Response Surface Methodology-Based Optimization for Enhancing the Viability of Microencapsulated Lactobacillus plantarum in Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Probiotic Cells
2.2. Production of Bacterial Cellulose (BC)
2.3. Microencapsulation of Lactobacillus plantarum
2.3.1. Dispersion Preparation
2.3.2. Emulsion Preparation
2.4. Experimental Design and Optimization of the Microcapsules
2.5. Statistical Analysis
2.6. Microcapsules Size
2.7. Efficiency of Microencapsulation
2.8. Determination of Lactobacillus plantarum Viability in Microcapsules
3. Results and Discussion
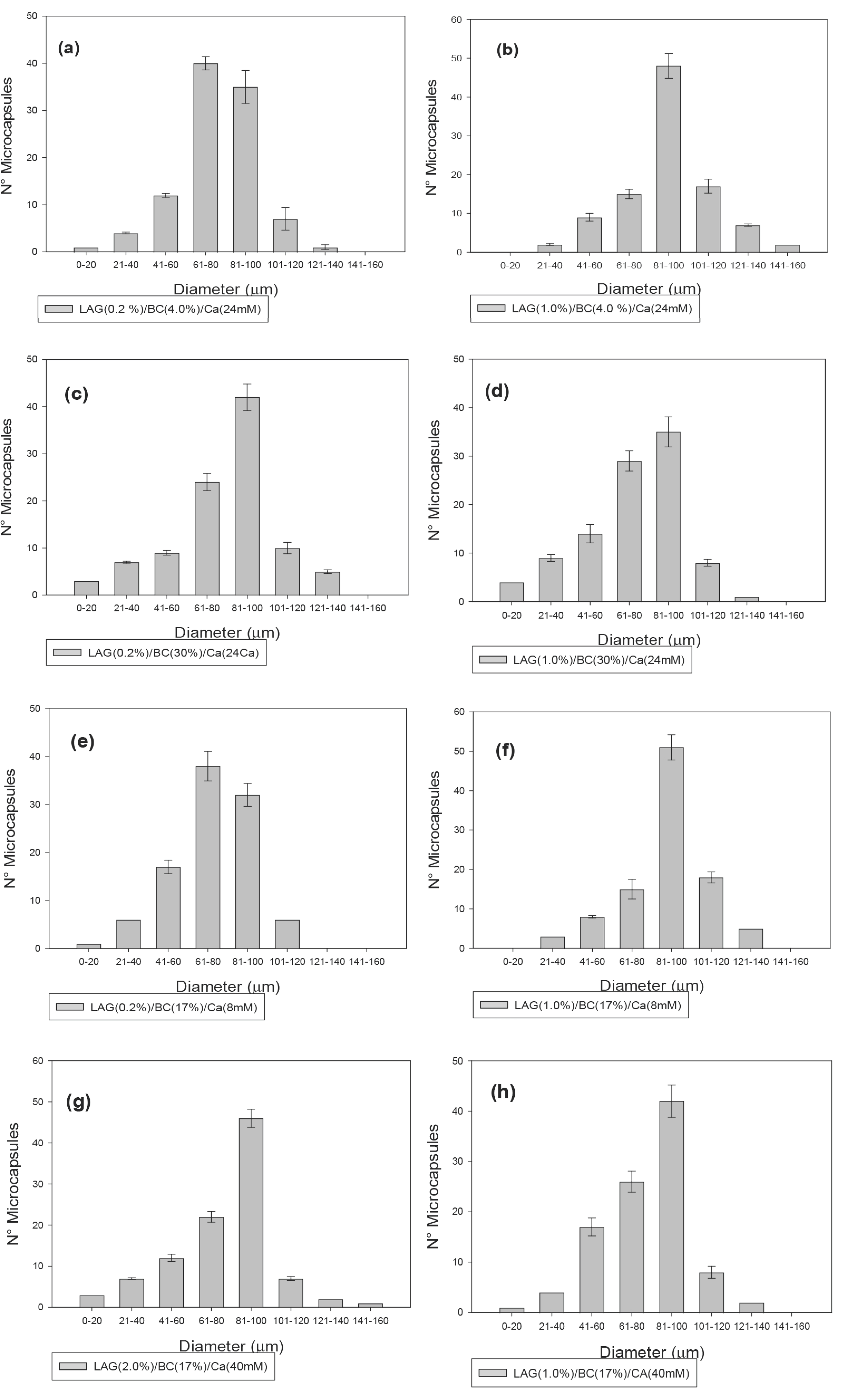
3.1. Microcapsule Size
3.2. Optimization of the Probiotic Microencapsulation
3.3. Response Surface Plots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herdiana, Y. Functional Food in Relation to Gastroesophageal Reflux Disease (GERD). Nutrients 2023, 15, 3583. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, F.; Sciortino, C.; Baviera-Puig, A.; Modica, F. Analyzing Consumer Trends in Functional Foods: A Cluster Analysis Approach. J. Agric. Food Res. 2024, 15, 101041. [Google Scholar] [CrossRef]
- Kheto, A.; Bist, Y.; Awana, A.; Kaur, S.; Kumar, Y.; Sehrawat, R. Utilization of Inulin as a Functional Ingredient in Food: Processing, Physicochemical Characteristics, Food Applications, and Future Research Directions. Food Chem. Adv. 2023, 3, 100443. [Google Scholar] [CrossRef]
- Martirosyan, D.M.; Singh, J. A New Definition of Functional Food by FFC: What Makes a New Definition Unique? Funct. Foods Health Dis. 2015, 5, 209. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olaniran, A.F.; Owa, S.O. Unveiling the Role of Functional Foods with Emphasis on Prebiotics and Probiotics in Human Health: A Review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Duan, C.; Yan, G. Effects of Mixed Starters of Plant- and Wine-Derived L. plantarum on Hawthorn Juice Fermentation: Physicochemical Properties, Phenolic and Volatile Profiles. Food Biosci. 2023, 56, 103363. [Google Scholar] [CrossRef]
- FAO/OMS. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/OMS: Rome, Italy, 2002. [Google Scholar]
- Le, B.; Yang, S.H. Efficacy of Lactobacillus plantarum in Prevention of Inflammatory Bowel Disease. Toxicol. Rep. 2018, 5, 314–317. [Google Scholar] [CrossRef]
- de Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum-Survival, Functional and Potential Probiotic Properties in the Human Intestinal Tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Luxananil, P.; Promchai, R.; Wanasen, S.; Kamdee, S.; Thepkasikul, P.; Plengvidhya, V.; Visessanguan, W.; Valyasevi, R. Monitoring Lactobacillus plantarum BCC 9546 Starter Culture during Fermentation of Nham, a Traditional Thai Pork Sausage. Int. J. Food Microbiol. 2009, 129, 312–315. [Google Scholar] [CrossRef]
- Palomino, J.M.; del Arbol, J.T.; Benomar, N.; Abriouel, H.; Cañamero, M.M.; Gálvez, A.; Pulido, R.P. Application of Lactobacillus plantarum Lb9 as Starter Culture in Caper Berry Fermentation. LWT-Food Sci. Technol. 2014, 60, 788–794. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Wang, Y.; Xie, L.; Liang, S.; Li, D.; Wang, Y.; Wang, J.; Zhan, X. Carboxymethyl Konjac Glucomannan-Chitosan Complex Nanogels Stabilized Double Emulsions Incorporated into Alginate Hydrogel Beads for the Encapsulation, Protection and Delivery of Probiotics. Carbohydr. Polym. 2022, 289, 119438. [Google Scholar] [CrossRef] [PubMed]
- Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients 2022, 14, 3457. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An Alternative Way to Encapsulate Probiotics within Electrospun Alginate Nanofibers as Monitored under Simulated Gastrointestinal Conditions and in Kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in Microencapsulation of Probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved Digestive Stability of Probiotics Encapsulated within Poly (Vinyl Alcohol)/Cellulose Acetate Hybrid Fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef]
- Ermis, E. A Review of Drying Methods for Improving the Quality of Probiotic Powders and Characterization. Dry. Technol. 2022, 40, 2199–2216. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almonaitytė, A.; Jensen, I.-J.; Kurek, M.; Lerfall, J. Alginate Microbeads Incorporated with Anthocyanins from Purple Corn (Zea mays L.) Using Electrostatic Extrusion: Microencapsulation Optimization, Characterization, and Stability Studies. Int. J. Biol. Macromol. 2023, 246, 125684. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- González, R.; Ramos, G.; Cruz, A.; Salazar, A. Rheological Characterization and Activation Energy Values of Binary Mixtures of Gellan. Eur. Food Res. Technol. 2012, 234, 305–313. [Google Scholar] [CrossRef]
- Huang, H.; Yan, W.; Tan, S.; Zhao, Y.; Dong, H.; Liao, W.; Shi, P.; Yang, X.; He, Q. Frontier in Gellan Gum-Based Micro-Capsules Obtained by Emulsification: Core-Shell Structure, Interaction Mechanism, Intervention Strategies. Int. J. Biol. Macromol. 2024, 272, 132697. [Google Scholar] [CrossRef]
- Gomes, D.; Batista-Silva, J.; Sousa, A.; Passarinha, L. Progress and Opportunities in Gellan Gum-Based Materials: A Review of Preparation, Characterization and Emerging Applications. Carbohydr. Polym. 2023, 311, 120782. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of Bacterial Cellulose and Its Composites in Biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; Sarubbo, L.A. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics, and engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds Within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Yuan, H.; Li, W.; Chen, C.; Yu, H.; Huang, J.; Tian, H. Novel Cinnamon Essential Oil-Bacterial Cellulose Microcapsules for Enhanced Preservation of Prefabricated Meat. Int. J. Biol. Macromol. 2024, 282, 136851. [Google Scholar] [CrossRef]
- Fasolo, D.; Pippi, B.; Meirelles, G.; Zorzi, G.; Fuentefria, A.M.; Poser, G.; Teixeira, H.F. Topical Delivery of Antifungal Brazilian Red Propolis Benzophenones-Rich Extract by Means of Cationic Lipid Nanoemulsions Optimized by Means of Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 56, 101573. [Google Scholar] [CrossRef]
- Zain, Z.M.; Abdulhameed, A.S.; Jawad, A.H.; Alothman, Z.A.; Yaseen, Z.M. A pH-Sensitive Surface of Chitosan/Sepiolite Clay/Algae Biocomposite for the Removal of Malachite Green and Remazol Brilliant Blue R Dyes: Optimization and Adsorption Mechanism Study. J. Polym. Environ. 2023, 31, 501–518. [Google Scholar] [CrossRef]
- González-Cuello, R.; Parada-Castro, A.L.; Ortega-Toro, R. Application of a Multi-Component Composite Edible Coating for the Preservation of Strawberry Fruit. J. Compos. Sci. 2024, 8, 515. [Google Scholar] [CrossRef]
- Cheow, W.; Yi, K.; Hadinoto, K. Controlled Release of Lactobacillus rhamnosus Biofilm Probiotics from Alginate-Locust Bean Gum Microcapsules. Carbohydr. Polym. 2014, 103, 587–595. [Google Scholar] [CrossRef]
- González, R.E.; Salazar, J.A.; Pérez, J.A. Obtaining Size-Controlled Microcapsules by Ionic Gelation with High and Low Acyl Gellans Containing Lactococcus lactis. Rev. Colomb. Biotecnol. 2013, 15, 70–80. [Google Scholar]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A Review on Probiotic Microencapsulation and Recent Advances of Their Application in Bakery Products. Food Bioproc. Technol. 2022, 15, 1677–1699. [Google Scholar] [CrossRef]
- Zarali, M.; Sadeghi, A.; Jafari, S.M.; Ebrahimi, M.; Mahoonak, A.S. Enhanced Viability and Improved In Situ Antibacterial Activity of the Probiotic LAB Microencapsulated Layer-by-Layer in Alginate Beads Coated with Nisin. Food Biosci. 2023, 53, 102593. [Google Scholar] [CrossRef]
- Larwood, V.; Howlin, B.; Webb, G. Solvation Effects on the Conformational Behavior of Gellan and Calcium Ion Binding to Gellan Double Helices. J. Mol. Model. 1996, 2, 175–182. [Google Scholar] [CrossRef]
- Tang, J.; Tung, M.; Zeng, Y. Gelling Properties of Gellan Solutions Containing Monovalent and Divalent Cations. J. Food Sci. 1997, 62, 688–712. [Google Scholar] [CrossRef]
- Lacroix, C.; Grattepanche, F.; Doleyres, Y.; Bergmaier, D. Immobilised Cell Technologies for the Dairy Industry. In Applications of Cell Immobilisation Biotechnology; Springer: Dordrecht, The Netherlands, 2005; pp. 295–319. [Google Scholar] [CrossRef]
- Kim, S.; Cho, S.; Kim, S.; Song, O.; Shin, I.; Cha, D.; Park, H. Effect of Microencapsulation on Viability and Other Characteristics in Lactobacillus acidophilus ATCC 43121. LWT-Food Sci. Technol. 2008, 41, 493–500. [Google Scholar] [CrossRef]
- Tyle, P. Effect of Size, Shape and Hardness of Particles in Suspension on Oral Texture and Palatability. Acta Psychol. 1993, 84, 111–118. [Google Scholar] [CrossRef]
- Fu, J.F.; Zhao, Y.Q.; Xue, X.D.; Li, W.C.; Babatunde, A.O. Multivariate-Parameter Optimization of Acid Blue-7 Wastewater Treatment by Ti/TiO2 Photoelectrocatalysis via Box-Behnken Design. Desalination 2009, 243, 42–51. [Google Scholar] [CrossRef]
- Aureli, P.; Capurso, L.; Castellazzi, A.; Clerici, M.; Giovannini, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G. Probiotics and Health: An Evidence-Based Review. Pharmacol. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef]
- Salminen, S.; Kenifel, W.; Ouwehand, A. Probiotics, Applications in Dairy Products. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Holkem, T.; Raddatz, G.; Nunes, L.; Cichoski, A.; Jacob, E.; Grosso, R.; Ragagnin, C. Development and Characterization of Alginate Microcapsules Containing Bifidobacterium BB-12 Produced by Emulsification/Internal Gelation Followed by Freeze Drying. LWT-Food Sci. Technol. 2016, 71, 302–308. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Zhang, H.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in Reinforced Alginate Microspheres Prepared by Emulsification/Internal Gelation. Int. J. Food Sci. Technol. 2011, 46, 1672–1678. [Google Scholar] [CrossRef]
- Wang, K.; Ni, J.; Li, H.; Tian, X.; Tan, M.; Su, W. Survivability of Probiotics Encapsulated in Kelp Nanocellulose/Alginate Microcapsules on Microfluidic Device. Food Res. Int. 2022, 160, 111723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, B.; Mishra, I.M. Process Parametric Study for Ethene Carboxylic Acid Removal onto Powder Activated Carbon Using Box-Behnken Design. Chem. Eng. Technol. 2007, 30, 932–937. [Google Scholar] [CrossRef]
- Körbahti, B.K. Response Surface Optimization of Electrochemical Treatment of Textile Dye Wastewater. J. Hazard. Mater. 2007, 145, 277–286. [Google Scholar] [CrossRef]
- Montgomery, D.C. Introduction to Statistical Quality Control, 6th ed.; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Ravilumar, K.; Ramalingam, S.; Krishnan, S.; Balu, K. Application of Response Surface Methodology to Optimize the Process Variables for Reactive Red and Acid Brown Dye Removal Using a Novel Adsorbent. Dye. Pigment. 2006, 70, 18–26. [Google Scholar] [CrossRef]
- Henseler, J.; Sarstedt, M. Goodness-of-Fit Indices for Partial Least Squares Path Modeling. Comput. Stat. 2013, 28, 565–580. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; He, J.; Huang, Y. A New Insight to the Effect of Calcium Concentration on Gelation Process and Physical Properties of Alginate Films. J. Mater. Sci. 2016, 51, 5791–5801. [Google Scholar] [CrossRef]
- Thinkohkaew, K.; Jonjaroen, V.; Niamsiri, N.; Panya, A.; Suppavorasatit, I.; Potiyaraj, P. Microencapsulation of Probiotics in Chitosan-Coated Alginate/Gellan Gum: Optimization for Viability and Stability Enhancement. Food Hydrocoll. 2024, 151, 109788. [Google Scholar] [CrossRef]
- Lai, P.Y.; How, Y.H.; Pui, L.P. Microencapsulation of Bifidobacterium lactis Bi-07 with Galactooligosaccharides Using Co-Extrusion Technique. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2416. [Google Scholar] [CrossRef]
- Dong, K.; Xu, K.; Wei, N.; Fang, Y.; Qin, Z. Three-Dimensional Porous Sodium Alginate/Gellan Gum Environmentally Friendly Aerogel: Preparation, Characterization, Adsorption, and Kinetics Studies. Chem. Eng. Res. Des. 2022, 179, 227–236. [Google Scholar] [CrossRef]



| Variable | Factor Code | Range and Levels of Factors | |
|---|---|---|---|
| −1 | 1 | ||
| LAG (w/v) | X1 | 0.20 | 1 |
| BC (w/v) | X2 | 4 | 30 |
| Ca (mM) | X3 | 8 | 40 |
| Run | X1: LAG (w/v) | X2: BC (w/v) | X3: Ca (mM) | %EE | Viability (%) | ||
|---|---|---|---|---|---|---|---|
| Experimental Data | RSM Predicted | Experimental Data | RSM Predicted | ||||
| 1 | 0.20 | 4 | 24 | 57.90 ± 1.34 | 57.81 | 70.80 ± 1.41 | 70.55 |
| 2 | 1.00 | 4 | 24 | 75.70 ± 0.99 | 75.41 | 85.20 ± 2.17 | 85.62 |
| 3 | 0.20 | 30 | 24 | 61.80 ± 0.74 | 62.08 | 72.60 ± 0.94 | 72.17 |
| 4 | 1.00 | 30 | 24 | 76.40 ± 0.28 | 76.48 | 88.40 ± 1.74 | 88.65 |
| 5 | 0.20 | 17 | 8 | 53.30 ± 2.25 | 53.51 | 66.70 ± 1.73 | 67.26 |
| 6 | 1.00 | 17 | 8 | 62.70 ± 1.73 | 63.11 | 79.30 ± 2.11 | 79.18 |
| 7 | 0.20 | 17 | 40 | 55.20 ± 0.87 | 54.78 | 69.20 ± 1.08 | 69.31 |
| 8 | 1.00 | 17 | 40 | 77.40 ± 2.04 | 77.18 | 89.50 ± 1.94 | 88.93 |
| 9 | 0.60 | 4 | 8 | 64.70 ± 1.45 | 64.57 | 74.80 ± 2.04 | 74.48 |
| 10 | 0.60 | 30 | 8 | 67.40 ± 1.55 | 66.90 | 76.30 ± 1.60 | 76.16 |
| 11 | 0.60 | 4 | 40 | 71.40 ± 1.73 | 71.90 | 79.60 ± 1.71 | 79.73 |
| 12 | 0.60 | 30 | 40 | 74.80 ± 1.39 | 74.92 | 82.40 ± 1.92 | 82.71 |
| 13 | 0.60 | 17 | 24 | 83.20 ± 2.35 | 84.00 | 90.70 ± 2.07 | 91.46 |
| 14 | 0.60 | 17 | 24 | 82.50 ± 1.88 | 84.00 | 92.40 ± 2.33 | 91.46 |
| 15 | 0.60 | 17 | 24 | 86.30 ± 2.06 | 84.00 | 91.30 ± 3.04 | 91.46 |
| Factors | %EE | Viability | ||
|---|---|---|---|---|
| Coefficient δ | p-Value | Coefficient δ | p-Value | |
| Intercept | 84.00 | 0.00 | 91.46 | 0.00 |
| X1 (LAG) | 8.00 | 0.00 | 7.88 | 0.00 |
| X2 (BC) | 1.33 | 0.03 | 1.16 | 0.00 |
| X3 (Ca) | 3.83 | 0.00 | 2.95 | 0.00 |
| Interaction | ||||
| X1 X2 | −0.80 | 0.29 | 0.35 | 0.39 |
| X1 X3 | 3.20 | 0.00 | 1.92 | 0.00 |
| X2 X3 | 0.17 | 0.80 | 0.32 | 0.43 |
| Quadratic | ||||
| −11.73 | 0.00 | −7.15 | 0.00 | |
| −4.31 | 0.00 | −5.05 | 0.00 | |
| −10.11 | 0.00 | −8.13 | 0.00 | |
| p-value (Model) | 0.00 | |||
| p-value (Lack of fit) | 0.94 | 0.71 | ||
| R2 | 99.23% | 99.64% | ||
| Adjusted R2 | 98.45% | 99.29% | ||
| Predicted R2 | 97.49% | 98.07% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Response Surface Methodology-Based Optimization for Enhancing the Viability of Microencapsulated Lactobacillus plantarum in Composite Materials. J. Compos. Sci. 2025, 9, 189. https://doi.org/10.3390/jcs9040189
González-Cuello R, Hernández-Fernández J, Ortega-Toro R. Response Surface Methodology-Based Optimization for Enhancing the Viability of Microencapsulated Lactobacillus plantarum in Composite Materials. Journal of Composites Science. 2025; 9(4):189. https://doi.org/10.3390/jcs9040189
Chicago/Turabian StyleGonzález-Cuello, Rafael, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2025. "Response Surface Methodology-Based Optimization for Enhancing the Viability of Microencapsulated Lactobacillus plantarum in Composite Materials" Journal of Composites Science 9, no. 4: 189. https://doi.org/10.3390/jcs9040189
APA StyleGonzález-Cuello, R., Hernández-Fernández, J., & Ortega-Toro, R. (2025). Response Surface Methodology-Based Optimization for Enhancing the Viability of Microencapsulated Lactobacillus plantarum in Composite Materials. Journal of Composites Science, 9(4), 189. https://doi.org/10.3390/jcs9040189








