Investigating the Properties of Composite Cement-Based Mortar Containing High Volumes of GGBS and CCR
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Experimental Work
2.2.1. Samples Preparation
2.2.2. Testing
Characteristics of the Fresh Mix
Compressive Strength
Flexural Strengths
SEM and EDX
3. Results and Discussion
3.1. Fresh Characteristics
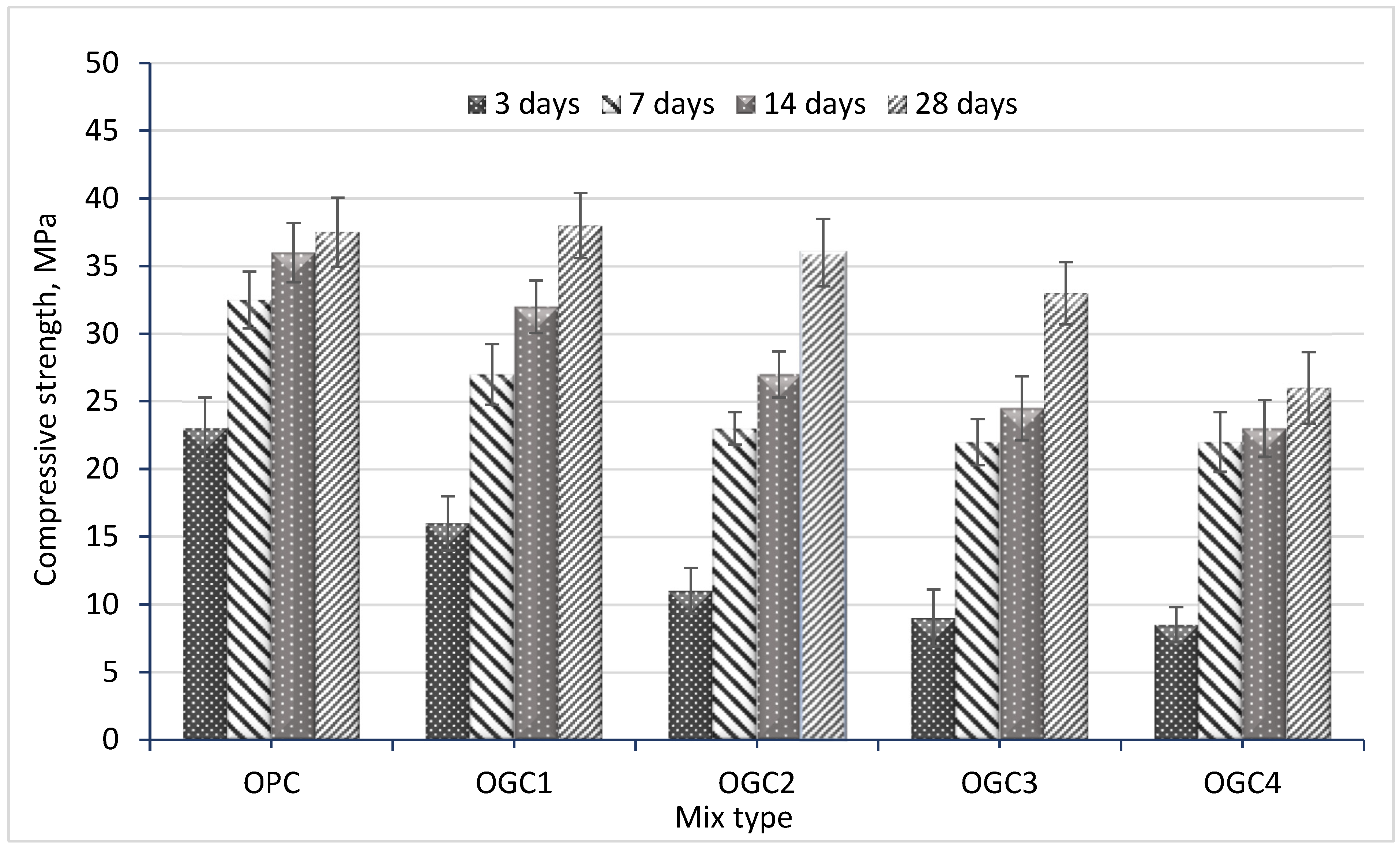
3.2. Compressive Strength Results
3.3. Flexural Strength Results
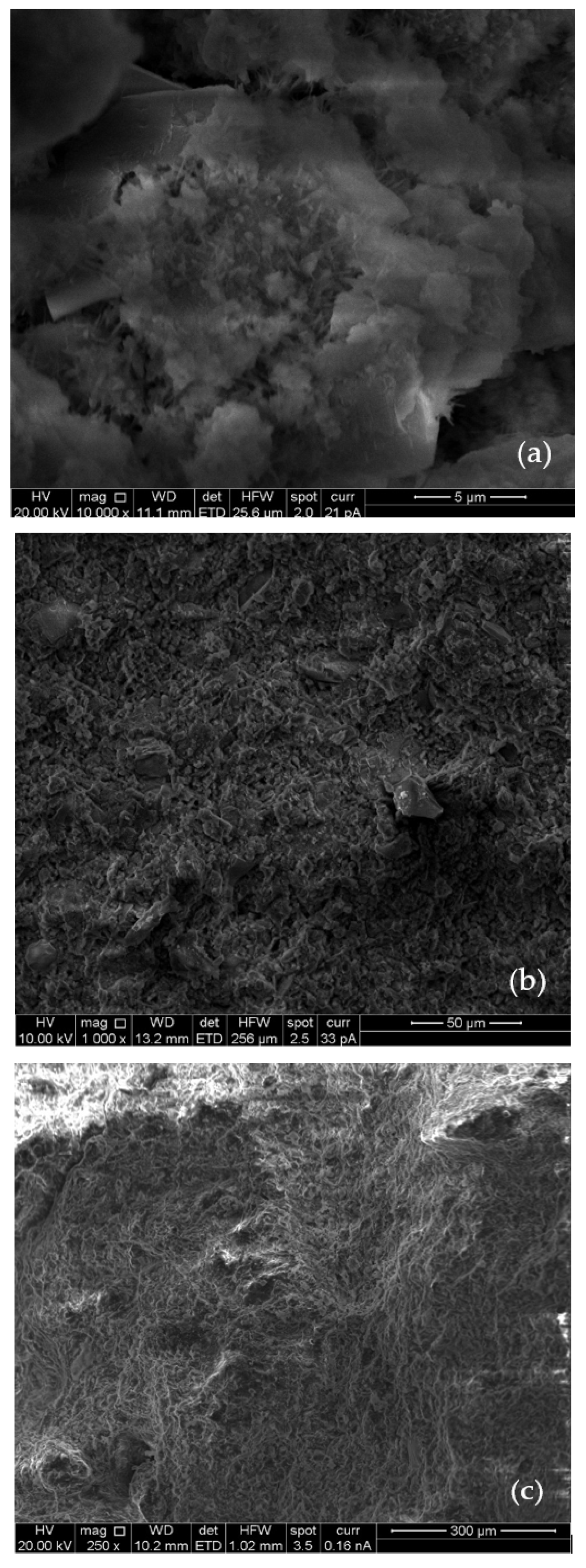
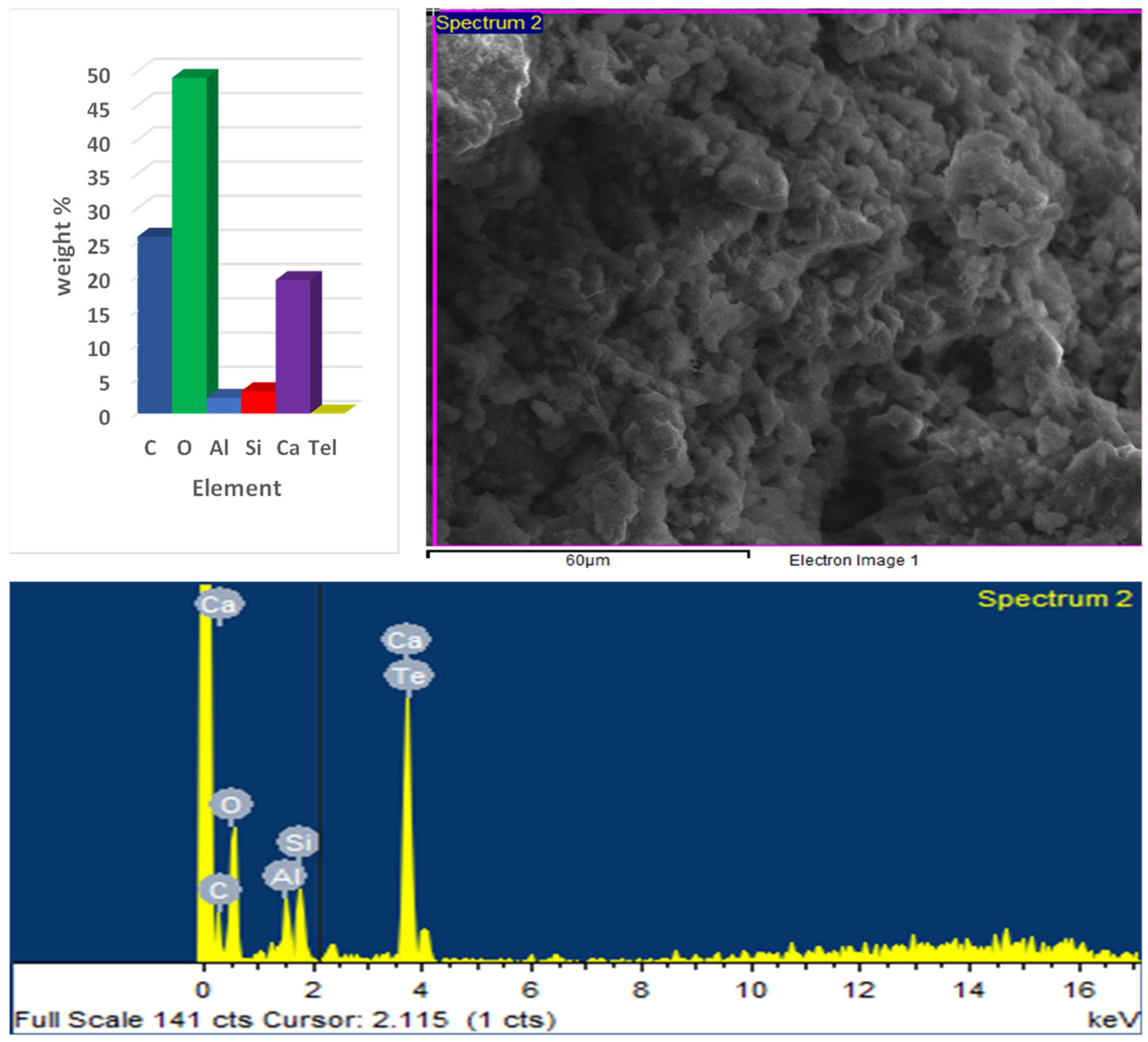
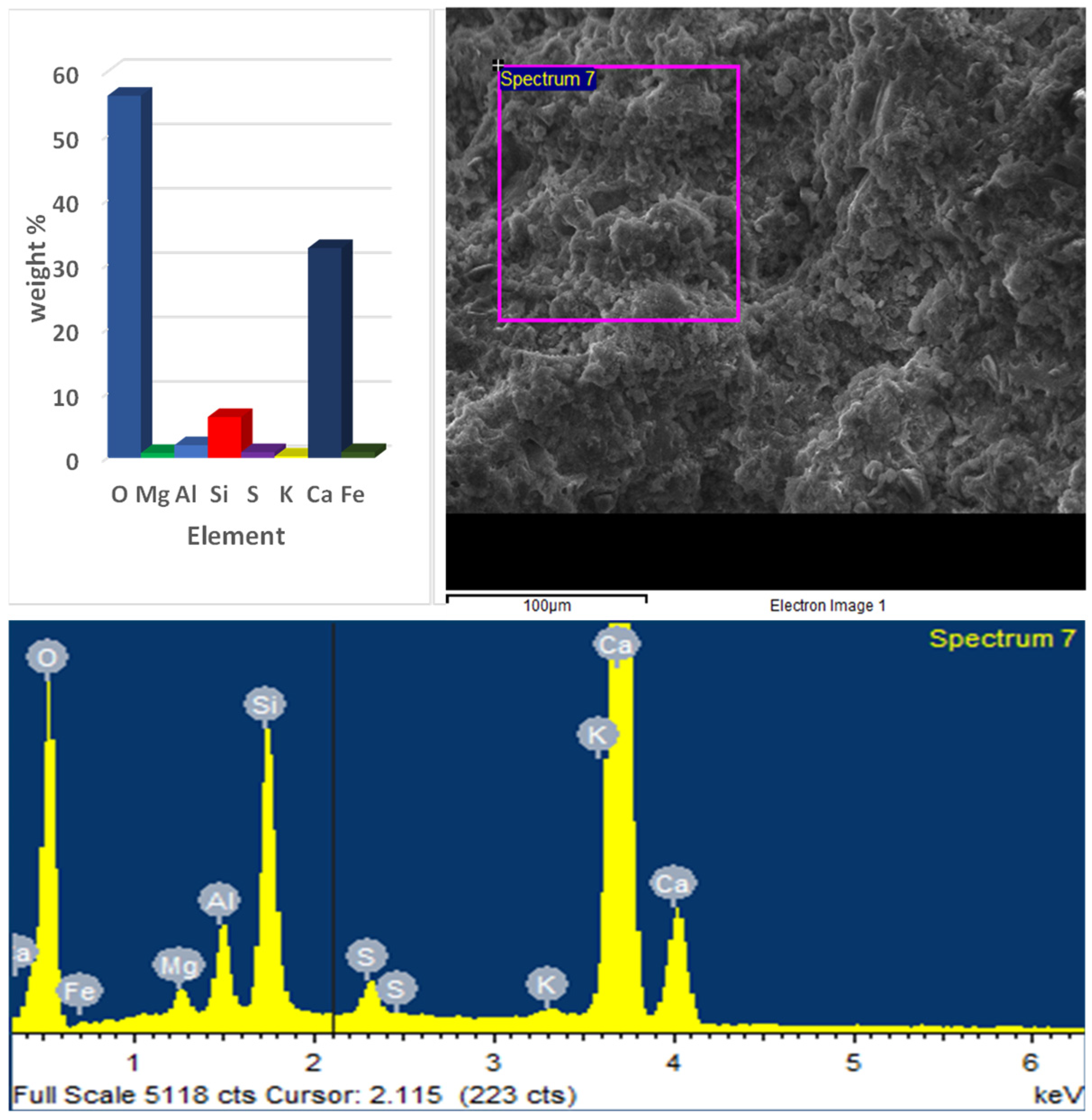
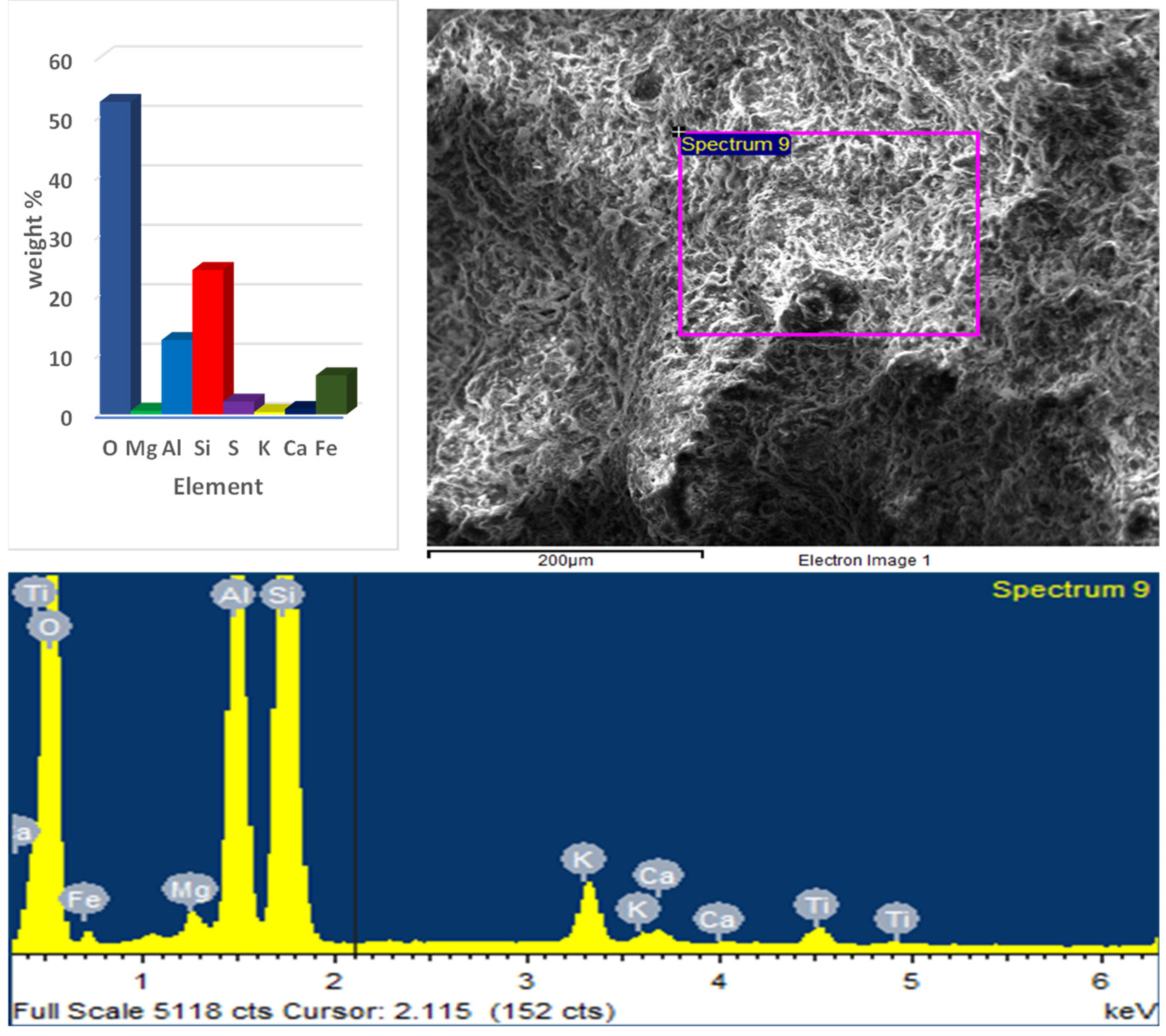
3.4. SEM and EDX Results
4. Conclusions
- -
- The increase in the replacement level of OPC in ternary binders by CCR-GGBS increased consistency, initial and final setting times;
- -
- Although the replacement of OPC by CCR-GGBS significantly reduced the early compressive and flexural strengths at 3 days, the strength development at 7 days was significantly greater than OPC mortars. The developed strength was comparable to OPC at 28 days of curing for all mixtures except for OGC4;
- -
- SEM images revealed the presence of ettringite, which contributes to early strength development, along with C–S–H and CH. At later curing stages, the formation of C–S–H and CH dominated, resulting in a denser and more compact microstructure;
- -
- EDX analysis of the optimum paste showed a significant presence of Ca at early ages, while later ages exhibited lower Ca content and higher concentrations of Si and Al. This indicated the activation of GGBS and the formation of C–S–H and ettringite;
- -
- It can thus be concluded that using CCR to activate GGBS holds significant potential for producing construction materials suitable for various applications.
- -
- While CCR effectively activates GGBS and enhances early strength, its high calcium hydroxide content—especially at substitution levels up to 26.5%—raises concerns about increased carbonation risk and long-term durability. The EDX results confirm elevated Ca levels. CCR may be better suited as a lower-dosage additive rather than a major cement replacement. Further studies on carbonation resistance, sulfate attack, and pore structure stability are needed to validate its long-term performance.
- -
- Among the tested binders, the OGC3 mixture with a 1:1:0.5 ratio of OPC:GGBS:CCR (40% OPC, 40% GGBS, 20% CCR) was identified as the optimal formulation. This blend provided the best balance of early and late-age mechanical performance, closely approaching the strength of OPC mortar at 28 days. It also avoided the reduction in strength observed in OGC4, where excessive CCR content likely led to microstructural inefficiencies. Moreover, OGC3 offers improved sustainability due to a 60% reduction in OPC content and the beneficial reuse of industrial waste materials, making it attractive for environmentally responsible construction applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammed, A.; Rafiq, S.; Mahmood, W.; Noaman, R.; Ghafor, K.; Qadir, W.; Kadhum, Q. Characterization and modeling the flow behavior and compression strength of the cement paste modified with silica nano-size at different temperature conditions. Constr. Build. Mater. 2020, 257, 119590. [Google Scholar] [CrossRef]
- Wu, S.; Shao, Z.; Andrew, R.M.; Bing, L.; Wang, J.; Niu, L.; Liu, Z.; Xi, F. Global CO₂ uptake by cement materials accounts 1930–2023. Sci. Data 2024, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Coudray, C.; Anger, B.; Moulin, I.; Colina, H.; Izoret, L.; Théry, F.; Smith, A. Beneficial reuse of dam fine sediments as clinker raw material. Constr. Build. Mater. 2019, 218, 365–384. [Google Scholar] [CrossRef]
- Her, S.; Park, T.; Zalnezhad, E.; Bae, S. Synthesis and characterization of cement clinker using recycled pulverized oyster and scallop shell as limestone substitutes. J. Clean. Prod. 2021, 278, 123987. [Google Scholar] [CrossRef]
- Chandrasiri, C.; Yehdego, T.; Peethamparan, S. Synthesis and characterization of bio-cement from conch shell waste. Constr. Build. Mater. 2019, 212, 775–786. [Google Scholar] [CrossRef]
- Patel, Y.J.; Shah, N. Enhancement of the properties of Ground Granulated Blast Furnace Slag based Self Compacting Geopolymer Concrete by incorporating Rice Husk Ash. Constr. Build. Mater. 2018, 171, 654–662. [Google Scholar] [CrossRef]
- Zakka, W.P.; Abdul Shukor Lim, N.H.; Chau Khun, M. A scientometric review of geopolymer concrete. J. Clean. Prod. 2021, 280, 124353. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A.; Gobinath, R. Role of recycling fine materials as filler for improving performance of concrete—A review. Aust. J. Civ. Eng. 2019, 17, 85–95. [Google Scholar] [CrossRef]
- Al Nageim, H.; Dulaimi, A.; Ruddock, F.; Seton, L. Development of a new cementitious filler for use in fast-curing cold binder course in pavement application. In Proceedings of the 38th International Conference on Cement Microscopy, Lyon, France, 17–21 April 2016. pp. 167–180.
- Wang, Q.; Li, J.; Yao, G.; Zhu, X.; Hu, S.; Qiu, J.; Chen, P.; Lyu, X. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.-K.; Wang, Z.-M.; Lyu, X.-J. Physical properties, hydration mechanism, and leaching evaluation of the Portland cement prepared from carbide residue. J. Clean. Prod. 2022, 366, 132777. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Fadele, O.; Otieno, M. Utilisation of supplementary cementitious materials from agricultural wastes: A review. Proc. Inst. Civ. Eng.—Constr. Mater. 2022, 175, 65–71. [Google Scholar] [CrossRef]
- Divsholi, B.S.; Lim, T.Y.D.; Teng, S. Durability Properties and Microstructure of Ground Granulated Blast Furnace Slag Cement Concrete. Int. J. Concr. Struct. Mater. 2014, 8, 157–164. [Google Scholar] [CrossRef]
- Singh, H.S.; Shukla, G. Regression analysis of properties of GGBS based bacillus subtilis bacterial concrete. Asian J. Civ. Eng. 2024, 25, 4461–4469. [Google Scholar] [CrossRef]
- Sumesh, M.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H.; Alnahhal, M.F. Incorporation of nano-materials in cement composite and geopolymer based paste and mortar—A review. Constr. Build. Mater. 2017, 148, 62–84. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Łukowski, P.; Salih, A. Durability of Mortars Containing Ground Granulated Blast-furnace Slag in Acid and Sulphate Environment. Procedia Eng. 2015, 108, 47–54. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Influence of slag characteristics on strength development and reaction products in a CaO-activated slag system. Cem. Concr. Compos. 2016, 72, 155–167. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Su, Y.; Yang, J.; Strnadel, B.; Wang, X. Efficiency of wet-grinding on the mechano-chemical activation of granulated blast furnace slag (GBFS). Constr. Build. Mater. 2019, 199, 185–193. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]
- Yu, H.; Yi, Y.; Unluer, C. Heat of hydration, bleeding, viscosity, setting of Ca(OH)2-GGBS and MgO-GGBS grouts. Constr. Build. Mater. 2021, 270, 121839. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Shah, S.N.R.; Ramli Sulong, N.H.; Shariati, M.; Khan, R.; Jumaat, M.Z. Behavior of steel pallet rack beam-to-column connections at elevated temperatures. Thin-Walled Struct. 2016, 106, 471–483. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, X.; Guo, L.; Zhang, S.; Chen, J. Strength and hydration products of cemented paste backfill from sulphide-rich tailings using reactive MgO-activated slag as a binder. Constr. Build. Mater. 2019, 203, 111–119. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, Z.; Huo, W.; Yang, L.; Zhang, Y.; Sun, H.; Zhang, X. Assessing the viability of a high performance one-part geopolymer made from fly ash and GGBS at ambient temperature. J. Build. Eng. 2023, 75, 106978. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sustainable geopolymer concrete using ground granulated blast furnace slag and rice husk ash: Strength and permeability properties. J. Clean. Prod. 2018, 205, 49–57. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Ajao, A.O.; Saka, H.B.; Oladipo, A.C. Calcium-carbide residue: A precursor for the synthesis of CaO–Al2O3–SiO2–CaSO4 solid acid catalyst for biodiesel production using waste lard. Chem. Eng. J. Adv. 2020, 4, 100033. [Google Scholar] [CrossRef]
- Li Xin, C.H.; Huang, B. Simultaneous Determination of 9 Heavy Metal Elements in Calcium Carbide Slag by ICP-MS. J. Insp. Quar. 2020, 30, 24–28. [Google Scholar]
- Cao, Y.; Yang, Y.-D.; Ying, Y.-M. Ecological risk assessment and cause analysis of heavy metals in carbide slag dump. China Environ. Sci. 2021, 41, 1293. [Google Scholar]
- Huo, H.; Liu, X.; Wen, Z.; Lou, G.; Dou, R.; Su, F.; Zhou, W.; Jiang, Z. Case study of a novel low rank coal to calcium carbide process based on techno-economic assessment. Energy 2021, 228, 120566. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Kampala, A.; Jitsangiam, P.; Horpibulsuk, S. Performance and evaluation of calcium carbide residue stabilized lateritic soil for construction materials. Case Stud. Constr. Mater. 2020, 13, e00389. [Google Scholar] [CrossRef]
- Dulaimi, A.; Shanbara, H.K.; Jafer, H.; Sadique, M. An evaluation of the performance of hot mix asphalt containing calcium carbide residue as a filler. Constr. Build. Mater. 2020, 261, 119918. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, T.; Zhang, J.; Wang, Z.; Liu, J.; Cao, J.; Wang, C. Recycling and utilization of calcium carbide slag-current status and new opportunities. Renew. Sustain. Energy Rev. 2022, 159, 112133. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Orogbade, B.O.; Okoro, C.W. The potential of calcium carbide waste and termite mound soil as materials in the production of unfired clay bricks. J. Clean. Prod. 2021, 279, 123693. [Google Scholar] [CrossRef]
- Adamu, M.; Ibrahim, Y.E.; Al-Atroush, M.E.; Alanazi, H. Mechanical Properties and Durability Performance of Concrete Containing Calcium Carbide Residue and Nano Silica. Materials 2021, 14, 6960. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zhao, Q.; Song, R.; Liu, J. Mechanical properties and microstructure of binding material using slag-fly ash synergistically activated by wet-basis soda residue-carbide slag. Constr. Build. Mater. 2021, 269, 121301. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.; He, X.; Yang, W.; Deng, X. Utilization of carbide slag-granulated blast furnace slag system by wet grinding as low carbon cementitious materials. Constr. Build. Mater. 2020, 249, 118763. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y. Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater. 2020, 238, 117713. [Google Scholar] [CrossRef]
- European Committee for Standardization. Cement–Part 1: Composition, Specifications Conformity Criteria for Common Cements; British Standards Institution: London, UK, 2011. [Google Scholar]
- BS EN 1008:2002; Mixing Water for Concrete—Specification for Sampling, Testing and Assessing the Suitability of Water, Including Water Recovered from Processes in the Concrete Industry, as Mixing Water for Concrete. BSI: London, UK, 2002.
- Reddy Babu, G.; Reddy, B.M.; Venkata Ramana, N. Quality of mixing water in cement concrete “a review”. Mater. Today Proc. 2018, 5 Pt 1, 1313–1320. [Google Scholar] [CrossRef]
- Celik, O.; Damsi, E.; Pişkin, S. Characterization of fly ash and its effect on the compressive strength properties of portland cement. Indian J. Eng. Mater. Sci. 2008, 15, 433–440. [Google Scholar]
- Chaunsali, P.; Peethamparan, S. Evolution of strength, microstructure and mineralogical composition of a CKD–GGBFS binder. Cem. Concr. Res. 2011, 41, 197–208. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Damrongwiriyanupap, N. Comparative study using Portland cement and calcium carbide residue as a promoter in bottom ash geopolymer mortar. Constr. Build. Mater. 2017, 133, 128–134. [Google Scholar] [CrossRef]
- Hester, D.; McNally, C.; Richardson, M. A study of the influence of slag alkali level on the alkali–silica reactivity of slag concrete. Constr. Build. Mater. 2005, 19, 661–665. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R. A comprehensive review on compressive strength and microstructure properties of GGBS-based geopolymer binder systems. Constr. Build. Mater. 2024, 417, 135242. [Google Scholar] [CrossRef]
- Dom, A.A.M.; Jamaluddin, N.; Hamid, N.A.A.; Hoon, C.S. A Review: GGBS as a Cement Replacement in Concrete. IOP Conf. Ser. Earth Environ. Sci. 2022, 1022, 012044. [Google Scholar]
- BS EN 196-3 and A1; Method of Testing Cement. Determination of Setting Time and Soundness. British Standards Institution: London, UK, 2008.
- BS EN 196-1; Methods of Testing Cement–Part 1: Determination of Strength. British Standards Institution: London, UK, 2005.
- BS EN 12390-5; Testing Hardened Concrete. Flexural Strength of Test Specimens. British Standards Institution: London, UK, 2019.
- Salih, M.A.; Farzadnia, N.; Abang Ali, A.A.; Demirboga, R. Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient temperature. Constr. Build. Mater. 2015, 93, 289–300. [Google Scholar] [CrossRef]
- Dave, N.; Misra, A.K.; Srivastava, A.; Kaushik, S.K. Experimental analysis of strength and durability properties of quaternary cement binder and mortar. Constr. Build. Mater. 2016, 107, 117–124. [Google Scholar] [CrossRef]
- Terzić, A.; Radulović, D.; Pezo, L.; Andrić, L.; Miličić, L.; Stojanović, J.; Grigorova, I. The effect of mechano-chemical activation and surface treatment of limestone filler on the properties of construction composites. Compos. Part B Eng. 2017, 117, 61–73. [Google Scholar] [CrossRef]
- Attari, A.; McNally, C.; Richardson, M.G. A probabilistic assessment of the influence of age factor on the service life of concretes with limestone cement/GGBS binders. Constr. Build. Mater. 2016, 111, 488–494. [Google Scholar] [CrossRef]
- Jafer, H.; Atherton, W.; Sadique, M.; Ruddock, F.; Loffill, E. Stabilisation of soft soil using binary blending of high calcium fly ash and palm oil fuel ash. Appl. Clay Sci. 2018, 152, 323–332. [Google Scholar] [CrossRef]
- Dulaimi, A.; Shanbara, H.K.; Al-Rifaie, A. The mechanical evaluation of cold asphalt emulsion mixtures using a new cementitious material comprising ground-granulated blast-furnace slag and a calcium carbide residue. Constr. Build. Mater. 2020, 250, 118808. [Google Scholar] [CrossRef]
- Cheah, C.B.; Chung, K.Y.; Ramli, M.; Lim, G.K. The engineering properties and microstructure development of cement mortar containing high volume of inter-grinded GGBS and PFA cured at ambient temperature. Constr. Build. Mater. 2016, 122, 683–693. [Google Scholar] [CrossRef]
- Sadique, M.; Al-Nageim, H.; Atherton, W.; Seton, L.; Dempster, N. Mechano-chemical activation of high-Ca fly ash by cement free blending and gypsum aided grinding. Constr. Build. Mater. 2013, 43, 480–489. [Google Scholar] [CrossRef]
- Sadique, M.; Al Nageim, H.; Atherton, W.; Seton, L.; Dempster, N. A new composite cementitious material for construction. Constr. Build. Mater. 2012, 35, 846–855. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. A calorimetric study of early hydration of alkali-slag cements. Cem. Concr. Res. 1995, 25, 1333–1346. [Google Scholar] [CrossRef]
- Poon, C.S.; Kou, S.C.; Lam, L.; Lin, Z.S. Activation of fly ash/cement systems using calcium sulfate anhydrite (CaSO4). Cem. Concr. Res. 2001, 31, 873–881. [Google Scholar] [CrossRef]
- Nassar, A.I.; Mohammed, M.K.; Thom, N.; Parry, T. Mechanical, durability and microstructure properties of Cold Asphalt Emulsion Mixtures with different types of filler. Constr. Build. Mater. 2016, 114, 352–363. [Google Scholar] [CrossRef]
- Dulaimi, A.; Al Nageim, H.; Ruddock, F.; Seton, L. High performance cold asphalt concrete mixture for binder course using alkali-activated binary blended cementitious filler. Constr. Build. Mater. 2017, 141, 160–170. [Google Scholar] [CrossRef]

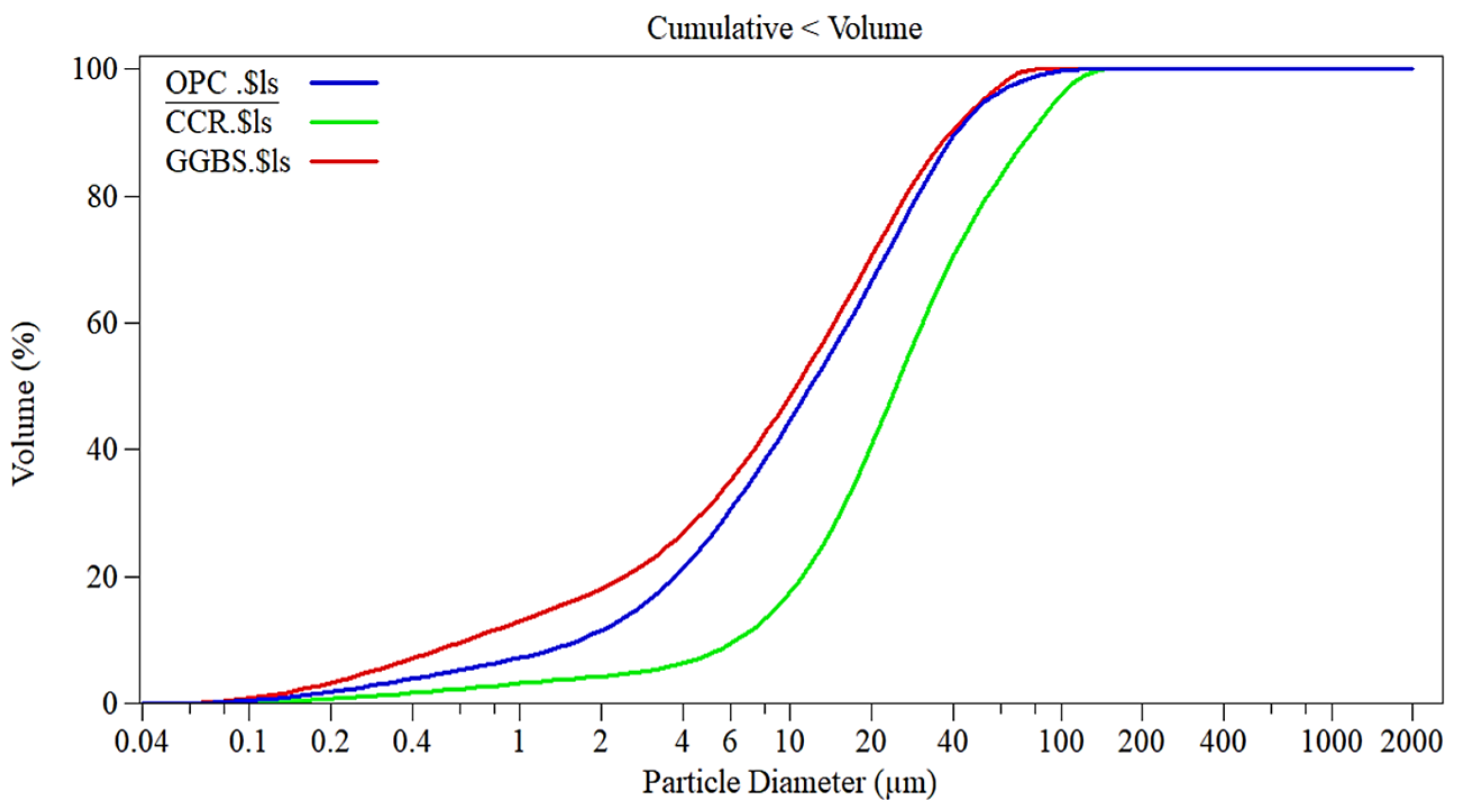
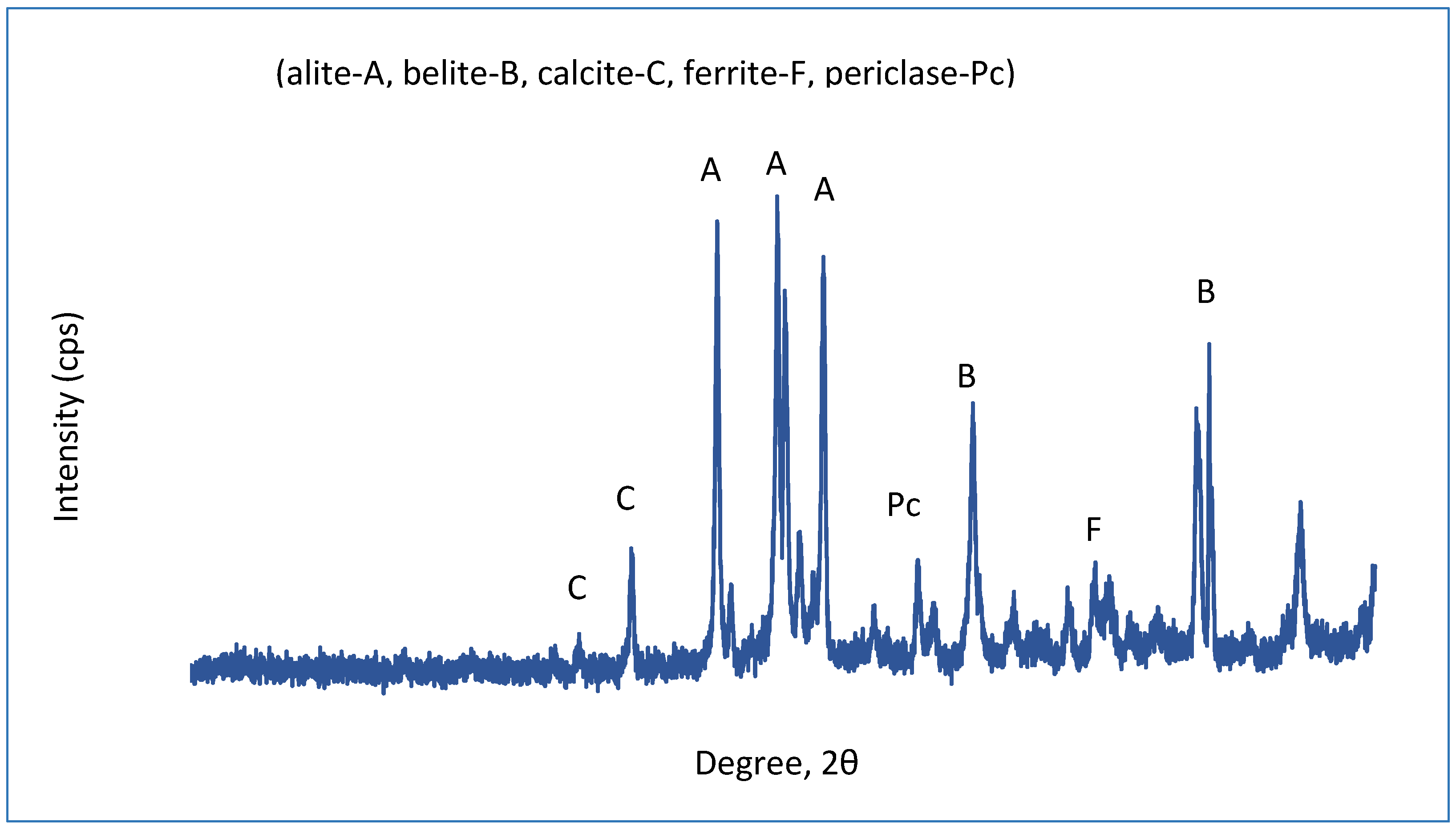

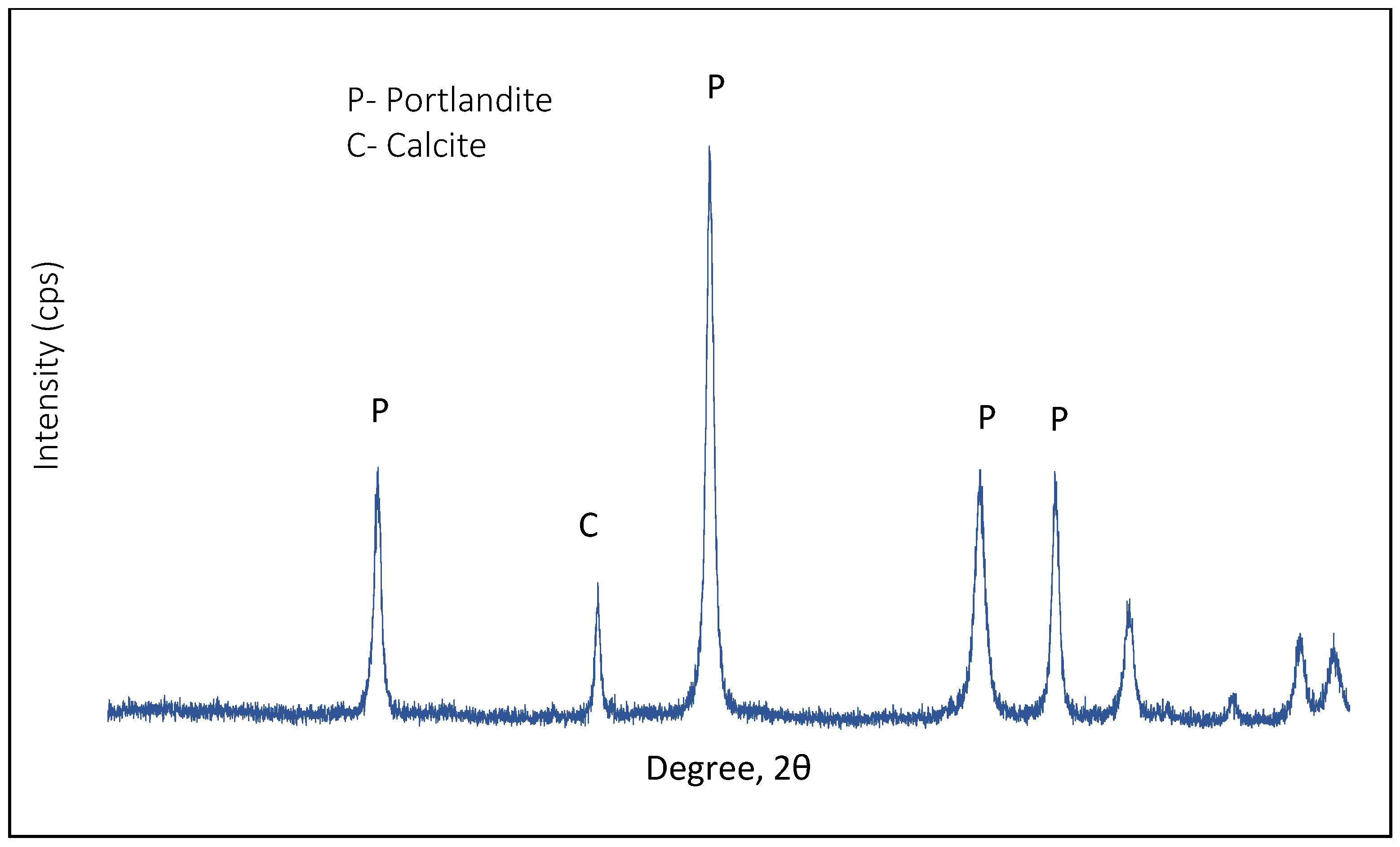
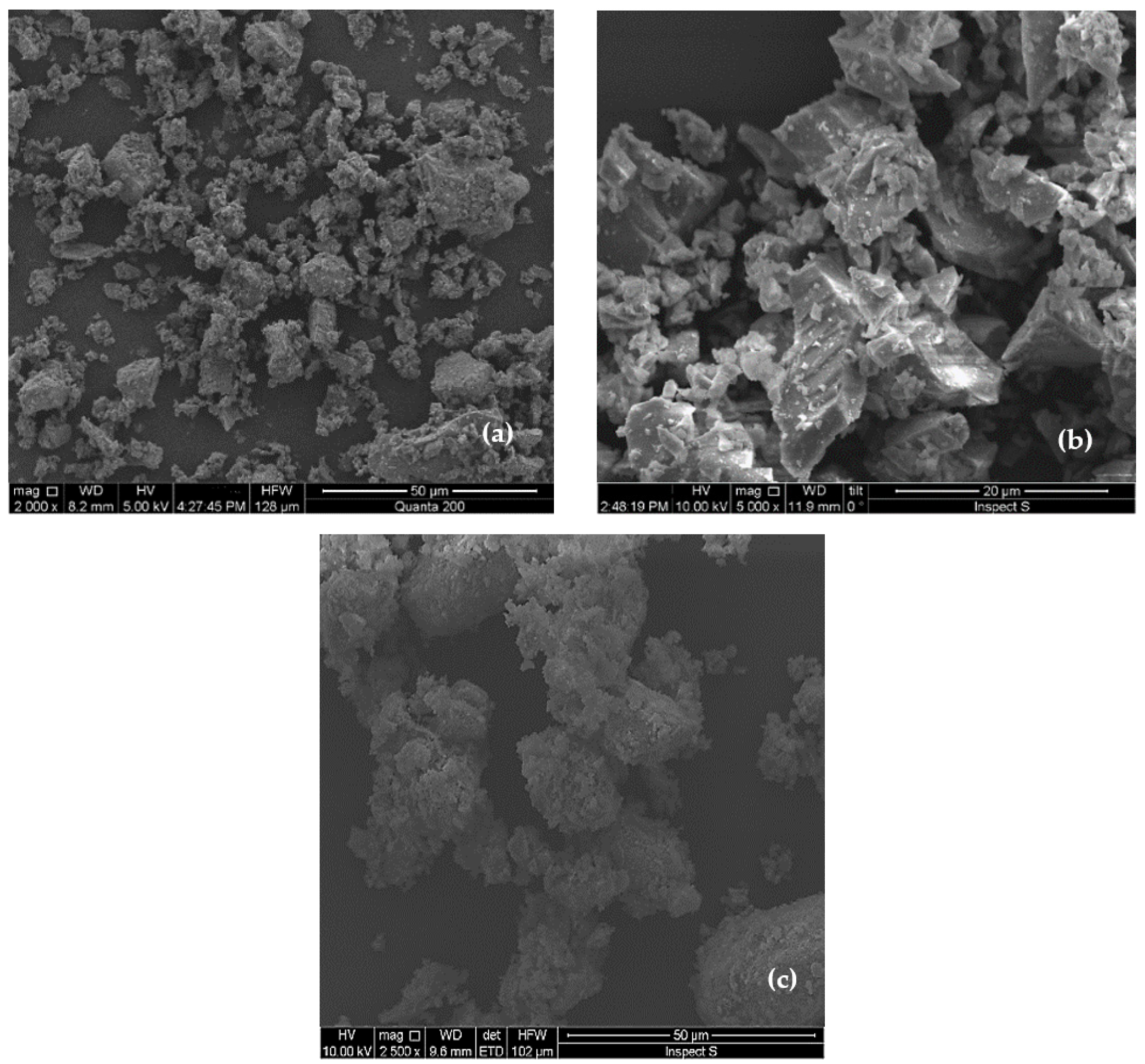


| Material | D10 (µm) | D50 (µm) | D90 (µm) |
|---|---|---|---|
| OPC | 1.0 | 10 | 40 |
| CCR | 2.0 | 20 | 80 |
| GGBS | 1.5 | 12 | 50 |
| Material | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | K2O | TiO2 | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| OPC | 62.379 | 26.639 | 2.435 | 1.572 | 1.745 | 2.588 | 0.724 | 0.385 | 1.533 |
| GGBS | 41.562 | 38.265 | 5.426 | 3.942 | 0.121 | 0.00 | 0.536 | 0.624 | 2.721 |
| CCR | 81.84 | 14.08 | 0.90 | 0.77 | 0.00 | 0.77 | 0.20 | 0.12 | 1.32 |
| Mix ID | OPC (%) | GGBS (%) | CCR (%) | Binder (g) | Water (g) | Sand (g) | Water/Binder Ratio | Binder/Sand Ratio |
|---|---|---|---|---|---|---|---|---|
| OPC | 100 | 0 | 0 | 150 | 60 | 375 | 0.40 | 2.5 |
| OGC1 | 80 | 13.3 | 6.6 | 150 | 60 | 375 | 0.40 | 2.5 |
| OGC2 | 60 | 26.6 | 13.3 | 150 | 60 | 375 | 0.40 | 2.5 |
| OGC3 | 40 | 40 | 20 | 150 | 60 | 375 | 0.40 | 2.5 |
| OGC4 | 20 | 53.3 | 26.6 | 150 | 60 | 375 | 0.40 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jwaida, Z.; Jadooe, A.; Dulaimi, A.; Almuhanna, R.R.A.; Hawesah, H.A.; Bernardo, L.F.A.; Andrade, J.M.d.A. Investigating the Properties of Composite Cement-Based Mortar Containing High Volumes of GGBS and CCR. J. Compos. Sci. 2025, 9, 301. https://doi.org/10.3390/jcs9060301
Jwaida Z, Jadooe A, Dulaimi A, Almuhanna RRA, Hawesah HA, Bernardo LFA, Andrade JMdA. Investigating the Properties of Composite Cement-Based Mortar Containing High Volumes of GGBS and CCR. Journal of Composites Science. 2025; 9(6):301. https://doi.org/10.3390/jcs9060301
Chicago/Turabian StyleJwaida, Zahraa, Awad Jadooe, Anmar Dulaimi, Raid R. A. Almuhanna, Hayder Al Hawesah, Luís Filipe Almeida Bernardo, and Jorge Miguel de Almeida Andrade. 2025. "Investigating the Properties of Composite Cement-Based Mortar Containing High Volumes of GGBS and CCR" Journal of Composites Science 9, no. 6: 301. https://doi.org/10.3390/jcs9060301
APA StyleJwaida, Z., Jadooe, A., Dulaimi, A., Almuhanna, R. R. A., Hawesah, H. A., Bernardo, L. F. A., & Andrade, J. M. d. A. (2025). Investigating the Properties of Composite Cement-Based Mortar Containing High Volumes of GGBS and CCR. Journal of Composites Science, 9(6), 301. https://doi.org/10.3390/jcs9060301










