Structural Insights: In Situ Synthesis of Titanium Carbide by Magnesiothermic Method Using Carbon Nanotubes and Turbostratic Carbon as Carbon Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
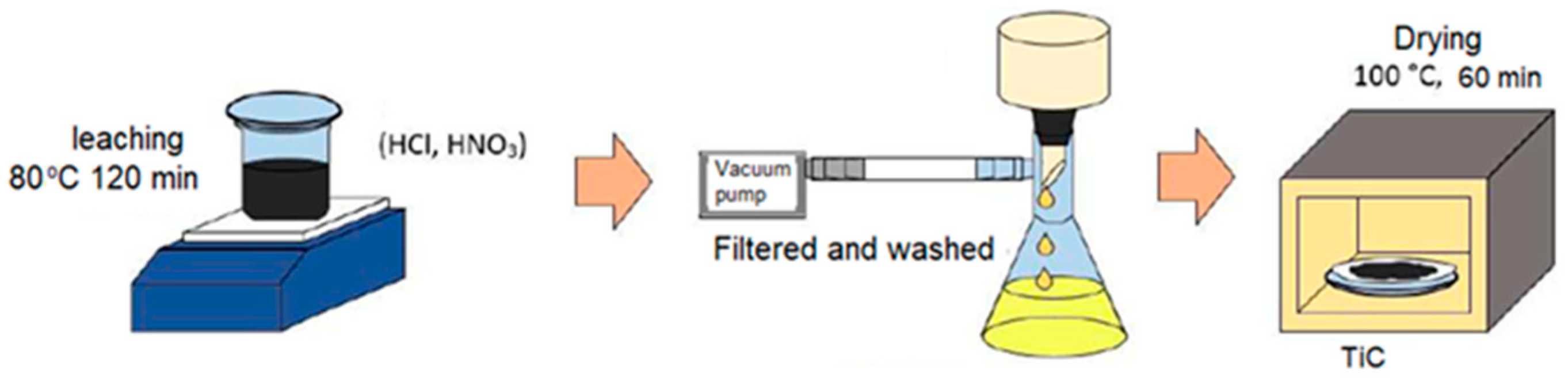
2.2. Experimental Procedure
2.3. Characterization
3. Results and Discussion
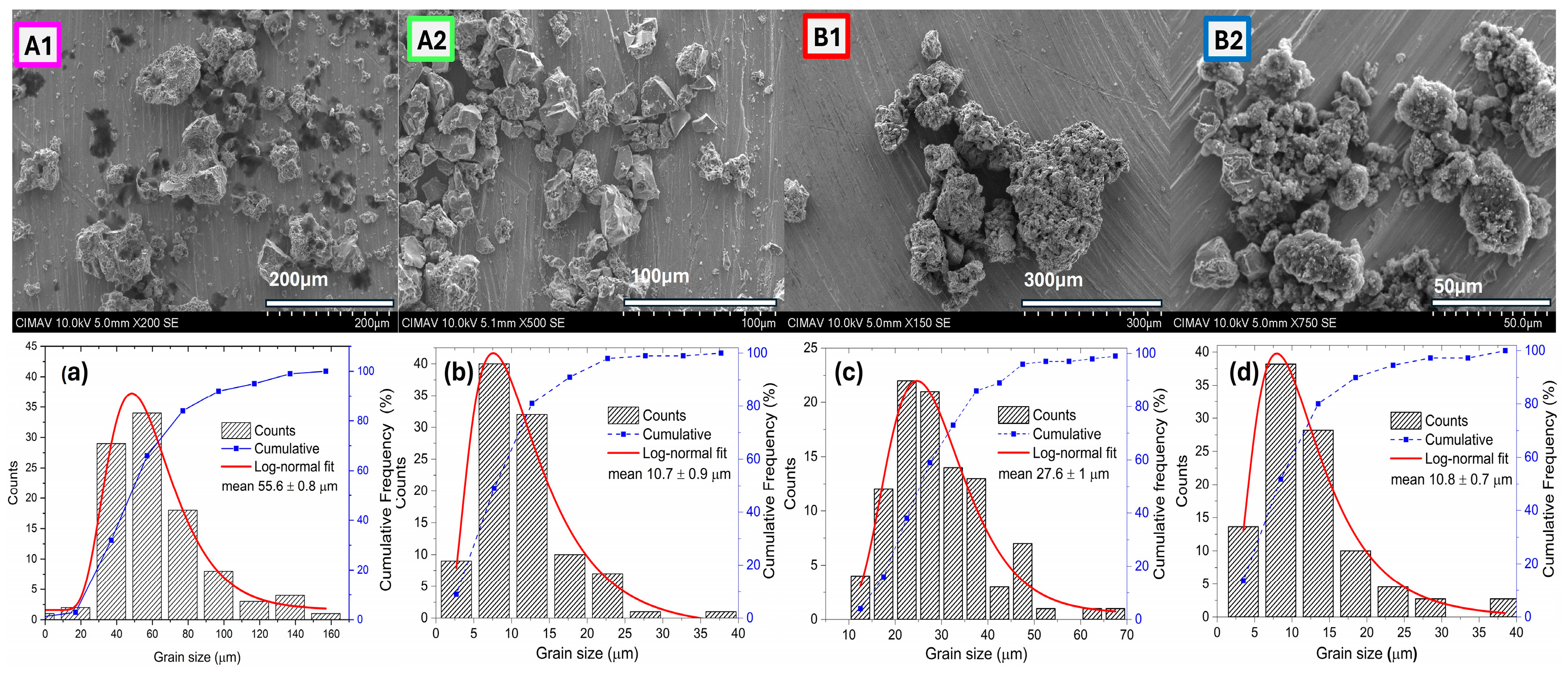
3.1. Scanning Electron Microscopy (SEM) Analysis
3.1.1. Initial Materials
3.1.2. Sources of Carbon
3.1.3. Morphology of the Composites
3.2. X-Ray Diffraction (XRD) Analysis
Rietveld Refinement Method
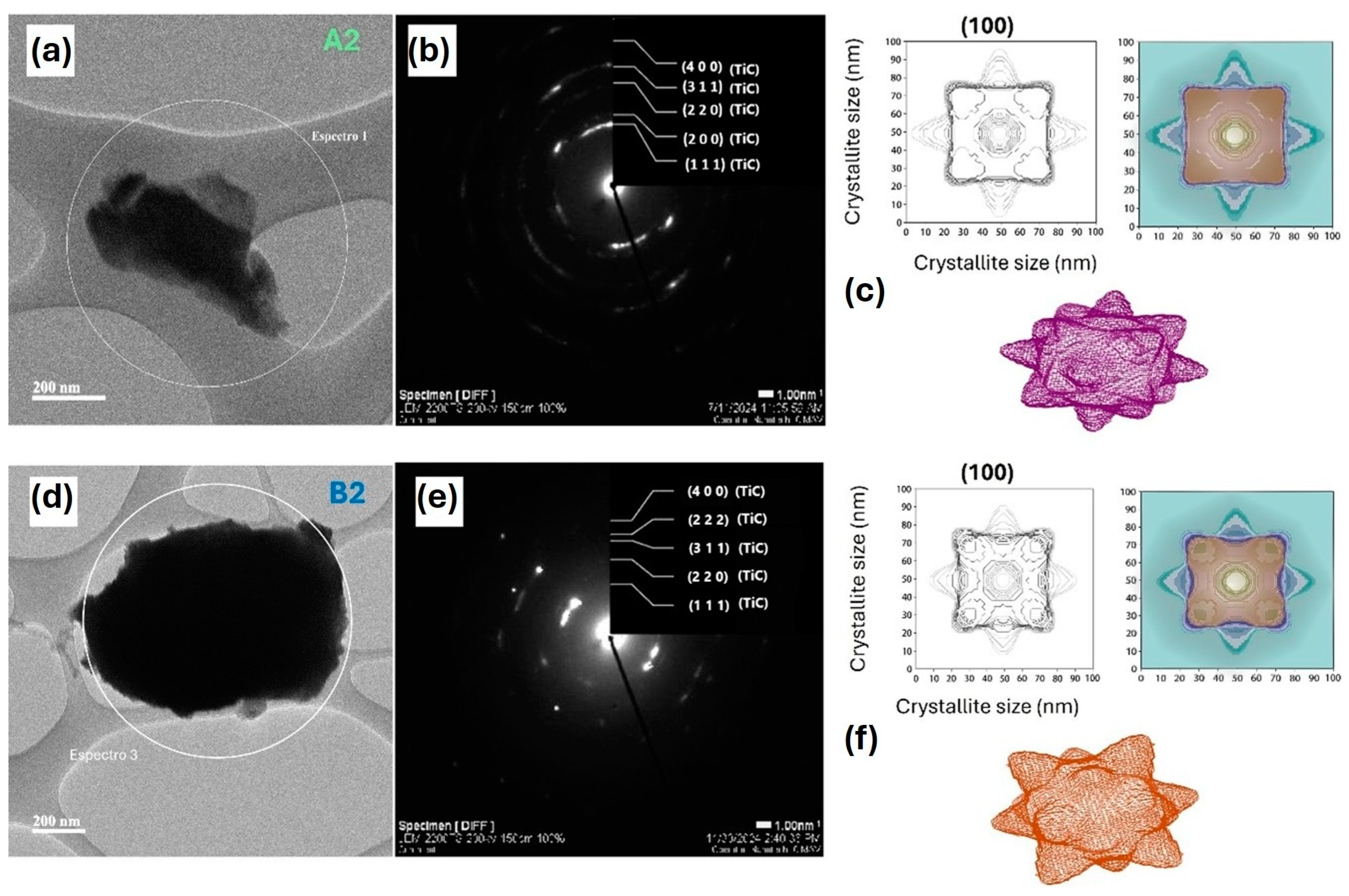
3.3. Bright-Field Transmission Electron Microscopy (BF-TEM) Micrographs
3.4. Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mhadhbi, M. Titanium Carbide: Synthesis, Properties and Applications. Brill. Eng. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Rao, V.R.; Ramanaiah, N.; Sarcar, M.M.M. Tribological properties of Aluminium Metal Matrix Composites (AA7075 Reinforced with Titanium Carbide (TiC) Particles). Int. J. Adv. Sci. Technol. 2016, 88, 13–26. [Google Scholar]
- He, B.; Ma, D.; Ma, F.; Xu, K. Microstructures and wear properties of TiC coating produced by laser cladding on Ti-6Al-4V with TiC and carbon nanotube mixed powders. Ferroelectrics 2019, 547, 217–225. [Google Scholar]
- Zemtsova, P.; Morozov, P.; Sidorova, Y.V.; Morozov, N.F.; Semenov, B.N.; Smirnov, V.M. The effect of carbide nanostructures of the reinforcing phase on the mechanical properties of an aluminum composite obtained by injection molding. Procedia Struct. Integr. 2024, 65, 317–323. [Google Scholar]
- Kovačević, S.; Mikheev, R.; Kalashnikov, I.; Kamyshev, K.V. Aluminium matrix hybrid composite materials reinforced with carbides and intermetallics. E3S Web Conf. 2023, 376, 01011. [Google Scholar]
- Dwivedi, S.P.; Chaudhary, V.; Sharma, S.; Pattanaik, A.; Kumar, P.V.; Bisht, Y.S.; Singh, V.; Abbas, M.; Kozak, D.; Lozanovic, J.; et al. Synthesis and purification of nano-sized titanium carbide particles through vacuum carbothermal reduction for enhanced mechanical, microstructural morphology, and tribological properties in friction stir processed A356-based aluminum composites. J. Mater. Res. Technol. 2024, 33, 5874–5887. [Google Scholar]
- Cochepin, B.; Gauthier, V.; Vrel, D.; Dubois, S. Crystal growth of TiC grains during SHS reactions. J. Cryst. Growth 2007, 304, 481–486. [Google Scholar]
- Zhang, L.; Hu, J.; Voevodin, A.A.; Fong, H. Synthesis of continuous TiC nanofibers and/or nanoribbons through electrospinning followed by carbothermal reduction. Nanoscale 2010, 2, 1670–1673. [Google Scholar] [CrossRef]
- Camacho-Ríos, M.L.; Herrera-Pérez, G.; Esparza-Rodríguez, M.A.R.; Pérez-Bustamante, R.; García-Herrera, J.E.; Betancourt-Cantera, J.A.; Lardizábal-Gutiérrez, D. Optimization of in situ formation of a titanium carbide nanohybrid via mechanical alloying using stearic acid and carbon nanotubes as carbon sources. J. Compos. Sci. 2023, 7, 502. [Google Scholar] [CrossRef]
- Yang, L.; Song, R.; Wan, D.; Ji, S.; Liu, J.; Hu, W.; Zhong, C. Magnesiothermic reduction SiO coated with vertical carbon layer as high-performance anode for lithium-ion batteries. J. Energy Storage 2024, 99, 113440. [Google Scholar]
- Ma, X.; Fei, W.; Liu, J.; Zhang, X.; Ji, J.; Zhou, X. Energetic characteristics of highly reactive Si nanoparticles prepared by magnesiothermic reduction of mesoporous SiO2. Chem. Eng. J. 2024, 481, 148542. [Google Scholar] [CrossRef]
- Smith, W.R. HSC Chemistry for Windows, 2.0. J. Chem. Inf. Comput. Sci. 1996, 36, 151–152. [Google Scholar]
- Davoodi, D.; Hassanzadeh-Tabrizi, S.A.; Emami, A.H.; Salahshour, S. A low temperature mechanochemical synthesis of nanostructured ZrC powder by a magnesiothermic reaction. Ceram. Int. 2015, 41, 8397–8401. [Google Scholar] [CrossRef]
- Hovhannisyan, A.A.; Dolukhanyan, S.K.; Ter-Galstyan, O.P.; Mnatsakanyan, N.L.; Asatryan, K.V.; Mnatsakanyan, S.E.; Mardanyan, S.S.; Muradyan, G.N. Synthesis of non-stoichiometric carbides and carbohydrides of Ti and Ti-Nb using self-propagating high-temperature synthesis technique. Materialia 2023, 30, 101820. [Google Scholar] [CrossRef]
- Cuadros-Lugo, E.; Piñon-Espitia, M.; Martinez-Rodriguez, H.A.; Lardizabal-Gutierrez, D.; Estrada-Guel, I.; Herrera-Ramirez, J.M.; Carreno-Gallardo, C. Turbostratic Carbon/Graphene Prepared via the Dry Ice in Flames Method and Its Purification Using Different Routes: A Comparative Study. Materials 2022, 15, 2501. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, T.; Chen, Y.; Niu, Y.; Tang, J.; Qin, L.C. Synthesis of Graphene from Dry Ice in Flames and Its Application in Supercapacitors. Chem. Phys. Lett. 2014, 591, 78–81. [Google Scholar] [CrossRef]
- Jones, R.F. A Demonstration of Burning Magnesium and Dry Ice. J. Chem. Educ. 1978, 55, 450–451. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Antúnez, W.; Alonso, G.; Delgado, F.P.; Espinosa, F.; Miki-Yoshida, M. Study of carbon nanotubes synthesis by spray pyrolysis and model of growth. Diam. Relat. Mater. 2006, 15, 1329–1335. [Google Scholar] [CrossRef]
- Valenzuela-Muñiz, A.M.; Alonso-Nuñez, G.; Miki-Yoshida, M.; Botte, G.G.; Verde-Gómez, Y. High electroactivity performance in Pt/MWCNT and PtNi/MWCNT electrocatalysts. Int. J. Hydrogen Energy 2013, 38, 12640–12647. [Google Scholar] [CrossRef]
- Rosado, G.; Verde, Y.; Valenzuela-Muñiz, A.M.; Barbosa, R.; Yoshida, M.M.; Escobar, B. Catalytic activity of Pt-Ni nanoparticles supported on multi-walled carbon nanotubes for the oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 23260–23271. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Qiu, F.; Liu, T.S.; Chu, J.G.; Zhao, Q.L.; Jiang, Q.C. Effects of Carbon Source on TiC Particles’ Distribution, Tensile, and Abrasive Wear Properties of In Situ TiC/Al-Cu Nanocomposites Prepared in the Al-Ti-C System. Nanomaterials 2018, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, Z.; Qi, Z.; Liu, G.; Bian, X. Formation of nanocrystalline TiC from titanium and different carbon sources by mechanical alloying. J. Alloys Compd. 2009, 472, 97–103. [Google Scholar]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffraction 2014, 29 (Suppl. 2), S13–S18. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar]
- Rodriguez-Carbajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Uribe-Chavira, J.S.; Herrera-Pérez, G.; Santillán-Rodríguez, C.R.; Sáenz-Hernández, R.J.; Matutes-Aquino, J.A.; Grijalva-Castillo, M.C. X-ray diffraction analysis by Rietveld refinement of FeAl alloys doped with terbium and its correlation with magnetostriction. J. Rare Earths 2023, 41, 1217–1224. [Google Scholar]
- Gonzales-Platas, J.; Rodriguez-Carvajal, J. GFourier: A Windows/Linux Program to Calculate and Display Fourier Maps (version 04.06); Program Available Within the FullProf Suite. Available online: https://scholar.google.com/scholar_lookup?title=GFourier+Program&author=Gonzales-Platas,+J.&author=Rodriguez-Carvajal,+J.&publication_year=2007&journal=Incl.+Full-Prof+Suite+Packag.&volume=26&pages=12%E2%80%9319#d=gs_cit&t=1743399622461&u=%2Fscholar%3Fq%3Dinfo%3A8SlBr80Vy5MJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den (accessed on 25 March 2025).
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar]
- Levenberg, K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. SIAM J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Origin(Pro), version 2018; OriginLab Corporation: Northampton, MA, USA, 2018.
- Kumar, Y.A.; Koyyada, G.; Ramachandran, T.; Kim, J.H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review. Nanomaterials 2023, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, B.; Wang, X.; Stickle, W.F.; Ji, X. Production of graphene by reduction using a magnesiothermic reaction. Chem. Commun. 2013, 49, 10676–10678. [Google Scholar]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar]
- Chakrabarti, A.; Lu, J.; Skrabutenas, J.C.; Xu, T.; Xiao, Z.; Maguire, J.A.; Hosmane, N.S. Conversion of carbon dioxide to few-layer graphene. J. Mater. Chem. 2011, 21, 9491–9493. [Google Scholar]
- Fang, Z.; Patterson, B.R.; Turner, M.E., Jr. Modeling Particle Size Distributions by the Weibull Distribution Function. Mater. Charact. 1993, 31, 177–182. [Google Scholar]
- Kim, T.; Kang, S. On the quantitative analysis of secondary carbide and carbon in (Ti1−xMx)C solid solutions via XRD measurements. Int. J. Refract. Met. Hard Mater. 2008, 26, 444–448. [Google Scholar] [CrossRef]
- Lipatnikov, V.N.; Gusev, A.I. Effect of ordering on the structure and specific heat of nonstoichiometric titanium carbide. JETP Lett. 1999, 69, 669–675. [Google Scholar]
- Gusev, A.I. Phase equilibria, phases and compounds in the Ti–C system. Russ. Chem. Rev. 2002, 71, 439–463. [Google Scholar]
- Horak, P.; Bakardjieva, S.; Vacik, J.; Rui, X.; Klie, R. Preparation of Ti2C MXene phase by ion beam sputtering and ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 469, 49–51. [Google Scholar]
- Aydinyan, S. Synthesis of Ti2AlC MAX phase and Ti2C MXene by activated combustion. Ceram. Int. 2024, 50, 12263–12269. [Google Scholar]
- Mondal, K.; Ghosh, P. Exfoliation of Ti2C and Ti3C2 Mxenes from bulk trigonal phases of titanium carbide: A theoretical prediction. Solid State Commun. 2019, 299, 113657. [Google Scholar]
- He, R.; Wan, Y.; Zhao, P.; Guo, P.; Jiang, Z.; Zheng, J. First-principles investigation of native point defects in two-dimensional Ti3C2. Comput. Theor. Chem. 2019, 1150, 26–39. [Google Scholar]
- Jiang, C.; Jiang, W. Pressure–composition phase diagram of Ti–C from first principles. Phys. Status Solidi (B) 2013, 251, 533–536. [Google Scholar]
- Korzhavyi, P.A.; Pourovskii, L.V.; Hugosson, H.W.; Ruban, A.V.; Johansson, B. Ab initio study of phase equilibria in TiC(x). Phys. Rev. Lett. 2002, 88, 015505. [Google Scholar]
- Tashmetov, M.Y.; Em, V.T.; Lee, C.H.; Shim, H.S.; Choi, Y.N.; Lee, J.S. Neutron diffraction study of the ordered structures of nonstoichiometric titanium carbide. Physica B 2002, 311, 318–325. [Google Scholar]
- de Novion, C.H.; Beuneu, B.; Priem, T.; Lorenzelli, N.; Finel, A. Defect Structures and Order-Disorder Transfromations in Transition Metal Carbides and Nitrides. In The Physics and Chemistry of Carbides, Nitrides and Borides; Freer, R., Ed.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1990; Volume 185, pp. 423–440. [Google Scholar]
- Ma, C.; Gu, D.; Dai, D.; Yu, G.; Xia, M.; Chen, H. Thermodynamic behaviour and formation mechanism of novel titanium carbide dendritic crystals within a molten pool of selective laser melting TiC/Ti–Ni composites. CrystEngComm 2017, 19, 1089–1099. [Google Scholar]
- Shen, X.; Su, T.; Fan, Q.; Xu, S.; Yang, L.; Gong, H.; Yan, Q.; Cai, C. Preparation of millimeter-scale hard layer on the surface of titanium alloy via self-propagating high temperature synthesis combined with hot pressing sintering process. J. Mater. Res. Technol. 2022, 21, 4012–4022. [Google Scholar]
- Hassanbeygi, V.; Shafyei, A. Investigation on Microstructure and Grain Refining Performance of a New Type of Al-3Ti-1C Master Alloy. Open J. Met. 2014, 4, 49–55. [Google Scholar] [CrossRef][Green Version]
- Twardowska, A.; Rajchel, B.; Jaworska, L. Low temperature Ti-Si-C thin film deposition by ion beam assisted methods. IOP Conf. Ser. Mater. Sci. Eng. 2010, 15, 012040. [Google Scholar]
- Saba, F.; Sajjadi, S.A.; Haddad-Sabzevar, M.; Zhang, F. Formation mechanism of nano titanium carbide on multi-walled carbon nanotube and influence of the nanocarbides on the load-bearing contribution of the nanotubes inner-walls in aluminum-matrix composites. Carbon 2017, 115, 720–729. [Google Scholar] [CrossRef]
- Wulff, G. On the question of speed of growth and dissolution of crystal surfaces. Z Kryst. Miner. 1901, 34, 449–530. [Google Scholar]
- Zucker, R.V.; Chatain, D.; Dahmen, U.; Hagège, S.; Carter, W.C. New software tools for the calculation and display of isolated and attached interfacial-energy minimizing particle shapes. J. Mater. Sci. 2012, 47, 8290–8302. [Google Scholar]
- Vengrenovitch, R.D. On the Ostwald ripening theory. Acta Metall. 1982, 30, 1079–1086. [Google Scholar] [CrossRef]
- Voorhees, P.W. The Theory of Ostwald Ripening. Theory Ostwald Ripening 1985, 38, 231–252. [Google Scholar]
- Lohse, B.H.; Calka, A.; Wexler, D. Raman spectroscopy as a tool to study TiC formation during controlled ball milling. J. Appl. Phys. 2005, 97, 114912. [Google Scholar]
- Amer, M.; Barsoum, M.W.; El-Raghy, T.; Weiss, I.; Leclair, S.; Liptak, D. The Raman spectrum of Ti3SiC2. J. Appl. Phys. 1998, 84, 5817. [Google Scholar] [CrossRef]
- Klein, M.V.; Holy, J.A.; Williams, W.S. Raman scattering induced by carbon vacancies in TiCx. Phys. Rev. B 1978, 17, 1546. [Google Scholar] [CrossRef]
- Kaipoldayev, O.E.; Baigarinova, G.A.; Nemkayeva, R.R.; Guseinov, N.R.; Mukhametkarimov, Y.S.; Tauasarov, K.; Prikhodko, O.Y. Influence of Substrate Temperature on the Formation of Titanium Carbide Film. J. Nano-Electron. Phys. 2019, 11, 04003. [Google Scholar] [CrossRef] [PubMed]
- Dreiling, I.; Stiens, D.; Chassé, T. Raman spectroscopy investigations of TiBxCyNz coatings deposited by low pressure chemical vapor deposition. Surf. Coat. Technol. 2010, 205, 1339–1344. [Google Scholar] [CrossRef]
- Pellegrino, S.; Trocellier, P.; Thomé, L.; Miro, S.; Costantini, J.M.; Jouanny, E. Raman investigation of ion irradiated TiC and ZrC. Nucl. Instrum. Methods Phys. 2019, 454, 61–67. [Google Scholar] [CrossRef]
- Munir, K.S.; Qian, M.; Li, Y.; Oldfield, D.T.; Kingshott, P.; Zhu, D.M.; Wen, C. Quantitative Analyses of MWCNT-Ti Powder Mixtures using Raman Spectroscopy: The Influence of Milling Parameters on Nanostructural Evolution. Adv. Eng. Mater. 2015, 17, 1660–1669. [Google Scholar] [CrossRef]






| Carbon Sources | Samples | Ti (wt.%) | CS (wt.%) | Ti (g) | FC (g) |
|---|---|---|---|---|---|
| CTurbostratic | A1 | 90 | 10 | 0.9 | 0.10 |
| A2 | 80 | 20 | 0.8 | 0.2 | |
| CNTs | B1 | 90 | 10 | 0.9 | 0.10 |
| B2 | 80 | 20 | 0.8 | 0.2 |
| Samples | Lattice Parameters (Å) | Volume (Å 3) | Phase % | Rp (%) | Rwp (%) | χ2 |
|---|---|---|---|---|---|---|
| TiC bcc Reference 01-089-3828 | a = b = c = 4.3178 | 80.50 | 100 | - | - | - |
| A2 | a = b = c = 4.3104 | 80.08 | 93.85 | 5.80 | 7.53 | 4.92 |
| B2 | a = b = c = 4.2957 | 79.27 | 91.17 | 8.08 | 9.49 | 4.96 |
| TiC hcp Reference 01-079-0971 | a = b = 3.06 c = 14.91 | 120.91 | 100 | - | - | - |
| A2 | a = b = 3.0587 c = 14.97 | 121.33 | 6.15 | 5.80 | 7.53 | 4.92 |
| B2 | a = b = 3.0579 c = 14.994 | 121.43 | 8.83 | 8.08 | 9.49 | 4.96 |
| Samples | TA (cm−1) | LA (cm−1) | TO (cm−1) | LO (cm−1) |
|---|---|---|---|---|
| B2 | 276 | 328 | 410 | 600 |
| B1 | 243 | 323 | 412 | 574 |
| A2 | 255 | 361 | 413 | 607 |
| A1 | 270 | 329 | 407 | 611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Ríos, M.L.; Herrera-Pérez, G.; Rodriguez-Pacheco, L.C.; Luján-Aguilar, M.L.; Ramírez-de la Cruz, A.; Aguilar-Cisneros, N.W.; Esparza-Rodriguez, M.A.R.; Lardizabal-Gutierrez, D.; Pérez-Bustamante, R.; Betancourt-Cantera, J.A. Structural Insights: In Situ Synthesis of Titanium Carbide by Magnesiothermic Method Using Carbon Nanotubes and Turbostratic Carbon as Carbon Sources. J. Compos. Sci. 2025, 9, 171. https://doi.org/10.3390/jcs9040171
Camacho-Ríos ML, Herrera-Pérez G, Rodriguez-Pacheco LC, Luján-Aguilar ML, Ramírez-de la Cruz A, Aguilar-Cisneros NW, Esparza-Rodriguez MAR, Lardizabal-Gutierrez D, Pérez-Bustamante R, Betancourt-Cantera JA. Structural Insights: In Situ Synthesis of Titanium Carbide by Magnesiothermic Method Using Carbon Nanotubes and Turbostratic Carbon as Carbon Sources. Journal of Composites Science. 2025; 9(4):171. https://doi.org/10.3390/jcs9040171
Chicago/Turabian StyleCamacho-Ríos, María Luisa, Guillermo Herrera-Pérez, Luis Carlos Rodriguez-Pacheco, Mariana Lizbeth Luján-Aguilar, Antonio Ramírez-de la Cruz, Nathaly Withney Aguilar-Cisneros, Marco Antonio Ruiz Esparza-Rodriguez, Daniel Lardizabal-Gutierrez, Raúl Pérez-Bustamante, and José Antonio Betancourt-Cantera. 2025. "Structural Insights: In Situ Synthesis of Titanium Carbide by Magnesiothermic Method Using Carbon Nanotubes and Turbostratic Carbon as Carbon Sources" Journal of Composites Science 9, no. 4: 171. https://doi.org/10.3390/jcs9040171
APA StyleCamacho-Ríos, M. L., Herrera-Pérez, G., Rodriguez-Pacheco, L. C., Luján-Aguilar, M. L., Ramírez-de la Cruz, A., Aguilar-Cisneros, N. W., Esparza-Rodriguez, M. A. R., Lardizabal-Gutierrez, D., Pérez-Bustamante, R., & Betancourt-Cantera, J. A. (2025). Structural Insights: In Situ Synthesis of Titanium Carbide by Magnesiothermic Method Using Carbon Nanotubes and Turbostratic Carbon as Carbon Sources. Journal of Composites Science, 9(4), 171. https://doi.org/10.3390/jcs9040171










