Carbon Composite Derived from Spent Bleaching Earth for Rubbery Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Carbon Composite (CC)
2.3. Design of Experiments
2.3.1. Determination of Turbidity Removal Efficiency
2.3.2. Determination of Chemical Oxygen Demand (COD) Removal Efficiency
3. Results and Discussion
3.1. Characterization
3.1.1. X-Ray Diffraction (XRD)
3.1.2. BET Surface Area
3.1.3. Field Emission Scanning Electron Microscope (FESEM)
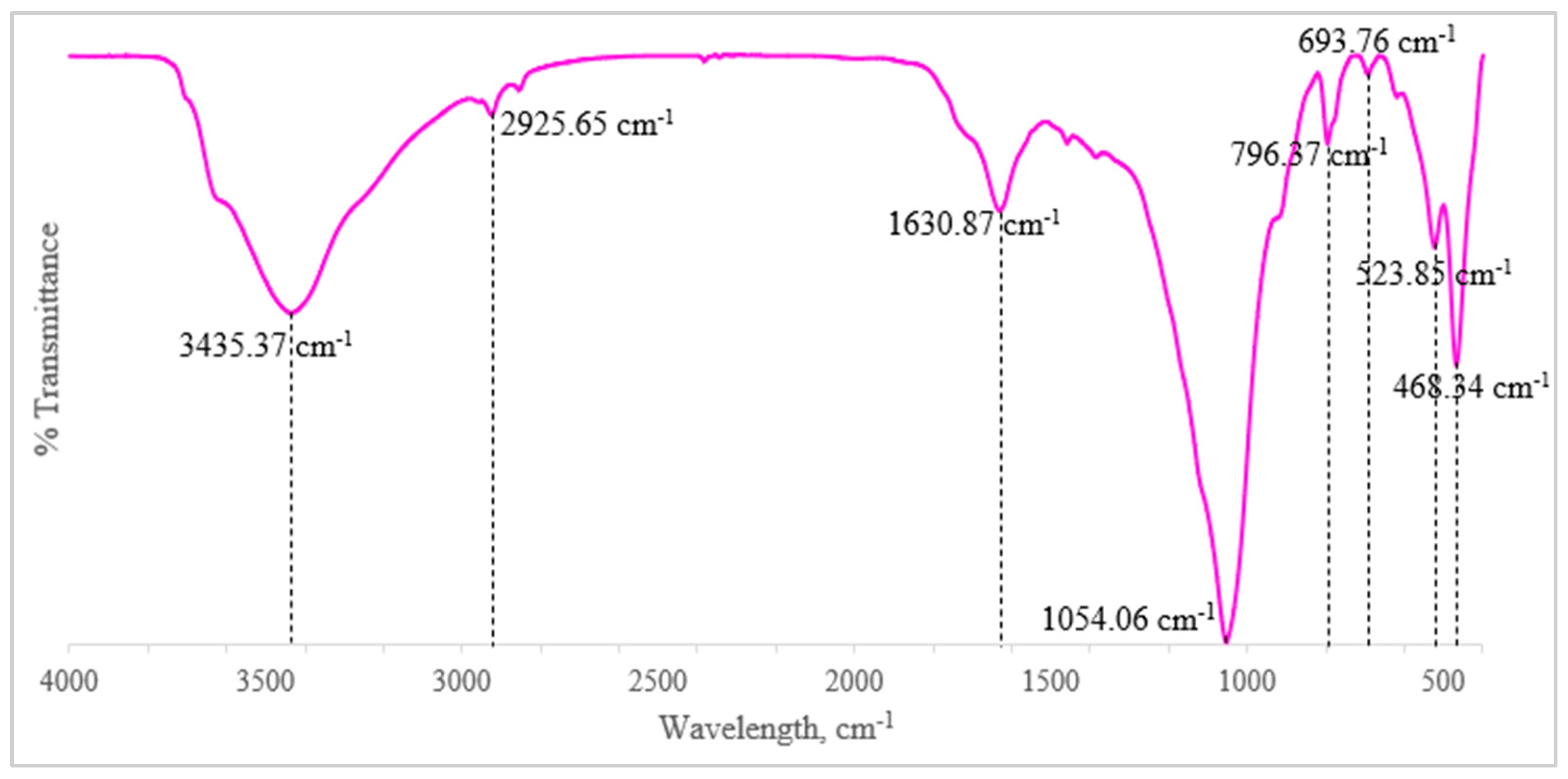
3.1.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Treatment of Rubber Wastewater
3.2.1. Visual Analysis of Carbon Composite (CC) Adsorption Performance
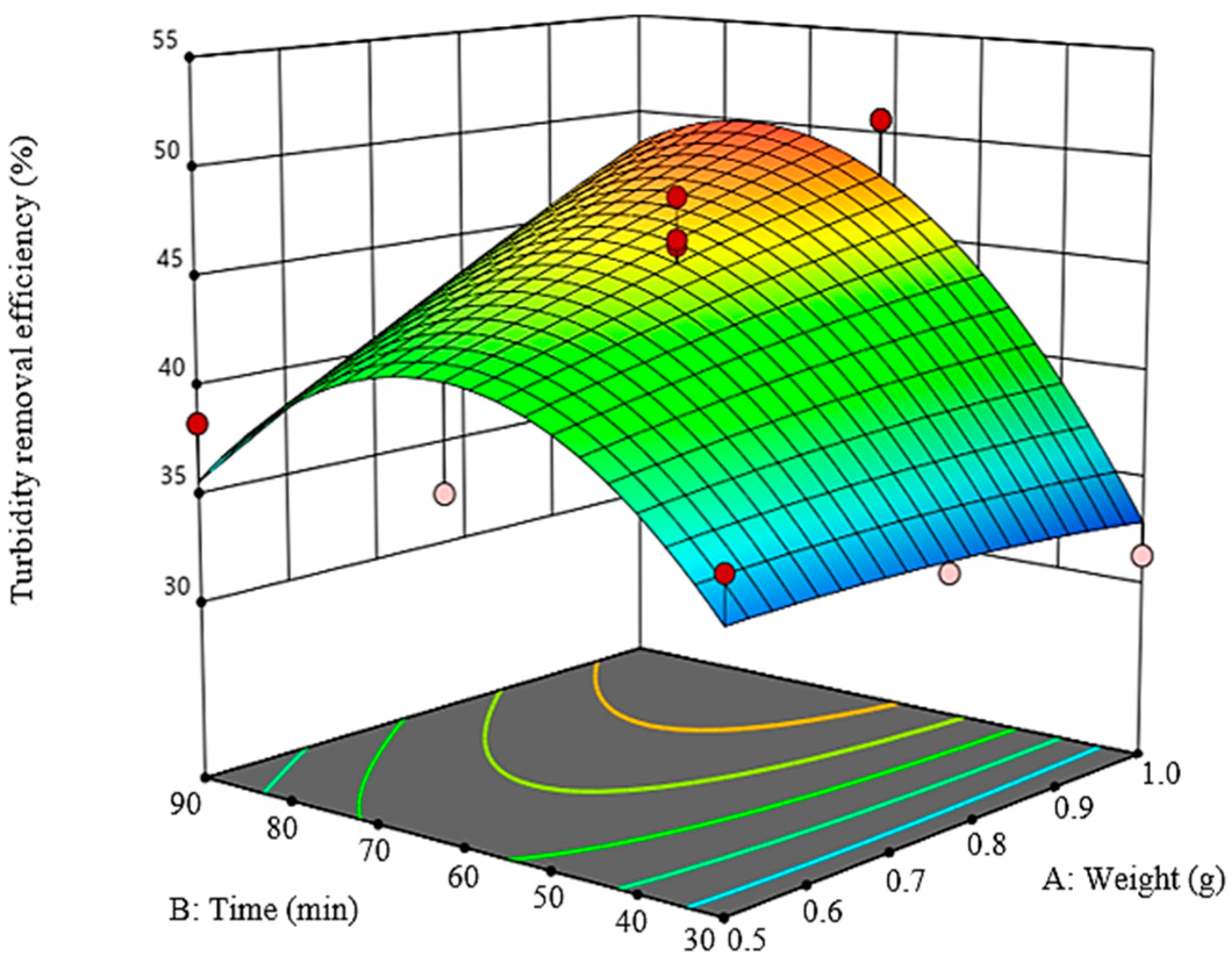
3.2.2. Analysis of Statistical Model
3.3. Numerical Optimization Study
3.4. Comparison with Reported Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| BOD | Biochemical Oxygen Demand |
| CC | Carbon Composite |
| COD | Chemical Oxygen Demand |
| FESEM | Field Emission Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| H2S | Hydrogen Sulfide |
| IUPAC | International Union of Pure and Applied Chemistry |
| RSM | Response Surface Methodology |
| SBE | Spent Bleaching Earth |
| XRD | X-Ray Diffraction |
References
- Zakaria, M.R.; Ahmad Farid, M.A.; Hafid, H.S.; Andou, Y.; Hassan, M.A. Practical role of oil palm fronds in Malaysia’s sustainable palm oil industry. Ind. Crop. Prod. 2024, 222, 119753. [Google Scholar] [CrossRef]
- Alam, A.S.A.F.; Er, A.C.; Begum, H. Malaysian oil palm industry: Prospect and problem. J. Food Agric. Environ. 2015, 13, 143–148. [Google Scholar]
- Sabour, M.R.; Shahi, M. Spent Bleaching Earth Recovery of Used Motor-Oil Refinery. Civ. Eng. J. 2018, 4, 572–584. [Google Scholar] [CrossRef]
- Keasavan, T.; Loh, S.K.; Jaafar, N.F.; Rahmawati, Z.; Abdullah, W.N.W. Synthesis of biodiesel from residual oil extracted from spent bleaching earth using spent bleaching earth-supported catalyst. Chem. Eng. Res. Des. 2023, 200, 716–728. [Google Scholar] [CrossRef]
- Majid, R.A.; Mat, C.R.C. Application of Regenerated Spent Bleaching Earth as Adsorbent for Treatment of Palm Oil Mill Effluentt. Palm Oil Eng. Bull. 2018, 127, 38–44. [Google Scholar]
- Massoudinejad, M.; Mehdipour, M.; Dehghani, M.H. Treatment of natural rubber industry wastewater through a combination of physicochemical and ozonation processes. J. Adv. Environ. Health Res. 2015, 3, 242–249. [Google Scholar]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Veerasamy, D. Membrane Distillation Technology for Treatment of Wastewater from Rubber Industry in Malaysia. Procedia CIRP 2015, 26, 792–796. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, D.S. Study of amount of Oxygen (BOD, OD, COD) in water and their effect on fishes. Nat. Sci. 2014, 7, 53–58. [Google Scholar]
- Akinnawo, S.O. Eutrophication: Causes, consequences; physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Mohammadi, M.; Man, H.C.; Hassan, M.A.; Yee, P.L. Treatment of wastewater from rubber industry in Malaysia. Afr. J. Biotechnol. 2010, 9, 6233–6243. [Google Scholar]
- Ho, K.C.; Chan, M.K.; Chen, Y.M.; Subhramaniyun, P. Treatment of rubber industry wastewater review: Recent advances and future prospects. J. Water Process. Eng. 2023, 52, 103559. [Google Scholar] [CrossRef]
- Wijerathna, W.; Wimalaweera, T.; Samarajeewa, D.; Lindamulla, L.; Rathnayake, R.; Nanayakkara, K.; Jegatheesan, V.; Wei, Y.; Jinadasa, K. Imperative assessment on the current status of rubber wastewater treatment: Research development and future perspectives. Chemosphere 2023, 338, 139512. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Issabayeva, G.; Chng, J.M.D. Rubber industry wastewater treatment: Adsorption of zinc. In Proceedings of the International Symposium on Green and Sustainable Technology (ISGST2019), Perak, Malaysia, 23–26 April 2019; p. 020012. [Google Scholar] [CrossRef]
- Agustina, T.E.; Sirait, E.J.; Silalahi, H. Treatment of rubber industry wastewater by using Fenton reagent and activated carbon. J. Teknol. 2017, 79, 11872. [Google Scholar] [CrossRef][Green Version]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef]
- Saeidi, N.; Parvini, M.; Niavarani, Z. High surface area and mesoporous graphene/activated carbon composite for adsorption of Pb(II) from wastewater. J. Environ. Chem. Eng. 2015, 3, 2697–2706. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El-Sayed, E.M.; Ali, S.; Elkady, M.F.; Elnouby, M.; Hamad, H.A. One-pot green synthesis of magnetic fullerene nanocomposite for adsorption characteristics. J. Water Process. Eng. 2020, 34, 101047. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Zheng, M.; Wang, A. Facile and green fabrication of magnetically recyclable carboxyl-functionalized attapulgite/carbon nanocomposites derived from spent bleaching earth for wastewater treatment. Chem. Eng. J. 2017, 322, 102–114. [Google Scholar] [CrossRef]
- Abu, N.E.F.; Yahya, N.Y.; Mansur, F.Z.; Muhammad, N. Removal of ammonia from rubber wastewater using rubber-sludge-based biochar to enhance biogas production. Process. Saf. Environ. Prot. 2024, 192, 378–385. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, Y.; Tan, Q.; Shen, Y.; Shen, L.; Sun, J.; Zhao, L.; Lin, H. Novel combination of iron-carbon composite and Fenton oxidation processes for high-concentration antibiotic wastewater treatment. J. Environ. Manag. 2024, 354, 120383. [Google Scholar] [CrossRef]
- Hermida, L.; Agustian, J.; Yeong, Y.F. Pelletizing of Attapulgite/carbon nanocomposite from used bleaching earth for continuous treatment of wastewater from a natural rubber factory. ASEAN J. Sci. Technol. Dev. 2024, 42, 5. [Google Scholar] [CrossRef]
- Shankar, D.; Sivakumar, D.; Thiruvengadam, M.; Manojkumar, M. Colour removal in a textile industry wastewater using coconut coir pith. Pollut. Res. 2014, 33, 499–503. [Google Scholar]
- Munien, C.; Tetteh, E.K.; Govender, T.; Jairajh, S.; Mguni, L.L.; Rathilal, S. Turbidity and COD Removal from Municipal Wastewater Using a TiO2 Photocatalyst—A Comparative Study of UV and Visible Light. Appl. Sci. 2023, 13, 4766. [Google Scholar] [CrossRef]
- Ke, Y.; Zhu, X.; Si, S.; Zhang, T.; Wang, J.; Zhang, Z. A Novel Adsorbent of Attapulgite & Carbon Composites Derived from Spent Bleaching Earth for Synergistic Removal of Copper and Tetracycline in Water. Int. J. Environ. Res. Public Health 2023, 20, 1573. [Google Scholar] [CrossRef] [PubMed]
- Yulikasari, A.; Nurhayati, E.; Utama, W.; Warmadewanthi, I. Characterization of Spent Bleaching Earth as an Adsorbent Material for Dye Removal. J. Ecol. Eng. 2022, 23, 96–104. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Shi, Y.; Wan, D.; Chen, J.; Xiao, S. Adsorption of toxic dye Eosin Y from aqueous solution by clay/carbon composite derived from spent bleaching earth. Water Environ. Res. 2021, 93, 159–169. [Google Scholar] [CrossRef]
- Naito, M.; Yokoyama, T.; Hosokawa, K.; Nogi, K. (Eds.) Chapter 2—Structural Control of Nanoparticles. In Nanoparticle Technology Handbook, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–107. [Google Scholar] [CrossRef]
- Song, Y.-J.; Li, H.-C.; Xiong, Z.-W.; Cheng, L.; Du, M.; Liu, Z.-Q.; Li, J.; Li, D.-Q. TiO2/carbon composites from waste sawdust for methylene blue photodegradation. Diam. Relat. Mater. 2023, 136, 109918. [Google Scholar] [CrossRef]
- Tang, J.; Zong, L.; Mu, B.; Zhu, Y.; Wang, A. Preparation and cyclic utilization assessment of palygorskite/carbon composites for sustainable efficient removal of methyl violet. Appl. Clay Sci. 2018, 161, 317–325. [Google Scholar] [CrossRef]
- Merikhy, A.; Heydari, A.; Eskandari, H.; Ghahraman-Rozegar, F. Carbonized spent bleaching earth as a low-cost adsorbent: A facile revalorization strategy via response surface methodology. Chem. Eng. Process. Process. Intensif. 2020, 158, 108167. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Eusoff, M.A.; Oladoye, P.O.; Adegoke, K.A.; Bello, O.S. Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel. Chem. Data Collect. 2020, 28, 100426. [Google Scholar] [CrossRef]
- Meziti, C.; Boukerroui, A. Removal of a Basic Textile Dye from Aqueous Solution by Adsorption on Regenerated Clay. Procedia Eng. 2012, 33, 303–312. [Google Scholar] [CrossRef]
- Daud, Z.; Ahmad, B.; Awang, H.; Abubakar, M.H.; Ridzuan, M.B.; Tajarudin, H.A. Removal of COD Using Delonix Regia Pods Activated Carbon Adsorbent for Natural Rubber Wastewater Treatment. Int. J. Integr. Eng. 2018, 10, 77–83. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, Y.; Lv, S.; Wang, R.; Hu, X.; Liu, Y.; Dong, Z.; Lin, K.; Liu, L. Chloride removal from sewage using bismuth trioxide: Characterization and optimization by response surface methodology (RSM). J. Environ. Chem. Eng. 2023, 11, 110868. [Google Scholar] [CrossRef]
- Lau, Y.L.; Yeong, Y.F. Optimization of Nitrate Removal from Aqueous Solution by Amine-functionalized MCM-41 Using Response Surface Methodology. Procedia Eng. 2016, 148, 1239–1246. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef]
- Kassahun, F.; Taddesse, A.M.; Teju, E.; Bogale, Y. Magnetic Al2O3/ZrO2/Fe3O4 nanocomposite: Synthesis, characterization, and application for the adsorptive removal of nitrate from aqueous solution. Groundw. Sustain. Dev. 2022, 20, 100873. [Google Scholar] [CrossRef]
- Aljohani, H.; Ahmed, Y.; El-Shafey, O.; El-Shafey, S.; Fouad, R.; Shoueir, K. Decolorization of turbid sugar juice from sugar factory using waste powdered carbon. Appl. Water Sci. 2018, 8, 48. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Utomo, D.P.; Kusumah, F.M.; Pratiwi, M.D. Performance of the crosslinked PVA coated PES-TiO2 nano hybrid membrane for the treatment of pretreated natural rubber wastewater involving sequential adsorption—Ozonation processes. J. Environ. Chem. Eng. 2021, 9, 104855. [Google Scholar] [CrossRef]
- Das, J.; Mondal, A.; Biswas, S.; Nag, S. The eco-friendly treatment of rubber industry effluent by using adsorbent derived from Moringa oleifera bark and Pseudomonas sp., cultured from effluent. Water Sci. Technol. 2022, 86, 2808–2819. [Google Scholar] [CrossRef]
- Kunarbekova, M.; Busquets, R.; Sailaukhanuly, Y.; Mikhalovsky, S.V.; Toshtay, K.; Kudaibergenov, K.; Azat, S.S. Carbon adsorbents for the uptake of radioactive iodine from contaminated water effluents: A systematic review. J. Water Process Eng. 2024, 67, 106174. [Google Scholar] [CrossRef]





| Run | Factor 1: Adsorbent Weight (g) | Factor 2: Contact Time (min) |
|---|---|---|
| 1 | 0.5 | 90 |
| 2 | 0.75 | 60 |
| 3 | 1 | 60 |
| 4 | 0.5 | 60 |
| 5 | 0.75 | 60 |
| 6 | 0.75 | 30 |
| 7 | 1 | 90 |
| 8 | 0.75 | 60 |
| 9 | 0.75 | 90 |
| 10 | 0.5 | 30 |
| 11 | 0.75 | 60 |
| 12 | 1 | 30 |
| 13 | 0.75 | 60 |
| Parameter | Result |
|---|---|
| BET surface area (m2/g) | 33.6695 |
| Pore volume (cm3/g) | 0.1051 |
| Pore size (nm) | 12.8717 |
| Run | Factor 1: Weight (g) | Factor 2: Time (min) | Response 1: COD Removal Efficiency (%) | Response 2: Turbidity Removal Efficiency (%) |
|---|---|---|---|---|
| 1 | 0.5 | 90 | 81.34 | 50.70 |
| 2 | 0.75 | 60 | 81.07 | 38.31 |
| 3 | 1 | 60 | 83.80 | 46.20 |
| 4 | 0.5 | 60 | 84.42 | 47.48 |
| 5 | 0.75 | 60 | 80.90 | 41.41 |
| 6 | 0.75 | 30 | 84.24 | 33.24 |
| 7 | 1 | 90 | 89.88 | 46.48 |
| 8 | 0.75 | 60 | 68.75 | 31.27 |
| 9 | 0.75 | 90 | 91.29 | 48.45 |
| 10 | 0.5 | 30 | 90.32 | 47.32 |
| 11 | 0.75 | 60 | 86.71 | 40.85 |
| 12 | 1 | 30 | 81.69 | 37.18 |
| 13 | 0.75 | 60 | 89.61 | 36.06 |
| ANOVA Result | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks | |||
| Model | 336.28 | 5 | 67.26 | 5.05 | 0.0281 | Significant | |||
| A: Weight | 23.84 | 1 | 23.84 | 1.79 | 0.2229 | ||||
| B: Time | 40.04 | 1 | 40.04 | 3.00 | 0.1266 | ||||
| AB | 226.65 | 1 | 226.65 | 17.01 | 0.0044 | ||||
| A2 | 40.21 | 1 | 40.21 | 3.02 | 0.1260 | ||||
| B2 | 0.0575 | 1 | 0.0575 | 0.0043 | 0.9494 | ||||
| Lack of fit | 16.93 | 3 | 5.64 | 0.2957 | 0.8277 | Not significant | |||
| Pure error | 76.37 | 4 | 19.09 | ||||||
| Cor total | 429.58 | 12 | |||||||
| Fit statistics | |||||||||
| R2 | 0.8828 | Adjusted R2 | 0.7277 | ||||||
| Adeq precision | 8.1531 | Predicted R2 | 0.7513 | ||||||
| ANOVA Result | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks | |||
| Model | 390.71 | 5 | 78.14 | 7.01 | 0.0119 | Significant | |||
| A: Weight | 52.45 | 1 | 52.45 | 4.71 | 0.0667 | ||||
| B: Time | 111.89 | 1 | 111.89 | 10.04 | 0.0157 | ||||
| AB | 47.61 | 1 | 47.61 | 4.27 | 0.0776 | ||||
| A2 | 0.4142 | 1 | 0.4142 | 0.0372 | 0.8526 | ||||
| B2 | 146.47 | 1 | 146.47 | 13.14 | 0.0085 | ||||
| Lack of fit | 50.64 | 3 | 16.88 | 2.47 | 0.2018 | Not significant | |||
| Pure error | 27.38 | 4 | 6.84 | ||||||
| Cor total | 468.73 | 12 | |||||||
| Fit statistics | |||||||||
| R2 | 0.8336 | Adjusted R2 | 0.7147 | ||||||
| Adeq precision | 6.8502 | Predicted R2 | 0.6389 | ||||||
| Run | Weight | Time | COD | Turbidity | Desirability |
|---|---|---|---|---|---|
| 1 | 0.750 | 90.560 | 90.296 | 49.017 | 0.958 |
| 2 | 1.000 | 90.000 | 90.124 | 48.448 | 0.953 |
| 3 | 1.000 | 89.646 | 90.173 | 48.517 | 0.919 |
| Material | Removal Efficiency (%) | References | |
|---|---|---|---|
| COD | Turbidity | ||
| Carbon composite (CC) derived from spent bleaching earth (SBE) | 90.3 | 49.2 | Present work |
| Combination of Fenton reagent and activated carbon | 95.0 | - | [15] |
| Activated carbon prepared from Delonix Regia pods | 70.7 | - | [34] |
| Bentonite granules | 37.5 | - | [40] |
| Moringa oleifera stem bark | 80.6 | 98.2 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamin, N.F.B.; Yeong, Y.F.; Agustian, J.; Hermida, L.; Liew, L.X. Carbon Composite Derived from Spent Bleaching Earth for Rubbery Wastewater Treatment. J. Compos. Sci. 2025, 9, 126. https://doi.org/10.3390/jcs9030126
Tamin NFB, Yeong YF, Agustian J, Hermida L, Liew LX. Carbon Composite Derived from Spent Bleaching Earth for Rubbery Wastewater Treatment. Journal of Composites Science. 2025; 9(3):126. https://doi.org/10.3390/jcs9030126
Chicago/Turabian StyleTamin, Nur Fatihah Binti, Yin Fong Yeong, Joni Agustian, Lilis Hermida, and Lih Xuan Liew. 2025. "Carbon Composite Derived from Spent Bleaching Earth for Rubbery Wastewater Treatment" Journal of Composites Science 9, no. 3: 126. https://doi.org/10.3390/jcs9030126
APA StyleTamin, N. F. B., Yeong, Y. F., Agustian, J., Hermida, L., & Liew, L. X. (2025). Carbon Composite Derived from Spent Bleaching Earth for Rubbery Wastewater Treatment. Journal of Composites Science, 9(3), 126. https://doi.org/10.3390/jcs9030126





