Facile Synthesis of Conductive Copolymers and Its Supercapacitor Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PANI, PANI-PPy, PANI-PTh, and PANI-PEDOT
2.3. Methods
3. Results and Discussion
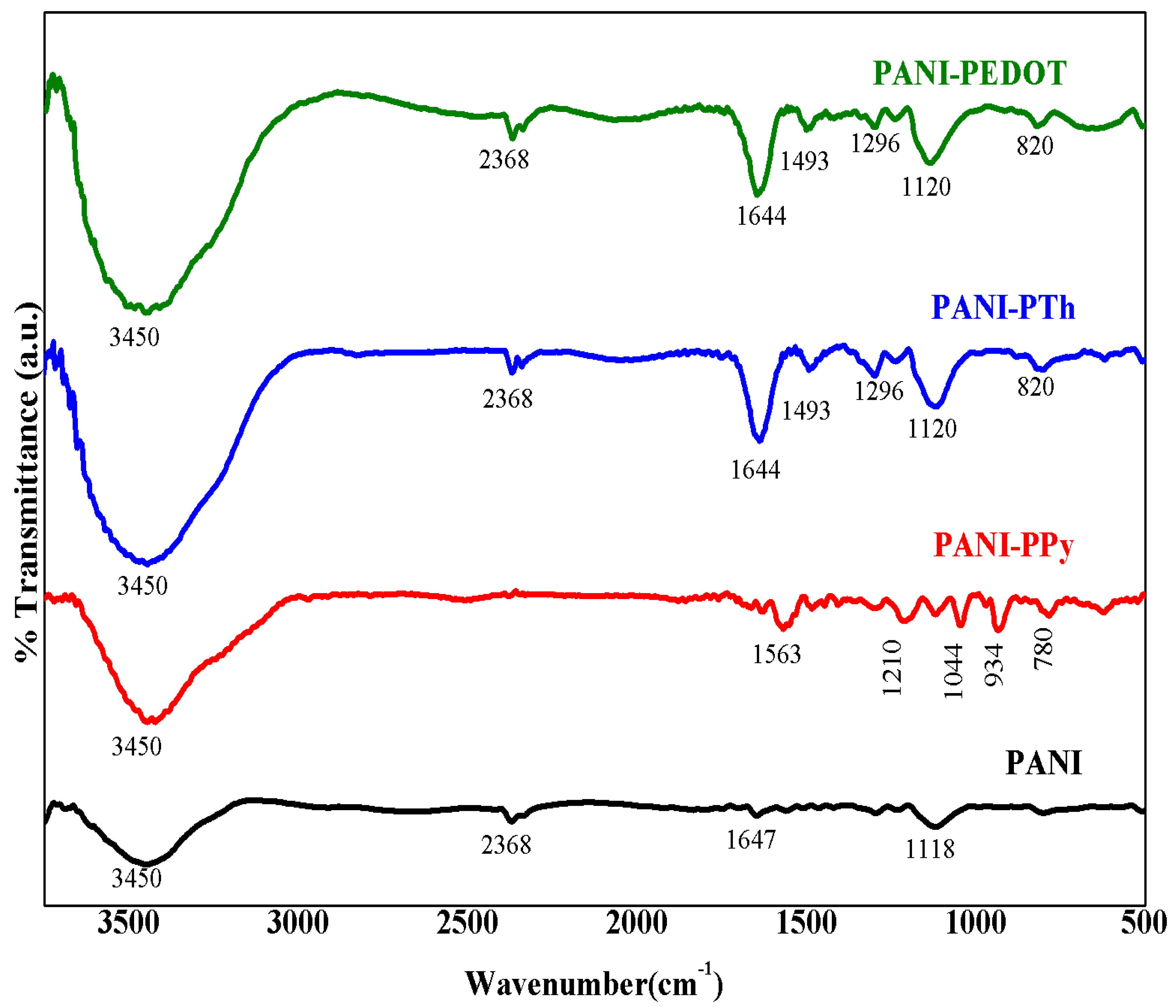
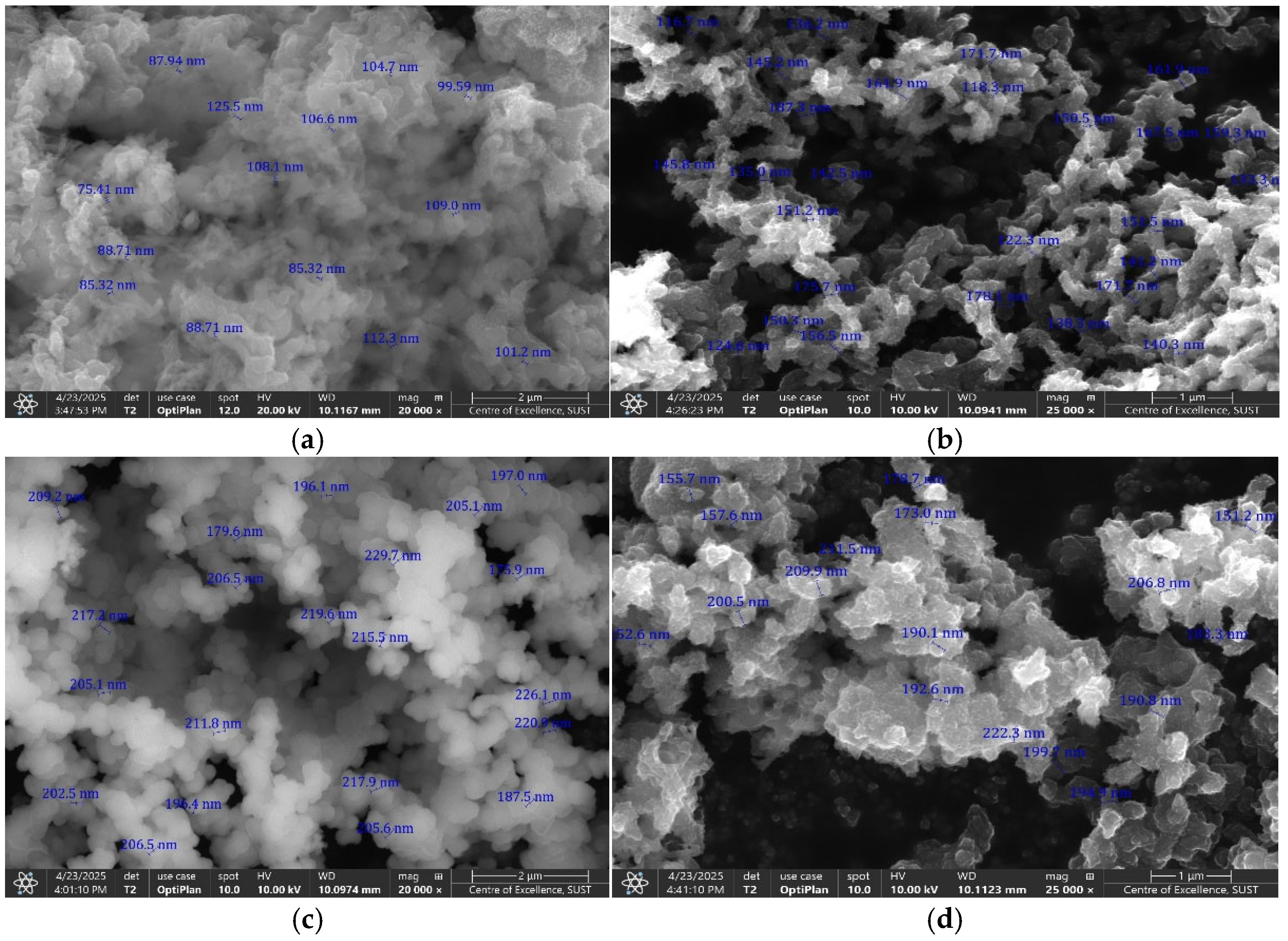
3.1. Structural and Morphological Properties
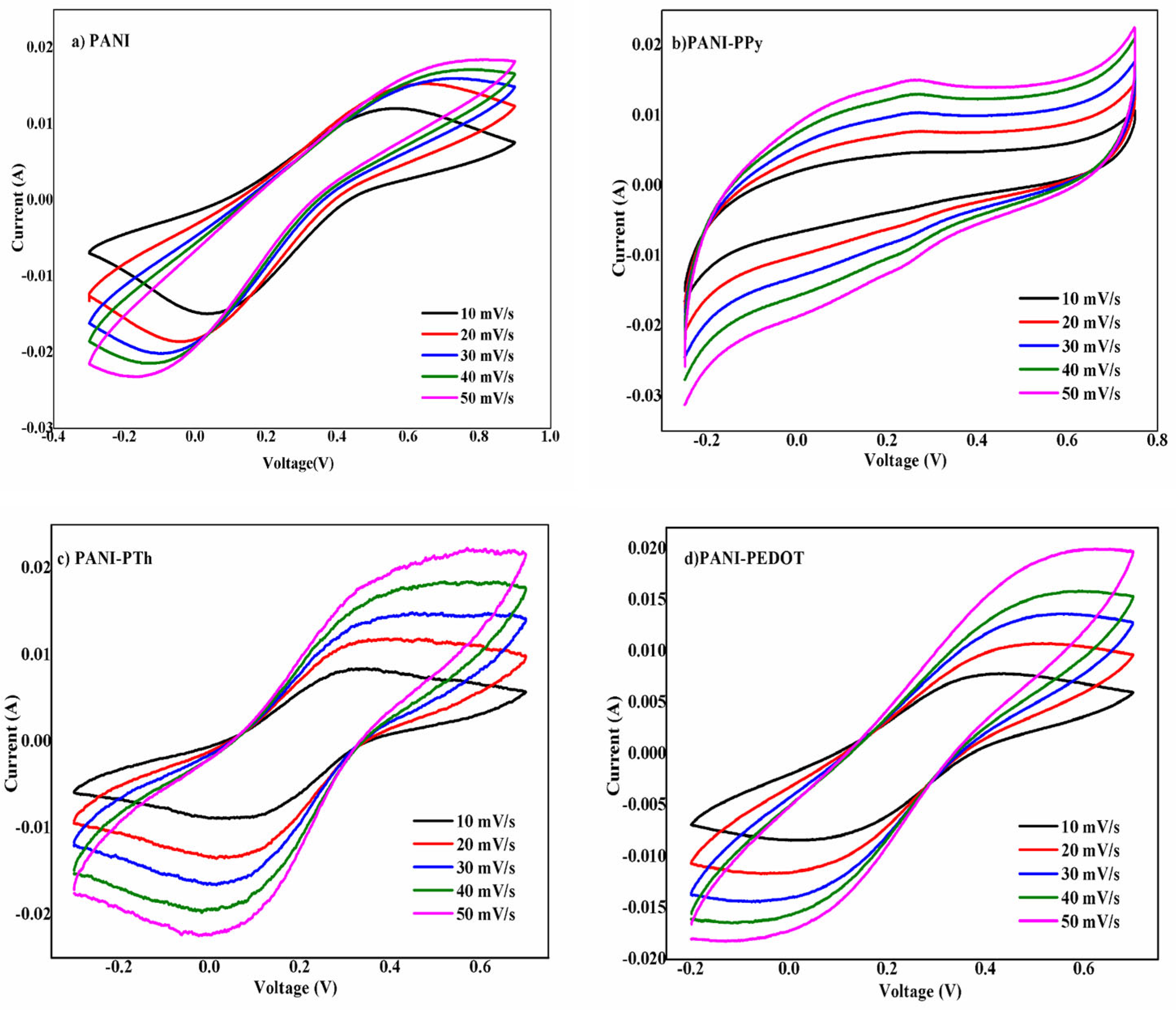
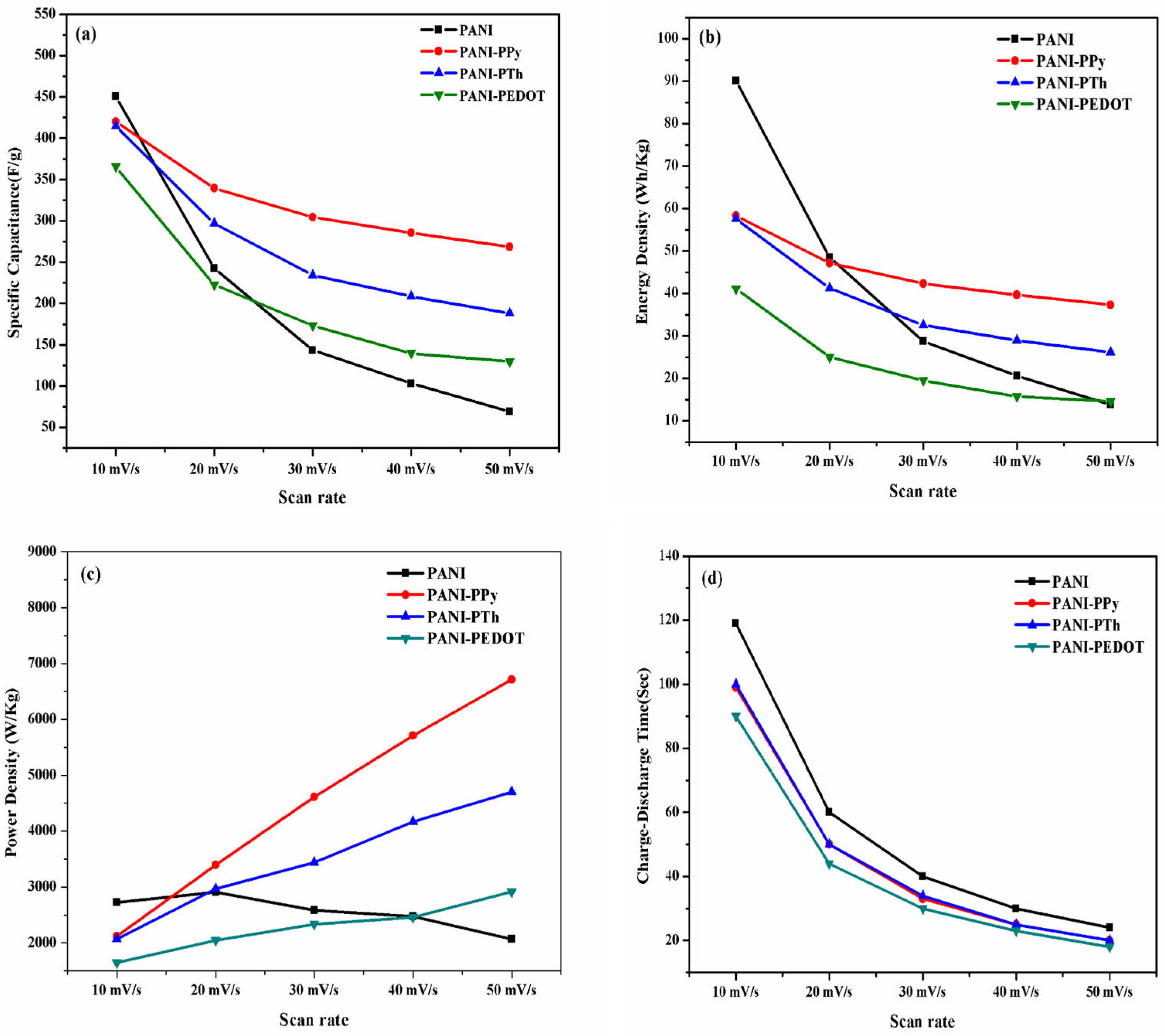
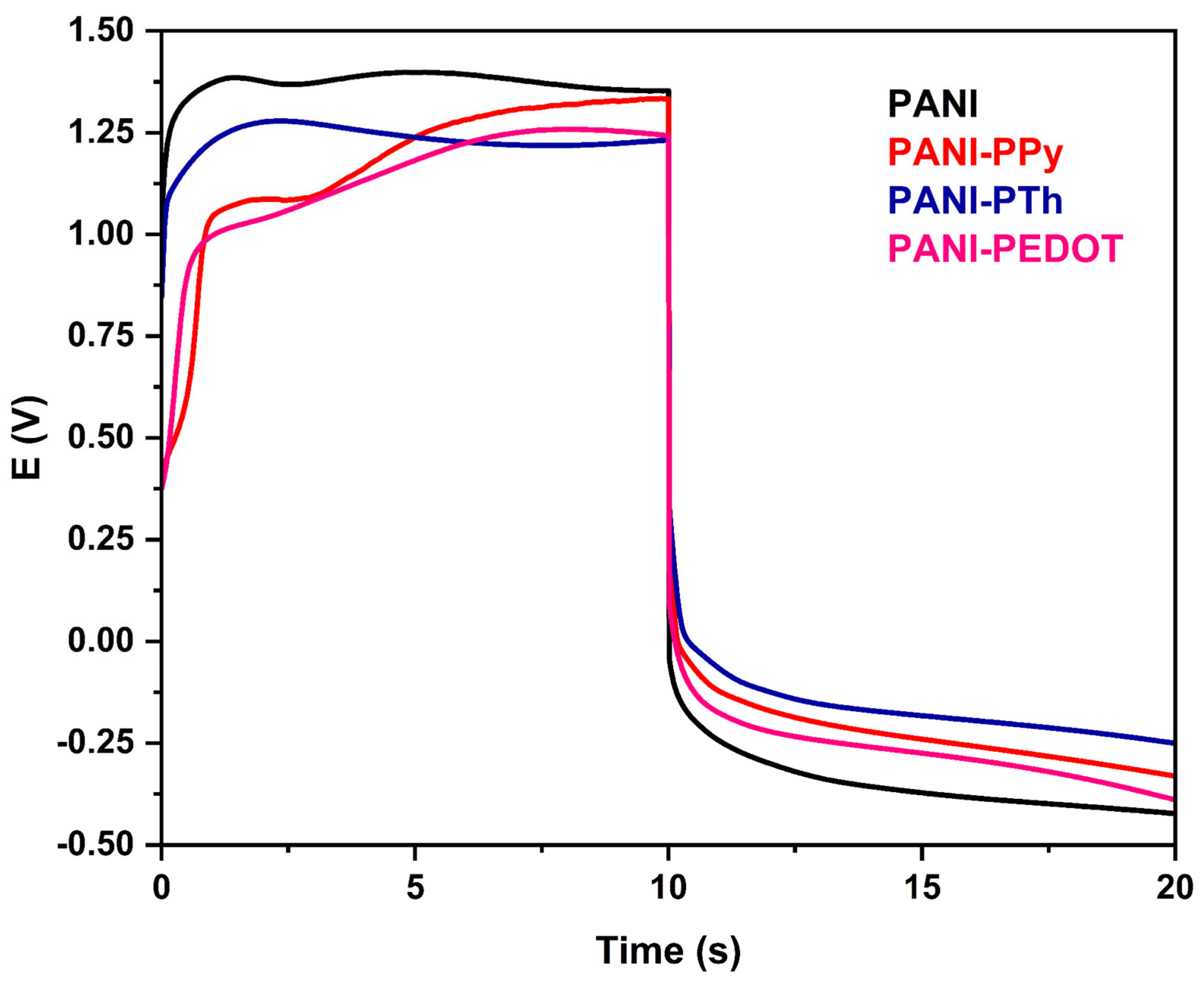
3.2. Electrochemical Properties Analysis
3.3. Impedance Spectroscopy
3.4. Fabrication of ASDs
Device Test
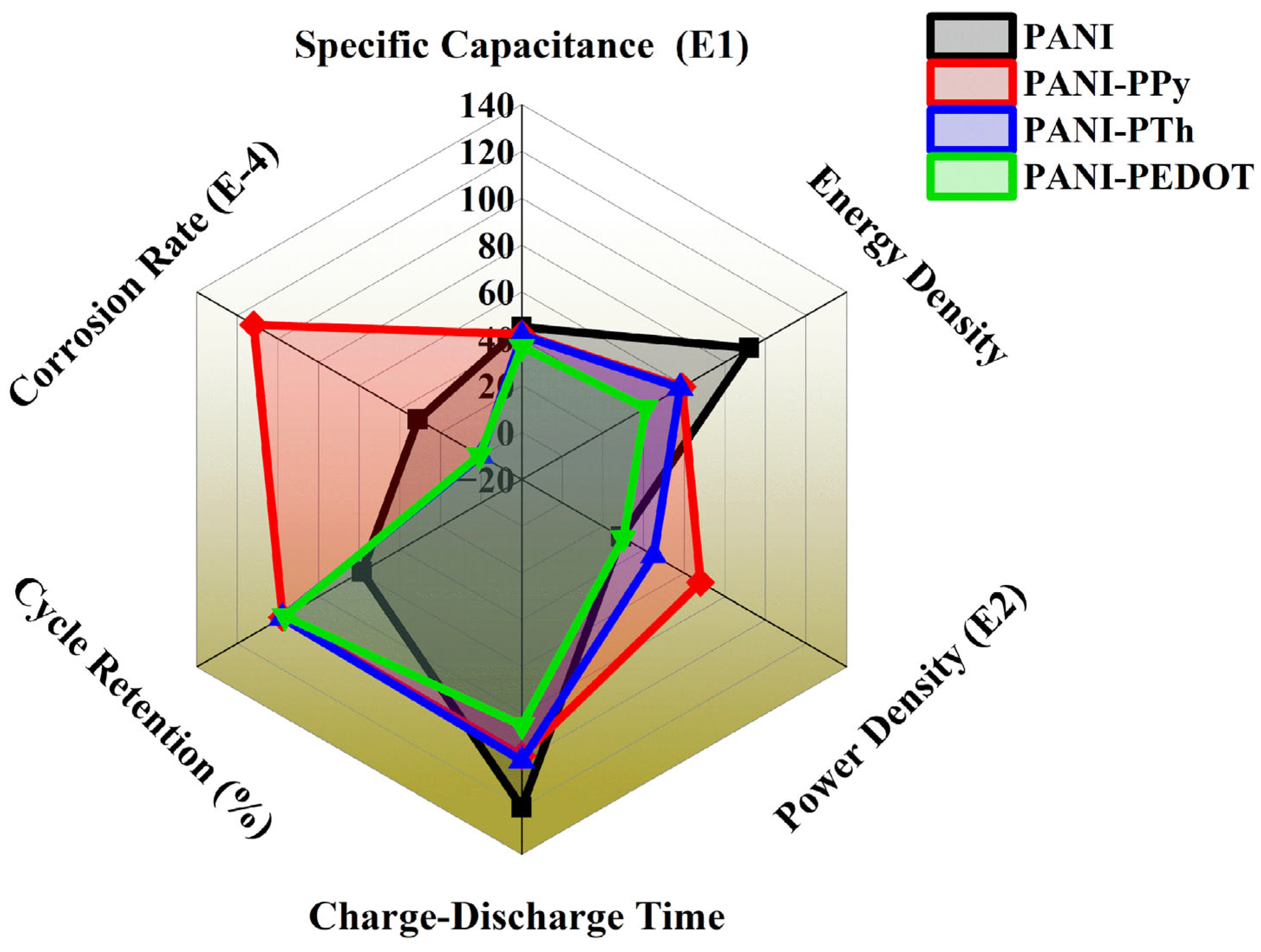
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Materials science: Electrochemical capacitors for energy management. Science (80) 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Bocklisch, T. Hybrid energy storage systems for renewable energy applications. Energy Procedia 2015, 73, 103–111. [Google Scholar] [CrossRef]
- Akande, I.G.; Ajayi, S.A.; Fajobi, M.A.; Oluwole, O.O.; Fayomi, O.S.I. Advancement in the Production and Applications of Conductive Polymers (CPs). Key Eng. Mater. 2021, 886, 12–29. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.C.; Wei, Y. One-dimensional conducting polymer nanocopolymers: Synthesis, properties, and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting Polymers: A New Era in Electrochemistry. J. Solid-State Electrochem. 2008, 14, 917. [Google Scholar]
- Goto, H.; Yoneyama, H.; Togashi, F.; Ohta, R.; Tsujimoto, A.; Kita, E.; Ohshima, K.I.; Rosenberg, D. Preparation of conducting polymers by electrochemical methods and demonstration of a polymer battery. J. Chem. Educ. 2008, 85, 1067–1070. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Liang, B.; Qin, Z.; Li, T.; Dou, Z.; Zeng, F.; Cai, Y.; Zhu, M.; Zhou, Z. Poly(aniline-co-pyrrole) on the surface of reduced graphene oxide as high-performance electrode materials for supercapacitors. Electrochim. Acta 2015, 177, 335–342. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Wang, L.Z.; Zhang, L.S.; Zhang, Y. Preparation and electrochemical capacitance of poly (pyrrole-co-aniline). J. Appl. Polym. Sci. 2010, 115, 1881–1885. [Google Scholar] [CrossRef]
- Mavundla, S.E.; Malgas, G.F.; Motaung, D.E.; Iwuoha, E.I. Physicochemical and morphological properties of poly(aniline-co-pyrrole). J. Mater. Sci. 2010, 45, 3325–3330. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Sun, W.; Wang, S. Capacitance properties of poly(3,4-ethylenedioxythiophene)/polypyrrole copolymers. J. Power Sources 2006, 159, 370–373. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Islam, M.; Amin, M.K.; Paul, S.K.; Rahman, S.; Talukder, M.M.; Rahman, M.M. Simplistic fabrication of aniline and pyrrole-based poly (Ani-co-Py) for efficient photocatalytic performance and supercapacitors. Int. J. Hydrogen Energy 2022, 47, 37860–37869. [Google Scholar] [CrossRef]
- Chuanqiang, Z.; Han, J.; Song, G.; Guo, R. Fabrication of Poly(aniline-co-pyrrole) Hollow Nanospheres with Triton X-100 Micelles as Templates. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3563–3572. [Google Scholar] [CrossRef]
- Ma, A.; Wang, B.; Zheng, Y.; Yang, H.; Ma, Q.; Deng, S. Electrochemical synthesis and energy storage study of aniline-pyrrole conductive copolymer electrode. J. Energy Storage 2023, 72, 108242. [Google Scholar] [CrossRef]
- Hacinecipoğlu, A.V.; Efeoğlu, S.; Kir, B.; Balik, B.; Genctin, M. Production and applications of lead (II) oxide/poly(aniline-co-thiophene) copolymer materials for enhanced supercapacitor performance. J. Mater. Sci. Mater. Electron. 2024, 35, 941. [Google Scholar] [CrossRef]
- Khan, S.; Alkhedher, M.; Raza, R.; Ahmad, M.A.; Majid, A.; Din, E.M.T.E. Electrochemical Investigation of PANI: PPy/AC and PANI: PEDOT/AC Copolymers as Electrode Materials in Supercapacitors. Polymers 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mahtab, T.; Mukhlish, M.Z.B.; Faruk, M.O.; Rahman, M.M. Enhancement of electrical properties of metal doped polyaniline synthesized by different doping techniques. Polym. Bull. 2021, 78, 5379–5397. [Google Scholar] [CrossRef]
- He, K.; Qin, C.; Wen, Q.; Wang, C.; Wang, B.; Yu, S.; Hao, C.; Chen, K. Facile fabrication of polyaniline/polypyrrole copolymer nanofibers with a rough surface and their electrorheological activities. J. Appl. Polym. Sci. 2018, 135, 6289. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M. Synthesis of Polyaniline without Metal Doping and Its Characterization. J. Mater. Sci. Surf. Eng. 2018, 6, 802–804. [Google Scholar]
- Tian, B.; Zerbi, G. Lattice-Dynamics and Vibrational Spectra of Polypyrrole. J. Chem. Phys. 2009, 92, 3886–3891. [Google Scholar] [CrossRef]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.K.; Samanta, S.B.; Malhotra, B.D.; Chand, S. Application of Electrochemically Prepared Polypyrrole Polyvinyl Sulphonate Films to DNA Biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schindler, J.L.; Kannewurf, C.R. Poly(3,4-ethylenedithiathiophene). A new soluble conductive polythiophene derivative. Chem. Mater. 1995, 7, 58–68. [Google Scholar] [CrossRef]
- Casado, J.; Hernandez, V.; Hotta, S. Vibrational spectra of charged defects in a series of α, α’-dimethyl end-capped oligothiophenes induced by chemical doping with iodine. J. Chem. Phys. 1998, 109, 10419–10429. [Google Scholar] [CrossRef]
- Yildiz, U.H.; Sahin, E.; Akhemdov, I.M. A new soluble conducting polymer and its electrochromic devices. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2215–2225. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hotta, S.; Heeger, A.J. Infrared photoexcitation and doping studies of poly(3-methyltrienolone). Phys. Rev. B 1987, 36, 7486–7490. [Google Scholar] [CrossRef]
- Furukawa, Y.; Akimoto, M.; Harada, I. Vibrational key bands and electrical conductivity of polythiophene. Synth. Met. 1987, 18, 151–156. [Google Scholar] [CrossRef]
- Bengt, S. Battery Technologies. In Hydrogen, Batteries, and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–79. [Google Scholar] [CrossRef]
- Kumar, A.S.; Dhaliwal, S. Cyclic voltammetry synthesis of polyaniline as supercapacitors electrode. Mater. Today Proc. 2020, 21, 1833–1839. [Google Scholar] [CrossRef]
- Yue, B.; Wang, C.; Wagner, P.; Yang, Y.; Ding, X.; Officer, D.L.; Wallace, G.G. Electrodeposition of pyrrole and 3-(4-tert-butylphenyl) thiophene copolymer for supercapacitor applications. Synth. Met. 2012, 162, 2216–2221. [Google Scholar] [CrossRef]
- Karaca, E.; Özgenç, G.; Pekmez, N.Ö.; Pekmez, K. Nano MnOx encapsulated pyrrole-carbazole copolymer and polyvinyl chloride/carbon-coated flexible electrodes for solid-state supercapacitor cell. J. Energy Storage 2022, 55, 105794. [Google Scholar] [CrossRef]
- Chang, X.; Qiu, Y.; Cheng, Z.; Luo, Q.; Tan, G.; Ren, B. Effect of topological structure on the supercapacitive performance of redox-active triblock copolymers electrode-based asymmetric supercapacitors. J. Organomet. Chem. 2023, 993, 122726. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Ma, G.; Bai, W.; Guan, X.; Li, C.; Wu, W.; Zhang, S. Aniline-co-pyrrole (ANPY)/MXene copolymers with high specific capacitance for flexible supercapacitors. J. Energy Storage 2024, 97, 112951. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossen, M.R.; Alam, I.; Rahman, M.H.; Faruk, O.; Nurbas, M.; Rahman, M.M.; Khan, M.M.R. Synthesis of hexagonal boron nitride based PANI/h-BN and PANI-PPy/hBN nanocopolymers for efficient supercapacitors. J. Alloys Compd. 2023, 947, 169471. [Google Scholar] [CrossRef]





| Sample | Cu Source, λ (Å) | Angle 2θ (º) | Full Width with Half Maximum, β | Degree of Crystallinity (%), Xc = (ΣVc/VT) × 100 | |
|---|---|---|---|---|---|
| PANI | 1.54187 | 25.34 | 1.62 | 0.911 | 37.84 |
| PANI-PPy | 1.54187 | 14.84 | 0.46 | 3.659 | 18.76 |
| PANI-PTh | 1.54187 | 25.28 | 0.95 | 1.8899 | 42.24 |
| PANI-PEDOT | 1.54187 | 25.22 | 0.91 | 0.72501 | 30.73 |
| Electrode | Sp. Capacitance, F/g | Capacitance Retention/Cycles | Energy Density, Wh/kg | Ref. |
|---|---|---|---|---|
| PANI film | 346 | 63%/1000 | - | [31] |
| PANI-PPy | 227 | - | - | [32] |
| PANI-PPy/AC | 586 | 92%/10,000 | 40 | [19] |
| Pyrrole and 3-(4-tert-butylphenyl) Thiophene copolymer | 291 | 91%/1000 | - | [33] |
| p(Py-co-Cz)/MnOx | 352 | 99%/1000 | - | [34] |
| ANPY/MXene | 451.75 | 91.8%/5000 | - | [35] |
| PANI-PPy/h-BN | 615 | 94.7%/100 | 105 | [36] |
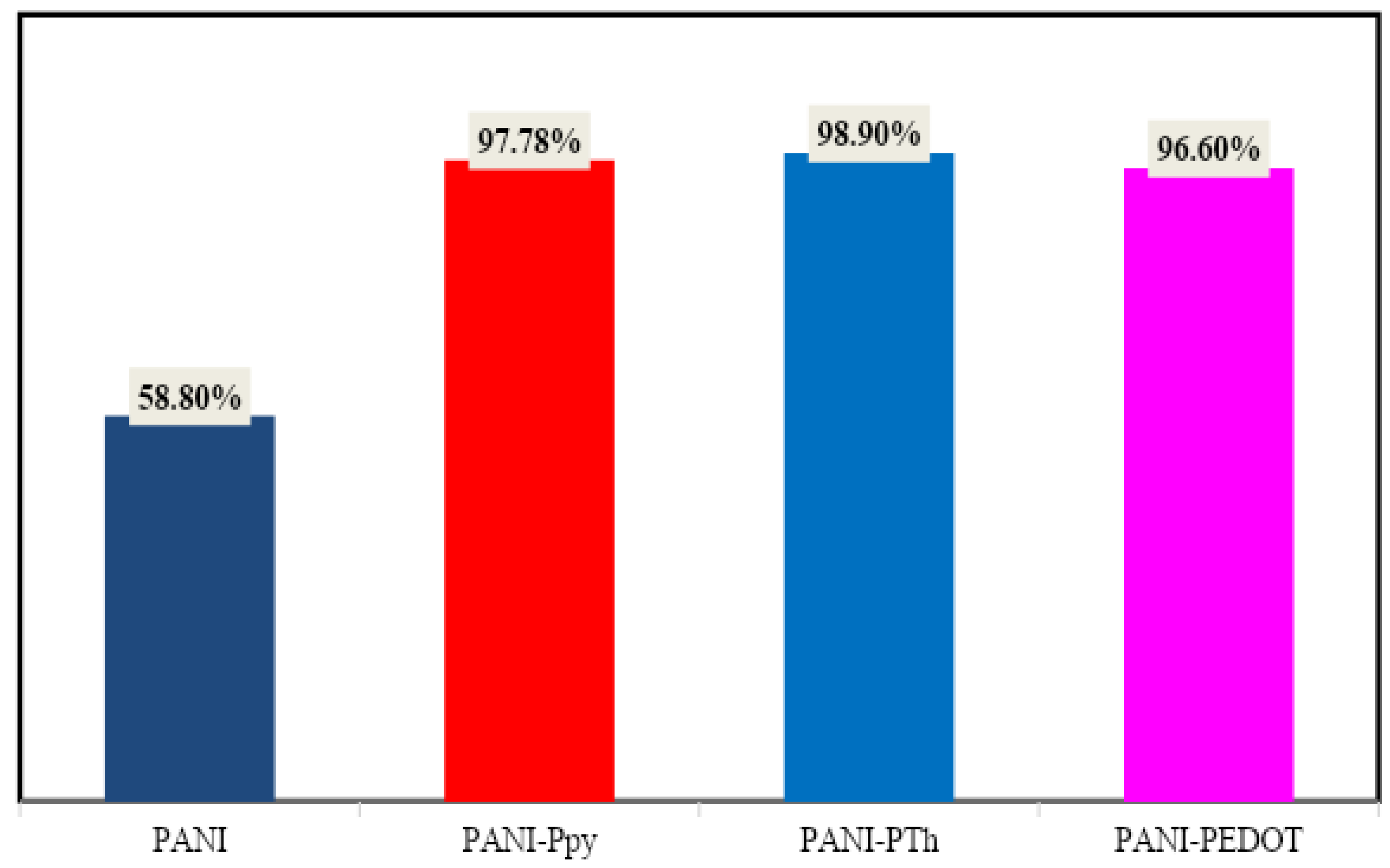
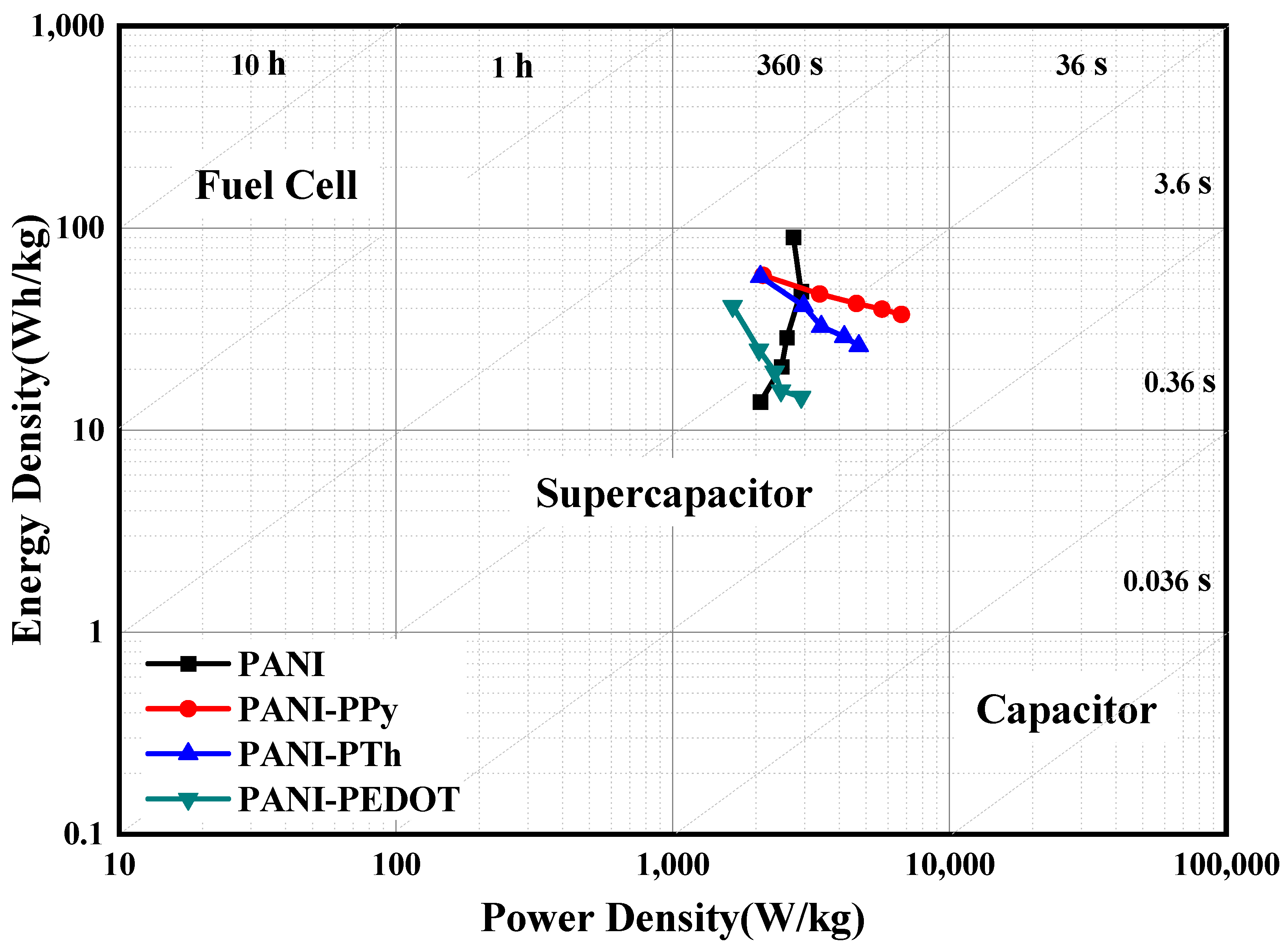
| PANI | 451 | 58.8%/100 | 90 | This Work |
| PANI-PPy | 420 | 97.78%/100 | 58 | This Work |
| PANI-PTh | 414 | 98.9%/100 | 58 | This Work |
| PANI-PEDOT | 366 | 96.6%/100 | 41 | This Work |
| Sample Name | Electrical Conductivity, G (S/m) | Capacitance, C (nF) | Reactance, X (Ω) | Impedance, Z (Ω) |
|---|---|---|---|---|
| PANI | 6.71 | 23.59 | −200.68 | 359.48 |
| PANI-PPy | 5.70 | 311.79 | −560.78 | 661.23 |
| PANI-PTh | 11.05 | 45.99 | −510.79 | 541.88 |
| PANI-PEDOT | 6.62 | 69.33 | −225.96 | 377.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Alam, I.; Hossen, M.R.; Azim, F.; Anjum, N.; Faruk, M.O.; Rahman, M.M.; Okoli, O.I. Facile Synthesis of Conductive Copolymers and Its Supercapacitor Application. J. Compos. Sci. 2025, 9, 253. https://doi.org/10.3390/jcs9050253
Rahman MM, Alam I, Hossen MR, Azim F, Anjum N, Faruk MO, Rahman MM, Okoli OI. Facile Synthesis of Conductive Copolymers and Its Supercapacitor Application. Journal of Composites Science. 2025; 9(5):253. https://doi.org/10.3390/jcs9050253
Chicago/Turabian StyleRahman, Md Mostafizur, Iftidul Alam, Md Rayhan Hossen, Farhan Azim, Nafiza Anjum, Muhammad Omar Faruk, Mohammed Mastabur Rahman, and Okenwa I. Okoli. 2025. "Facile Synthesis of Conductive Copolymers and Its Supercapacitor Application" Journal of Composites Science 9, no. 5: 253. https://doi.org/10.3390/jcs9050253
APA StyleRahman, M. M., Alam, I., Hossen, M. R., Azim, F., Anjum, N., Faruk, M. O., Rahman, M. M., & Okoli, O. I. (2025). Facile Synthesis of Conductive Copolymers and Its Supercapacitor Application. Journal of Composites Science, 9(5), 253. https://doi.org/10.3390/jcs9050253








