoM). Burst fraction (Fb), burst-phase rate constant (kb), and sustained-phase rate constant (ks) were obtained by fitting cumulative-release data to the biphasic exponential model. Degradation rate constants (kd) were determined from first-order mass-loss profiles. Table S2. Quadratic regression summary linking DoM to programmable-release metrics. Equations and coefficients of determination (R2) are shown for burst fraction (Fb), sustained-phase rate constant (ks), and cumulative release at 7 d and 14 d. Figure S1. Burst fraction (Fb) as a function of degree of methacrylation (DoM). Figure S2. Sustained-phase rate constant (ks) versus DoM. Figure S3. Correlation between degradation rate constant (kd) and sustained-phase release rate costant (ks).
Author Contributions
Conceptualization, M.O.; methodology, M.O.; validation, M.O., and D.S.M.-M.; formal analysis, M.O.; investigation, M.O.; resources, J.M.T., M.O.; data curation, M.O.; writing—original draft preparation, M.O.; writing—review and editing, J.M.T., M.O., and D.S.M.-M.; visualization, M.O.; supervision, J.M.T., M.O.; project administration, J.M.T., M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
A generative AI was used to enhance the language and readability of this manuscript. The AI did not contribute to the study’s design, data, or scientific conclusions. The authors retain full responsibility for the final content.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative Endodontics: A Comprehensive Review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp Vascularization during Tooth Development, Regeneration, and Therapy. J. Dent. Res. 2017, 96, 137–144. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of Angiogenesis in Endodontics: Contributions of Stem Cells and Proangiogenic and Antiangiogenic Factors to Dental Pulp Regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Hashemi, M.; Tayebi, L. Microfluidic Synthesis of PLGA/Carbon Quantum Dot Microspheres for Vascular Endothelial Growth Factor Delivery. RSC Adv. 2019, 9, 33246–33256. [Google Scholar] [CrossRef]
- Omidi, M.; Mansouri, V.; Mohammadi Amirabad, L.; Tayebi, L. Impact of Lipid/Magnesium Hydroxide Hybrid Nanoparticles on the Stability of Vascular Endothelial Growth Factor-Loaded PLGA Microspheres. ACS Appl. Mater. Interfaces 2021, 13, 24370–24384. [Google Scholar] [CrossRef]
- Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in Regenerative Dentistry: Are We Far Enough for Therapy? Int. J. Mol. Sci. 2021, 22, 929. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The Angiogenic Potential of DPSCs and SCAPs in an In Vivo Model of Dental Pulp Regeneration. Stem Cells Int. 2017, 2017, 2582080. [Google Scholar] [CrossRef]
- Zisch, A.H.; Lutolf, M.P.; Ehrbar, M.; Raeber, G.P.; Rizzi, S.C.; Davies, N.; Schmökel, H.; Bezuidenhout, D.; Djonov, V.; Zilla, P.; et al. Cell-Demanded Release of VEGF from Synthetic, Biointeractive Cell-Ingrowth Matrices for Vascularized Tissue Growth. FASEB J. 2003, 17, 2260–2262. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Ashoori, A.; Rajabi, S.; Pezeshki-Modaress, M.; Ayati, A.; Mousavi, M.R.; Ellini, M.R.; Kamali, A.; Azarpazhooh, A.; Kishen, A. Human Amniotic Membrane Extracellular Matrix Scaffold for Dental Pulp Regeneration in Vitro and in Vivo. Int. Endod. J. 2022, 55, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D.J. Effects of VEGF Temporal and Spatial Presentation on Angiogenesis. Biomaterials 2010, 31, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-Based Hydrogel for Vascular Endothelial Growth Factor Release in Peripheral Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 459–470. [Google Scholar] [CrossRef]
- Ouyang, L.; Dan, Y.; Shao, Z.; Yang, S.; Yang, C.; Liu, G.; Duan, D. MMP-sensitive PEG Hydrogel Modified with RGD Promotes BFGF, VEGF and EPC-mediated Angiogenesis. Exp. Ther. Med. 2019, 18, 2933–2941. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Bai, H.; Li, R.; Shang, J.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Wang, J.; et al. Sustained Release of VEGF to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front. Bioeng. Biotechnol. 2021, 9, 757767. [Google Scholar] [CrossRef]
- Jeon, O.; Powell, C.; Solorio, L.D.; Krebs, M.D.; Alsberg, E. Affinity-Based Growth Factor Delivery Using Biodegradable, Photocrosslinked Heparin-Alginate Hydrogels. J. Control. Release 2011, 154, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Yang, J.; Dang, H.; Xu, Y. Recent Advancement of Decellularization Extracellular Matrix for Tissue Engineering and Biomedical Application. Artif. Organs 2022, 46, 549–567. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Kou, L.; Xu, H.L.; Zhao, Y.Z. Recent Development and Biomedical Applications of Decellularized Extracellular Matrix Biomaterials. Mater. Sci. Eng. C 2019, 104, 109942. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Shah, R.; Raghurama R Somala, S.; Anwar, K.N.; Shen, X.; An, S.; Omidi, M.; Rosenblatt, M.I.; Shokuhfar, T.; Djalilian, A.R. In-Situ Porcine Corneal Matrix Hydrogel as Ocular Surface Bandage. Ocul. Surf. 2021, 21, 27–36. [Google Scholar] [CrossRef]
- Basara, G.; Yue, X.; Zorlutuna, P. Dual Crosslinked Gelatin Methacryloyl Hydrogels for Photolithography and 3D Printing. Gels 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, Q.; Han, Y.; Chen, H.; Tan, Y. Doubly Dynamic Hydrogel Formed by Combining Boronate Ester and Acylhydrazone Bonds. Polymers 2020, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, G.; Jiang, Y.; Rabiee, B.; Omidi, M.; Rosenblatt, M.I.; Shokuhfar, T.; Pan, Y.; Naba, A.; Djalilian, A.R. Fabrication, Rheological, and Compositional Characterization of Thermoresponsive Hydrogel from Cornea. Tissue Eng. Part C Methods 2021, 27, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kafili, G.; Tamjid, E.; Niknejad, H.; Simchi, A. Development of Injectable Hydrogels Based on Human Amniotic Membrane and Polyethyleneglycol-Modified Nanosilicates for Tissue Engineering Applications. Eur. Polym. J. 2022, 179, 111566. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Shen, X.; Nguyen, T.; Anwar, K.N.; Jeon, O.; Jiang, Y.; Pachenari, M.; Pan, Y.; Shokuhfar, T.; Rosenblatt, M.I.; et al. A Light-Curable and Tunable Extracellular Matrix Hydrogel for In Situ Suture-Free Corneal Repair. Adv. Funct. Mater. 2022, 32, 2113383. [Google Scholar] [CrossRef]
- Jonidi Shariatzadeh, F.; Solouk, A.; Bagheri Khoulenjani, S.; Bonakdar, S.; Mirzadeh, H. Injectable and Reversible Preformed Cryogels Based on Chemically Crosslinked Gelatin Methacrylate (GelMA) and Physically Crosslinked Hyaluronic Acid (HA) for Soft Tissue Engineering. Colloids Surf. B Biointerfaces 2021, 203, 111725. [Google Scholar] [CrossRef]
- Song, J.; Gerecht, S. Hydrogels to Recapture Extracellular Matrix Cues That Regulate Vascularization. Arterioscler. Thromb. Vasc. Biol. 2023, 43, E291–E302. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org (accessed on 14 July 2025).
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, B.; Zou, L.; Huang, X.; Yang, F.; Lv, H. Decellularized Human Amniotic Membrane Scaffolds: Influence on the Biological Behavior of Dental Pulp Stem Cells. BMC Oral Health 2024, 24, 394. [Google Scholar] [CrossRef]
- de Oliveira, C.L.L.; Ferreira, F.M.; Puppin-Rontani, J.; Puppin-Rontani, R.M.; Pascon, F.M. Potential of Irrigants and Medicaments in Regenerative Endodontics: Insights from a Systematic Review on Dentin Growth Factor Release. Odontology 2025. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Rehab Khan, M.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef]
- Hong, S.; Li, L.; Cai, W.; Jiang, B. The Potential Application of Concentrated Growth Factor in Regenerative Endodontics. Int. Endod. J. 2019, 52, 646–655. [Google Scholar] [CrossRef]
- Roy, A.; Mantay, M.; Brannan, C.; Griffiths, S. Placental Tissues as Biomaterials in Regenerative Medicine. BioMed Res. Int. 2022, 2022, 6751456. [Google Scholar] [CrossRef]
- Salgado García, M.G.; Díaz, N.F.; García López, G.; Álvarez Maya, I.; Hernández Jimenez, C.; Roman Maldonado, Y.; Aguayo, D.J.M.; Martínez, N.E.D. Evaluation Methods for Decellularized Tissues: A Focus on Human Amniotic Membrane. J. Biosci. Bioeng. 2025, 139, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tauseef, H.; Ainuddin, J.A.; Zafar, M.; Khan, I.; Salim, A.; Mirza, M.R.; Mohiuddin, O.A. Assessment of the Proteome Profile of Decellularized Human Amniotic Membrane and Its Biocompatibility with Umbilical Cord-Derived Mesenchymal Stem Cells. J. Biomed. Mater. Res. Part A 2024, 112, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Coombe, D.R.; Kett, W.C. Heparan Sulfate-Protein Interactions: Therapeutic Potential through Structure-Function Insights. Cell. Mol. Life Sci. 2005, 62, 410–424. [Google Scholar] [CrossRef]
- Parmar, U.P.S.; Surico, P.L.; Scarabosio, A.; Barone, V.; Singh, R.B.; D’Ancona, F.; Zeppieri, M.; Parodi, P.C.; Mori, T.; Cutrupi, F.; et al. Amniotic Membrane Transplantation for Wound Healing, Tissue Regeneration and Immune Modulation. Stem Cell Rev. Rep. 2025, 21, 1428–1448. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and Application in the Evolution of Biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Parmeggiani, D.; Barbarisi, A. Smart Materials as Scaffolds for Tissue Engineering. J. Cell. Physiol. 2005, 203, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. Biomimetic Nanofibrous Scaffolds for Bone Tissue Engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).