Enhanced Electrocatalytic Performance of Nickel-Cobalt-Titanium Dioxide-Embedded Carbon Nanofibers for Direct Alcohol Fuel Cells
Abstract
1. Introduction
2. Experimental Studies
2.1. Preparation of Investigated Samples
2.2. Sample Characterization
3. Results and Discussion
3.1. Morphological Studies
3.2. Elemental Analysis
3.3. Surface Chemistry and Valence States of Ni-Co-TiO2/CNF Analysis via (XPS)
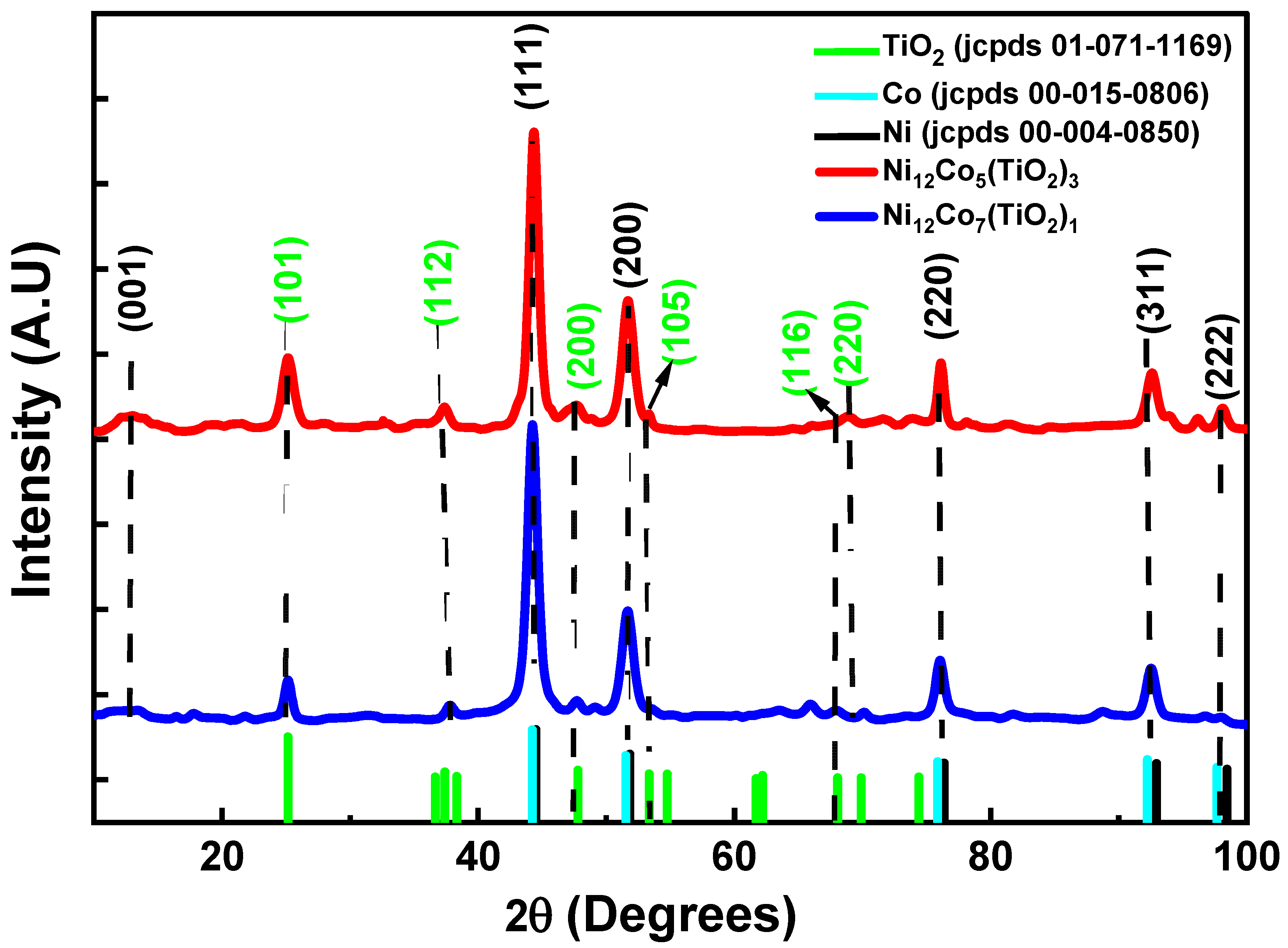
3.4. Characterization of Structure and Evaluation of Phases (XRD)
3.5. Electrooxidation Study
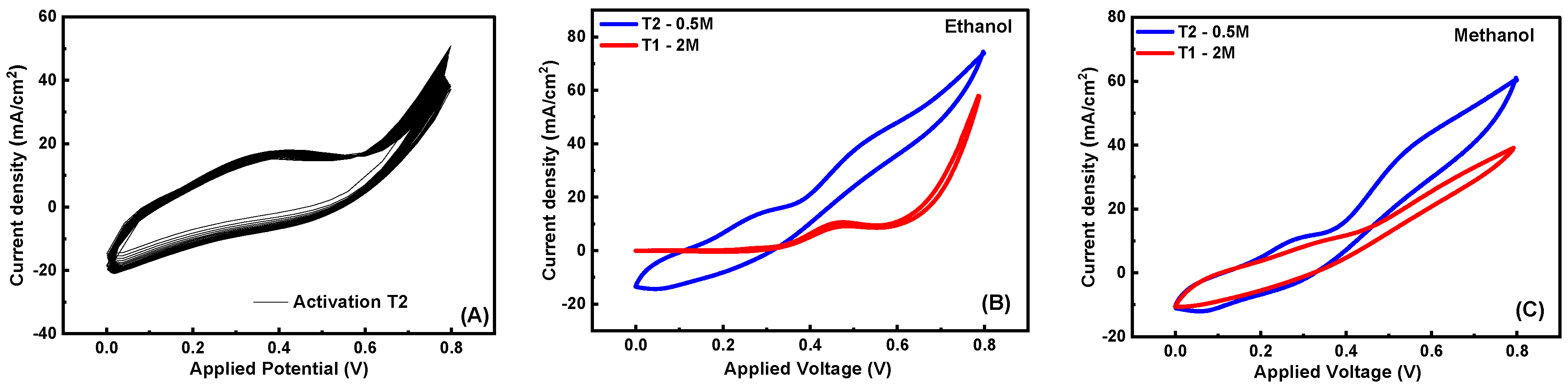
3.5.1. Surface Activation
3.5.2. Sample Composition Effect
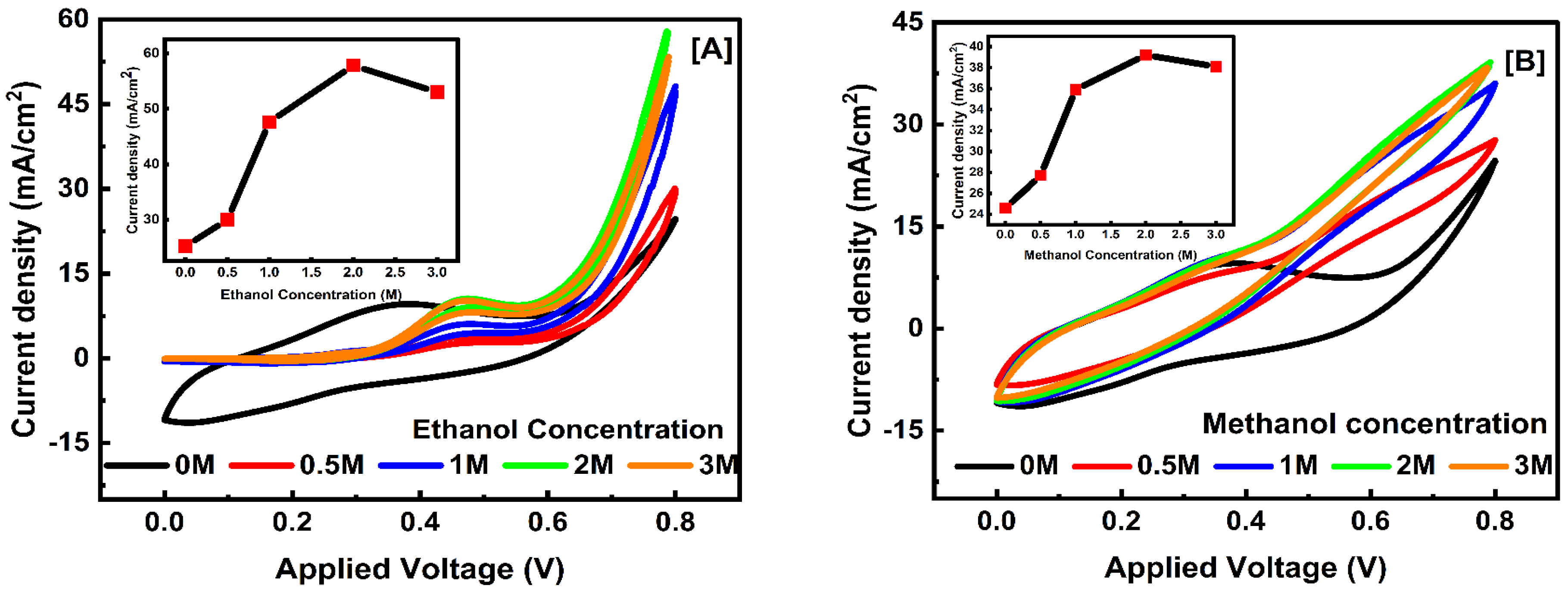
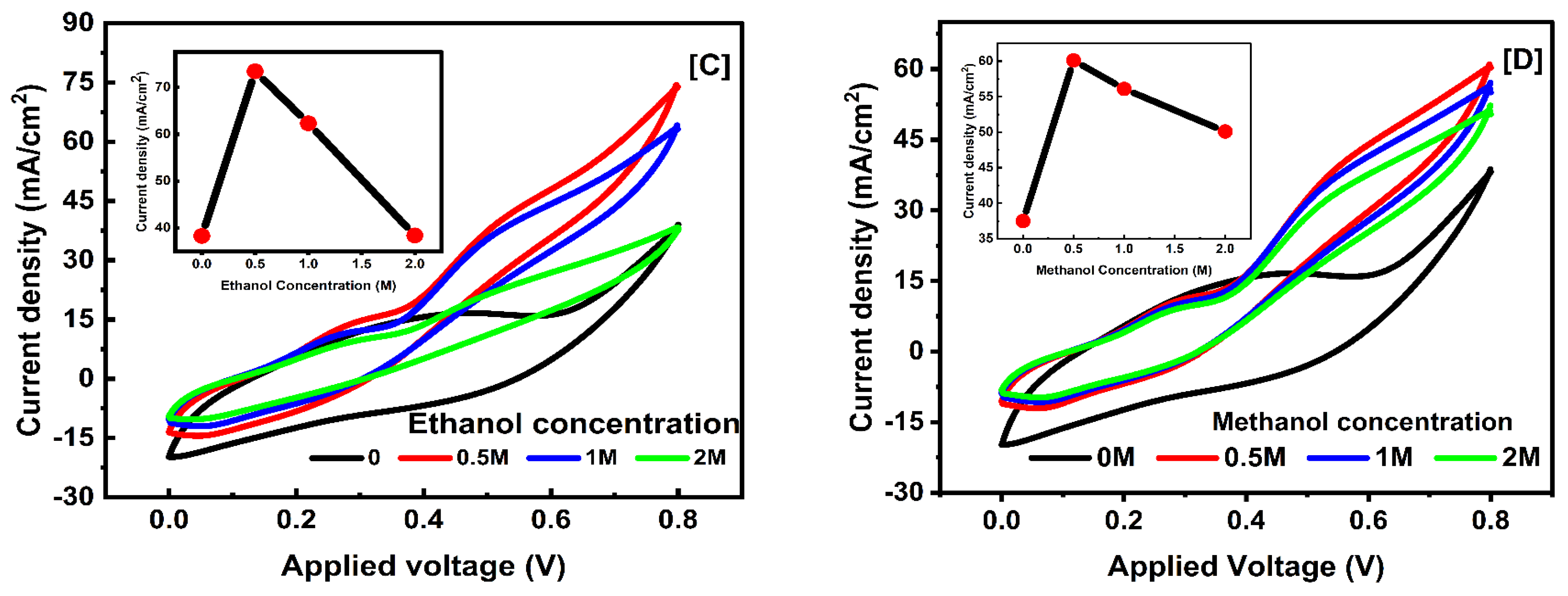
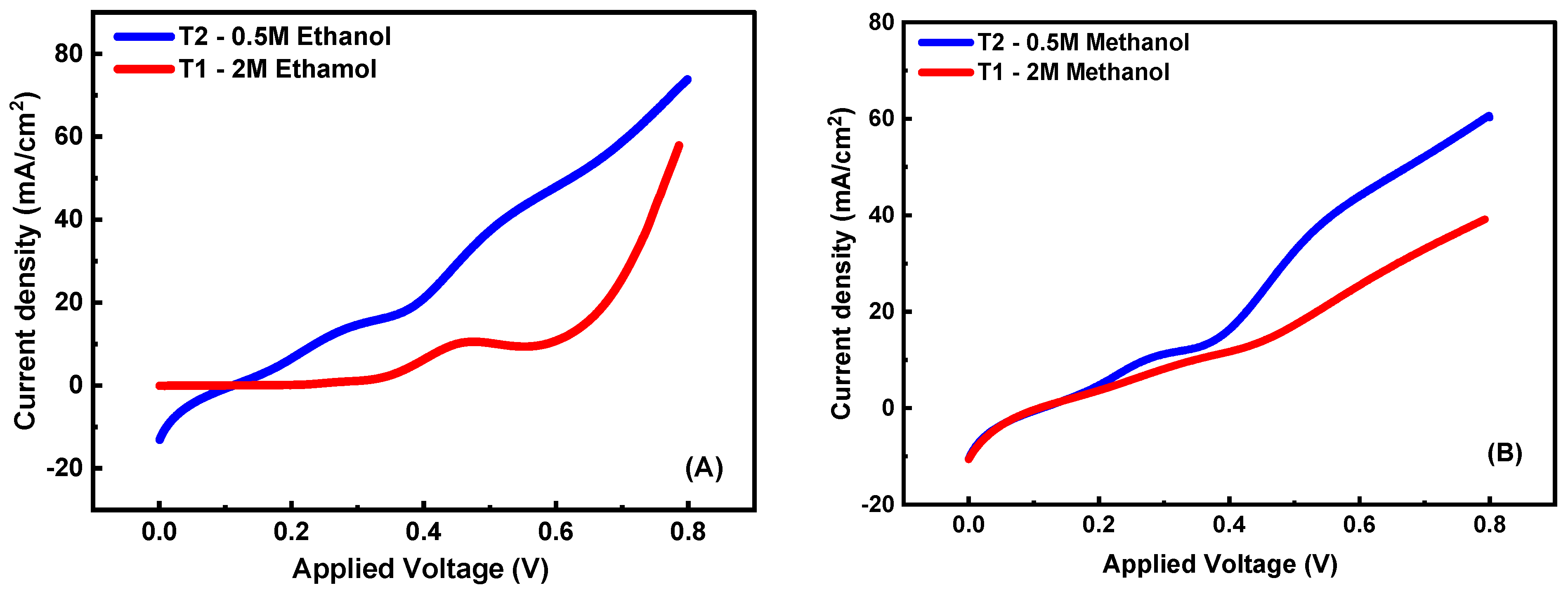
3.5.3. Concentration Effect of Electrolyte
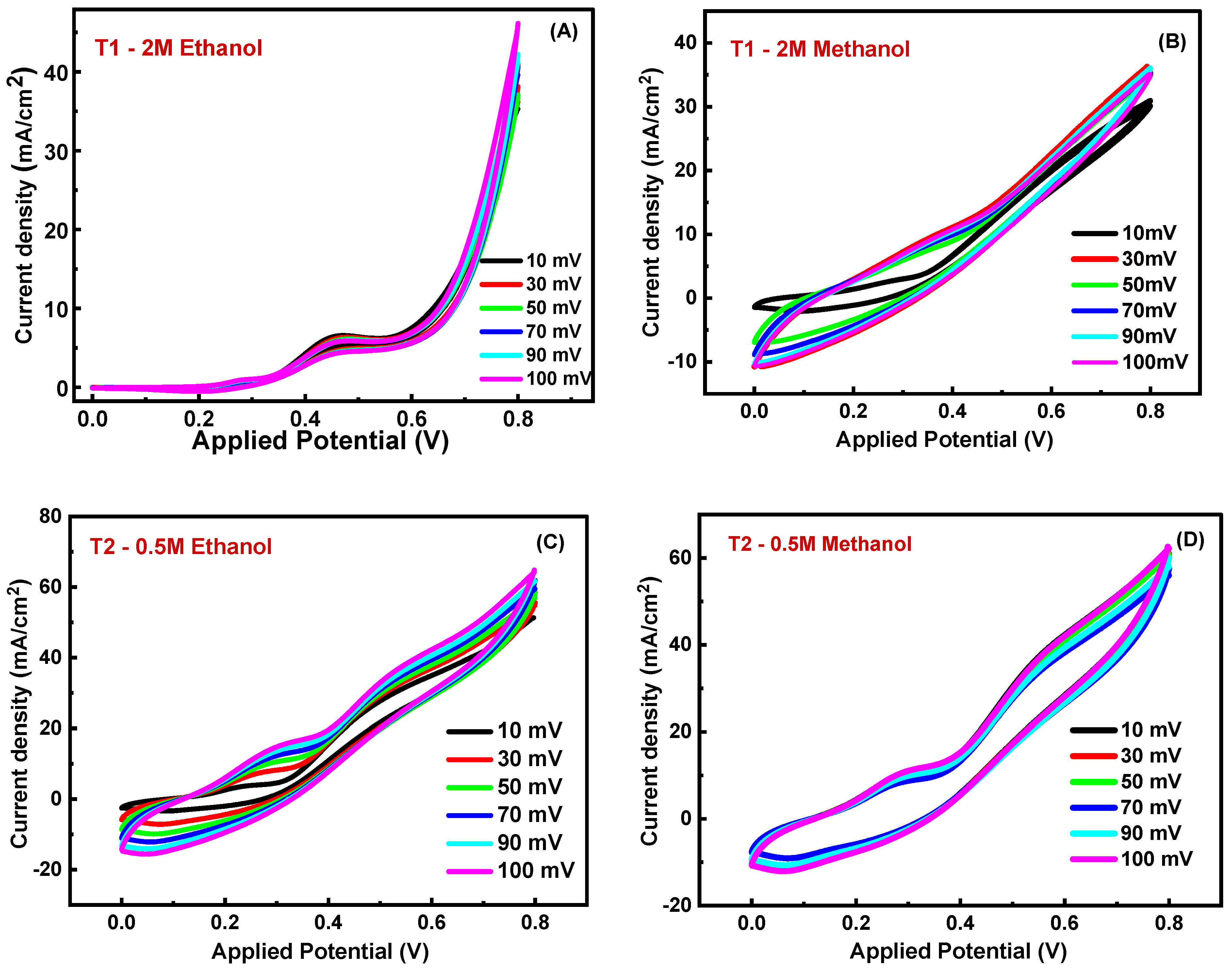
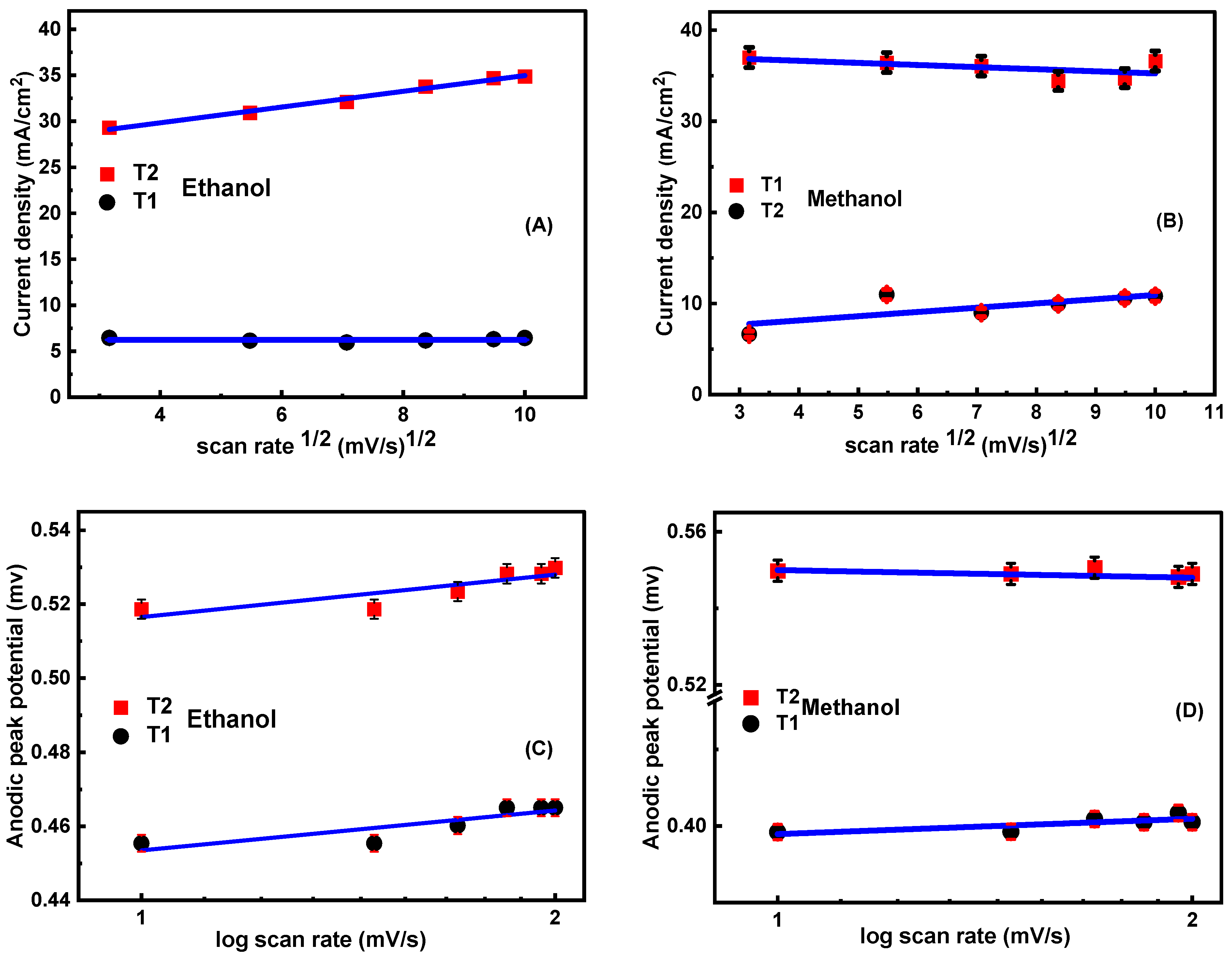
3.5.4. Influence of Scan Rate (SR)
3.5.5. Linear Sweep Voltammetry (LSV) and Tafel Slope
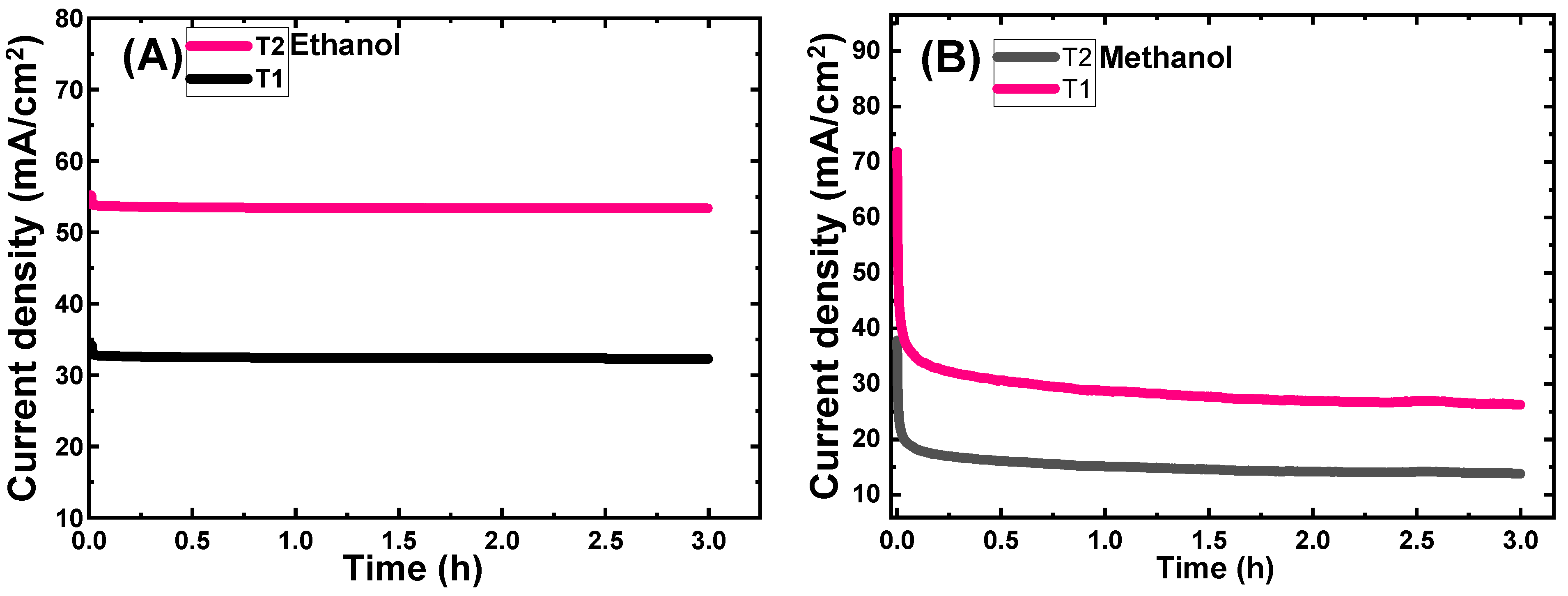
3.5.6. Stability Study
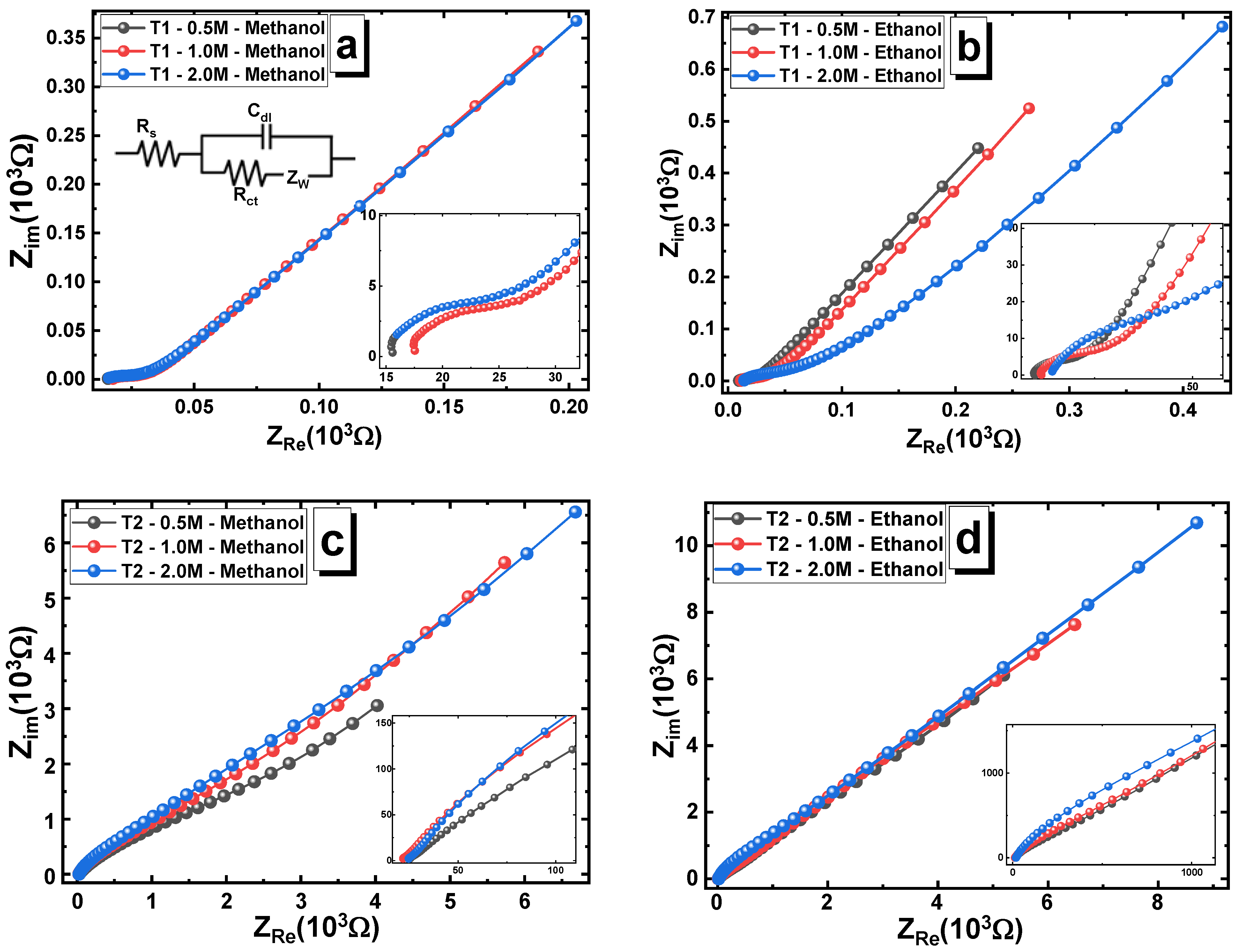
3.6. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Mohammed, W.M.; Awad, S.; Abdel-Hady, E.E.; Mohamed, H.F.; Elsharkawy, Y.S.; Elsharkawy, M.R. Nanostructure analysis and dielectric properties of PVA/sPTA proton exchange membrane for fuel cell applications: Positron lifetime study. Radiat. Phys. Chem. 2023, 208, 110942. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Marković, N.M. Just a dream—Or future reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Gao, Y. Understanding and approaches for the durability issues of Pt-based catalysts for PEM fuel cell. J. Power Sources 2007, 171, 558–566. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Awad, S.; Kaouach, H.; Mohammed, M.A.; Abdel-Hady, E.E.; Mohammed, W.M. Fabrication of bimetallic Ni-Ag/CNFs nanoparticles as a catalyst in direct alcohol fuel cells (DAFCs). Polym. Adv. Technol. 2023, 34, 1723–1738. [Google Scholar] [CrossRef]
- Abdel-Hady, E.E.; Gamal, A.; Hamdy, H.; Shaban, M.; Abdel-Hamed, M.O.; Mohammed, M.A.; Mohammed, W.M. Methanol electro oxidation on Ni–Pt–CrO/CNFs composite: Morphology, structural, and electrochemical characterization. Sci. Rep. 2023, 13, 4870. [Google Scholar] [CrossRef]
- Kongkanand, A.; Kuwabata, S.; Girishkumar, G.; Kamat, P. Single-wall carbon nanotubes supported platinum nanoparticles with improved electrocatalytic activity for oxygen reduction reaction. Langmuir 2006, 22, 2392–2396. [Google Scholar] [CrossRef]
- Chen, P.-J.; Wang, Y.-L.; Kuo, L.-M.; Lin, C.-F.; Chen, C.-Y.; Tsai, Y.-F.; Shen, J.-J.; Hwang, T.-L. Honokiol suppresses TNF-α-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IκBα. Sci. Rep. 2016, 6, 26554. [Google Scholar] [CrossRef]
- Saha, M.S.; Li, R.; Sun, X. High loading and monodispersed Pt nanoparticles on multiwalled carbon nanotubes for high performance proton exchange membrane fuel cells. J. Power Sources 2008, 177, 314–322. [Google Scholar] [CrossRef]
- Ferreira, P.; Shao-Horn, Y.; Morgan, D.; Makharia, R.; Kocha, S.; Gasteiger, H. Instability of Pt/C electrocatalysts in proton exchange membrane fuel cells: A mechanistic investigation. J. Electrochem. Soc. 2005, 152, A2256. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schüth, F.; Mayrhofer, K.J. Degradation mechanisms of Pt/C fuel cell catalysts under simulated start–stop conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Hikosaka, B.N. Fuel Cells: Current Technology Challenges and Future Research Needs; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kočí, K.; Obalová, L.; Lacný, Z. Photocatalytic reduction of CO2 over TiO2 based catalysts. Chem. Pap. 2008, 62, 1–9. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Sarwar, E.; Noor, T.; Iqbal, N.; Mehmood, Y.; Ahmed, S.; Mehek, R. Effect of Co-Ni ratio in graphene based bimetallic electro-catalyst for methanol oxidation. Fuel Cells 2018, 18, 189–194. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Wala, M.; Blacha-Grzechnik, A.; Stolarczyk, A.; Maciej, A.; Simka, W. Electrocatalytic methanol oxidation using Ni–Co–graphene composite electrodes. Int. J. Hydrogen Energy 2021, 46, 33272–33286. [Google Scholar] [CrossRef]
- Hassanzadeh, V.; Sheikh-Mohseni, M.A.; Habibi, B. Catalytic oxidation of ethanol by a nanostructured Ni-Co/RGO composite: Electrochemical construction and investigation. J. Electroanal. Chem. 2019, 847, 113200. [Google Scholar] [CrossRef]
- Roodbari, N.J.; Omrani, A.; Hosseini, S.R. Synthesis, characterization and performance of a novel Ni–Co/GO-TiO2 for electrooxidation of methanol and ethanol. Int. J. Hydrog. Energy 2022, 47, 6044–6058. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Weng, Y.-X.; Han, Y.-H.; Ye, R.-H.; Huang, D.-H. A novel pencil graphite electrode modified with an iron-based conductive metal-organic framework exhibited good ability in simultaneous sensing bisphenol A and bisphenol S. Ecotoxicol. Environ. Saf. 2024, 272, 116065. [Google Scholar] [CrossRef]
- Li, M.; Feng, L.G. Advances of phosphide promoter assisted Pt based catalyst for electrooxidation of methanol. J. Electrochem. 2022, 28, 2106211. [Google Scholar]
- Zhu, Z.; Men, Y.; Zhang, W.; Yang, W.; Wang, F.; Zhang, Y.; Zeng, X.; Xiao, J.; Tang, C.; Li, X.; et al. Versatile carbon-based materials from biomass for advanced electrochemical energy storage systems. Escience 2024, 4, 100249. [Google Scholar] [CrossRef]
- Long, N.V.; Asaka, T.; Matsubara, T.; Nogami, M. Shape-controlled synthesis of Pt–Pd core–shell nanoparticles exhibiting polyhedral morphologies by modified polyol method. Acta Mater. 2011, 59, 2901–2907. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Lee, K.-H.; Song, W.-J.; Cao, G.; Park, K.-W. Synthesis of monodispersed Pt-Ni alloy nanodendrites and their electrochemical properties. Int. J. Electrochem. Sci. 2013, 8, 2305–2312. [Google Scholar]
- Abdel-Hady, E.E.; Shaban, M.; Abdel-Hamed, M.O.; Gamal, A.; Yehia, H.; Ahmed, A.M. Synthesis and characterization of NiCoPt/CNFs nanoparticles as an effective electrocatalyst for energy applications. Nanomaterials 2022, 12, 492. [Google Scholar] [CrossRef]
- Barakat, N.A.; El-Newehy, M.H.; Yasin, A.S.; Ghouri, Z.K.; Al-Deyab, S.S. Ni & Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A Gen. 2016, 510, 180–188. [Google Scholar]
- Pant, B.; Pant, H.R.; Barakat, N.A.; Park, M.; Jeon, K.; Choi, Y.; Kim, H. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, L.; Tian, J.; Nie, S.; Ning, Z. Enhanced electrocatalytic oxidation of methanol on Pd/polypyrrole–graphene in alkaline medium. Electrochim. Acta 2011, 56, 1967–1972. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992; Volume 40, p. 221. [Google Scholar]
- Mohammed, W.M.; Gumarov, A.I.; Vakhitov, I.R.; Yanilkin, I.V.; Kiiamov, A.G.; Kharintsev, S.S.; Nikitin, S.I.; Tagirov, L.R.; Yusupov, R.V. (Eds.) Electrical properties of titanium nitride films synthesized by reactive magnetron sputtering. J. Phys. Conf. Ser. 2017, 927, 012036. [Google Scholar] [CrossRef]
- Madhugiri, S.; Sun, B.; Smirniotis, P.G.; Ferraris, J.P.; Balkus, K.J., Jr. Electrospun mesoporous titanium dioxide fibers. Microporous Mesoporous Mater. 2004, 69, 77–83. [Google Scholar] [CrossRef]
- Xu, J.; Ao, Y.; Fu, D.; Yuan, C. Synthesis of Gd-doped TiO2 nanoparticles under mild condition and their photocatalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 107–111. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Vaiano, V.; Ciambelli, P.; Sannino, D. Photocatalytic ethanol oxidative dehydrogenation over Pt/TiO2: Effect of the addition of blue phosphors. Int. J. Photoenergy 2012, 2012, 687262. [Google Scholar] [CrossRef]
- John, A.K.; Palaty, S.; Sharma, S.S. Greener approach towards the synthesis of titanium dioxide nanostructures with exposed {001} facets for enhanced visible light photodegradation of organic pollutants. J. Mater. Sci. Mater. Electron. 2020, 31, 20868–20882. [Google Scholar] [CrossRef]
- Soomro, S.A.; Gul, I.H.; Naseer, H.; Marwat, S.; Mujahid, M. Improved performance of CuFe2O4/rGO nanohybrid as an anode material for lithium-ion batteries prepared via facile one-step method. Curr. Nanosci. 2019, 15, 420–429. [Google Scholar] [CrossRef]
- Malas, A.; Das, C.K. Effect of graphene oxide on the physical, mechanical and thermo-mechanical properties of neoprene and chlorosulfonated polyethylene vulcanizates. Compos. Part B Eng. 2015, 79, 639–648. [Google Scholar] [CrossRef]
- Abdel-Hamed, M.O.; Draz, A.A.; Khala, M.; El-Hossary, F.M.; Mohamed, H.F.; Abdel-Hady, E.E. Effect of plasma pretreatment and graphene oxide ratios on the transport properties of PVA/PVP membranes for fuel cells. Sci. Rep. 2024, 14, 1092. [Google Scholar] [CrossRef]
- Mohamed, H.F.; Abdel-Hady, E.E.; Mohammed, W.M. Investigation of Transport Mechanism and Nanostructure of Nylon-6, 6/PVA Blend Polymers. Polymers 2022, 15, 107. [Google Scholar] [CrossRef]
- Raghuveer, V.; Manthiram, A.; Bard, A.J. Pd−Co−Mo electrocatalyst for the oxygen reduction reaction in proton exchange membrane fuel cells. J. Phys. Chem. B 2005, 109, 22909–22912. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Barbhuiya, T. Studies on the density and surface area of nanoparticles from Camellia sinensis, A natural source. J. Chem. Pharm. Res. 2014, 6, 608–610. [Google Scholar]
- Ramachandran, K.; Raj Kumar, T.; Babu, K.J.; Gnana Kumar, G. Ni-Co bimetal nanowires filled multiwalled carbon nanotubes for the highly sensitive and selective non-enzymatic glucose sensor applications. Sci. Rep. 2016, 6, 36583. [Google Scholar] [CrossRef]
- Barakat, N.A.; Moustafa, H.M.; Nassar, M.M.; Abdelkareem, M.A.; Mahmoud, M.S.; Almajid, A.A.; Khalil, K.A. Distinct influence for carbon nano-morphology on the activity and optimum metal loading of Ni/C composite used for ethanol oxidation. Electrochim. Acta 2015, 182, 143–155. [Google Scholar] [CrossRef]
- García-Rodríguez, S.; Rojas, S.; Pena, M.; Fierro, J.; Baranton, S.; Léger, J. An FTIR study of Rh-PtSn/C catalysts for ethanol electrooxidation: Effect of surface composition. Appl. Catal. B Environ. 2011, 106, 520–528. [Google Scholar] [CrossRef]
- Shenashen, M.A.; Hassen, D.; El-Safty, S.A.; Isago, H.; Elmarakbi, A.; Yamaguchi, H. Axially oriented tubercle vein and X-crossed sheet of N-Co3O4@ C hierarchical mesoarchitectures as potential heterogeneous catalysts for methanol oxidation reaction. Chem. Eng. J. 2017, 313, 83–98. [Google Scholar] [CrossRef]
- Zhang, J.; Tse, Y.-H.; Pietro, W.J.; Lever, A. Electrocatalytic activity of N, N′, N″, N‴-tetramethyl-tetra-3, 4-pyridoporphyrazinocobalt (II) adsorbed on a graphite electrode towards the oxidation of hydrazine and hydroxylamine. J. Electroanal. Chem. 1996, 406, 203–211. [Google Scholar] [CrossRef]
- Elsharkawy, M.R.M.; Ibrahim, S.; Mohammed, W.M.M. Effect of V2O5 incorporation on the Structural, Electrical, and Conduction Properties of sulfonated PVC/PMMA Blend: Positron Annihilation Study. Egypt. J. Phys. 2024, 52, 65–79. [Google Scholar] [CrossRef]
- Elsharkawy, M.R.; Mohammed, W.M. Effect of the electric field on the free volume investigated from positron annihilation lifetime and dielectric properties of sulfonated PVC/PMMA. Polym. Adv. Technol. 2024, 35, e6519. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Mohamed, I.M.; Yasin, A.S.; Barakat, N.A.; Song, S.A.; Lee, H.E.; Kim, S.S. Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl. Surf. Sci. 2018, 435, 122–129. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Liao, L.; Beine, A.K.; Palkovits, R. Stability and deactivation of OER electrocatalysts: A review. J. Energy Chem. 2022, 69, 301–329. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, G.; Singh, P.P.; Kaushal, S. Supported bimetallic nanoparticles as anode catalysts for direct methanol fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 15820–15849. [Google Scholar] [CrossRef]
- Siwal, S.S.; Thakur, S.; Zhang, Q.; Thakur, V.K. Electrocatalysts for electrooxidation of direct alcohol fuel cell: Chemistry and applications. Mater. Today Chem. 2019, 14, 100182. [Google Scholar] [CrossRef]
- Liu, C.; Yang, F.; Schechter, A.; Feng, L. Recent progress of Ni-based catalysts for methanol electrooxidation reaction in alkaline media. Adv. Sens. Energy Mater. 2023, 2, 100055. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhang, X.-H.; Zhao, D.-M.; Wang, S.-F. Electrochemical studies of bovine serum albumin immobilization onto the poly-o-phenylenediamine and carbon-coated nickel composite film and its interaction with papaverine. Sens. Actuators B Chem. 2011, 152, 88–93. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Abdel-Hamed, M.O.; Ibrahim, M.; Abdel-Hady, E.E.; Elsharkawy, Y.S. High-Performance Methanol Oxidation via Ni12-Metal8/CNF Catalyst for Fuel Cell Applications. Catalysts 2024, 14, 680. [Google Scholar] [CrossRef]
- Chen, M.; Du, C.; Yin, G.; Shi, P.; Zhao, T. Numerical analysis of the electrochemical impedance spectra of the cathode of direct methanol fuel cells. Int. J. Hydrogen Energy 2009, 34, 1522–1530. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Huang, W.-C.; Weng, J.-H.; Chen, L.-C.; Chou, C.-F.; Wei, P.-K. Impedimetric aptasensing using a symmetric Randles circuit model. Electrochim. Acta 2020, 337, 135750. [Google Scholar] [CrossRef]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, P.; Xu, S.; Yan, X.; Xue, Q. Free-standing three-dimensional graphene/manganese oxide hybrids as binder-free electrode materials for energy storage applications. ACS Appl. Mater. Interfaces 2014, 6, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, M.; Zhang, X.; Cai, J.; Lin, S. A novel octahedral MnO/RGO composite prepared by thermal decomposition as a noble-metal free electrocatalyst for ORR. J. Mater. Sci. 2017, 52, 6656–6669. [Google Scholar] [CrossRef]
- Chen, F.; Guo, S.; Yu, S.; Zhang, C.; Guo, M.; Li, C. Hierarchical N-doped carbon nanofiber-loaded NiCo alloy nanocrystals with enhanced methanol electrooxidation for alkaline direct methanol fuel cells. J. Colloid Interface Sci. 2023, 646, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Cheshideh, H.; Nasirpouri, F. Cyclic voltammetry deposition of nickel nanoparticles on TiO2 nanotubes and their enhanced properties for electro-oxidation of methanol. J. Electroanal. Chem. 2017, 797, 121–133. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Momeni, M.; Faraji, M. Highly active nickel nanoparticles supported on TiO2 nanotube electrodes for methanol electrooxidation. Electroanalysis 2010, 22, 2620–2625. [Google Scholar] [CrossRef]
- Hu, G.-H.; Zeng, H.-X.; Wei, Z.-G.; Pan, Z.-C.; Xie, S.-G.; Zou, Y.-D. Electrocatalytic Oxidation of Ethanol on Ni-P-TiO2 Composite Coating electrodes. J. Electrochem. 2012, 18, 16. [Google Scholar] [CrossRef]
- Mahapatra, S.; Datta, J. Characterization of Pt-Pd/C electrocatalyst for methanol oxidation in alkaline medium. Int. J. Electrochem. 2011, 2011, 563495. [Google Scholar] [CrossRef]
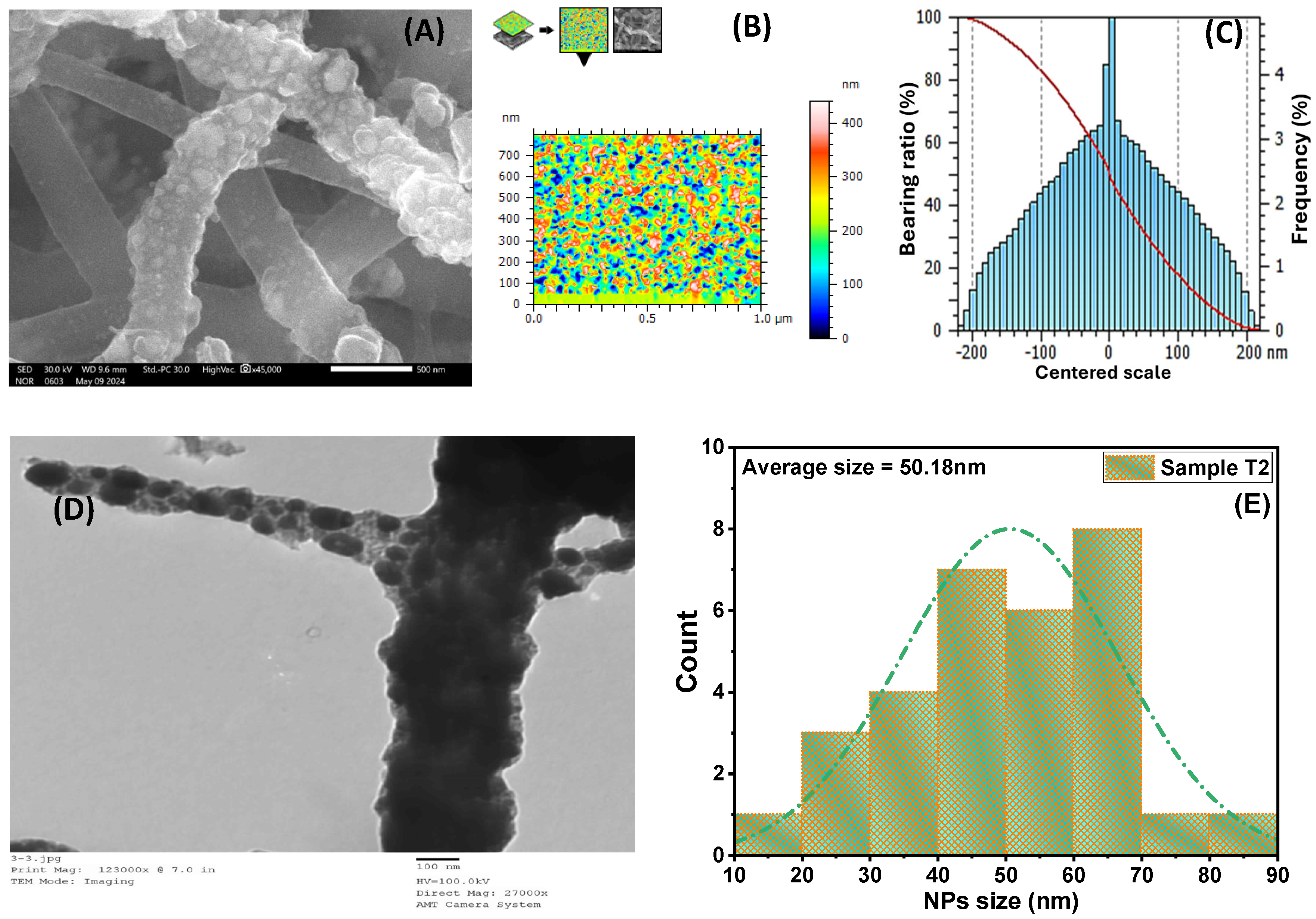
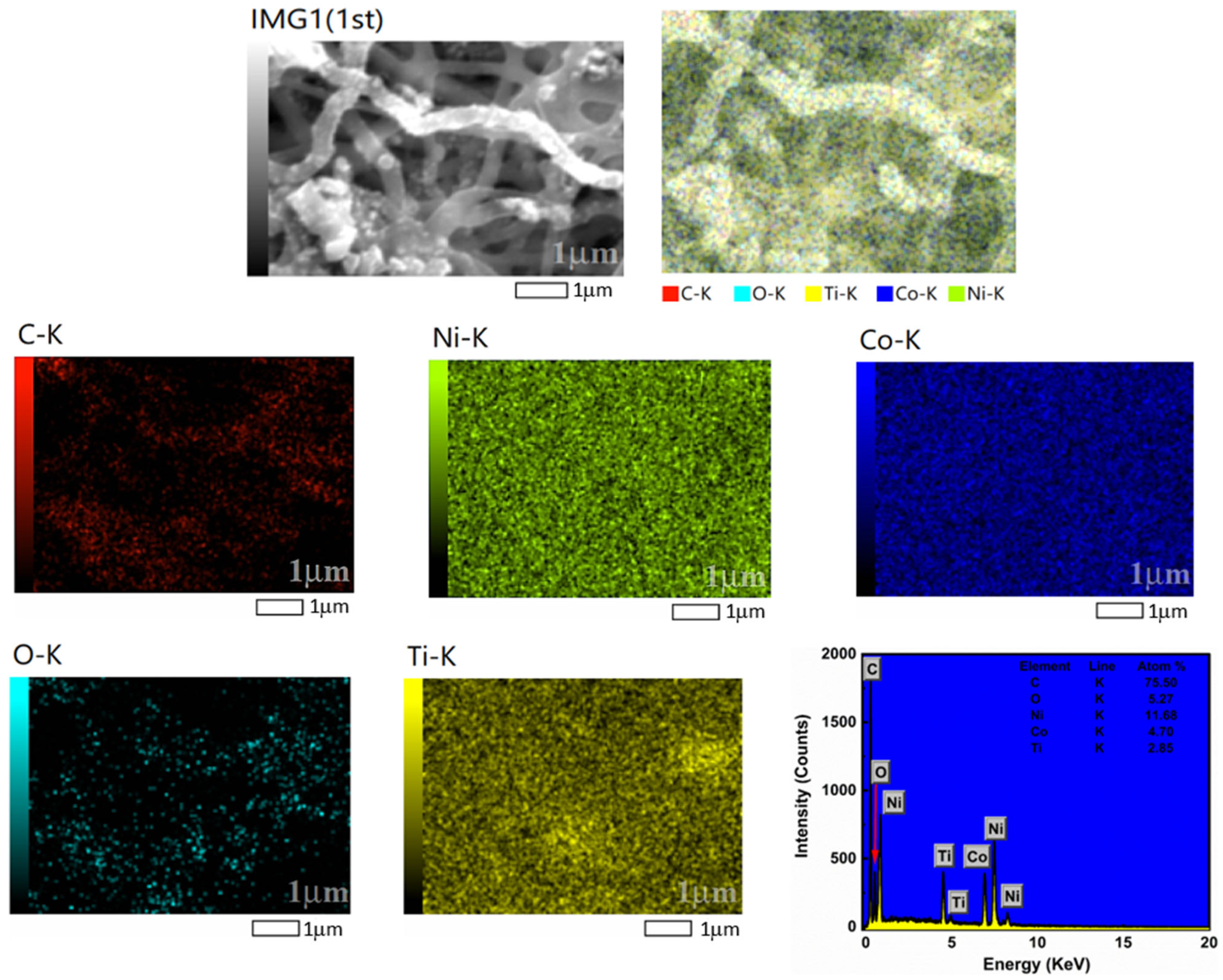
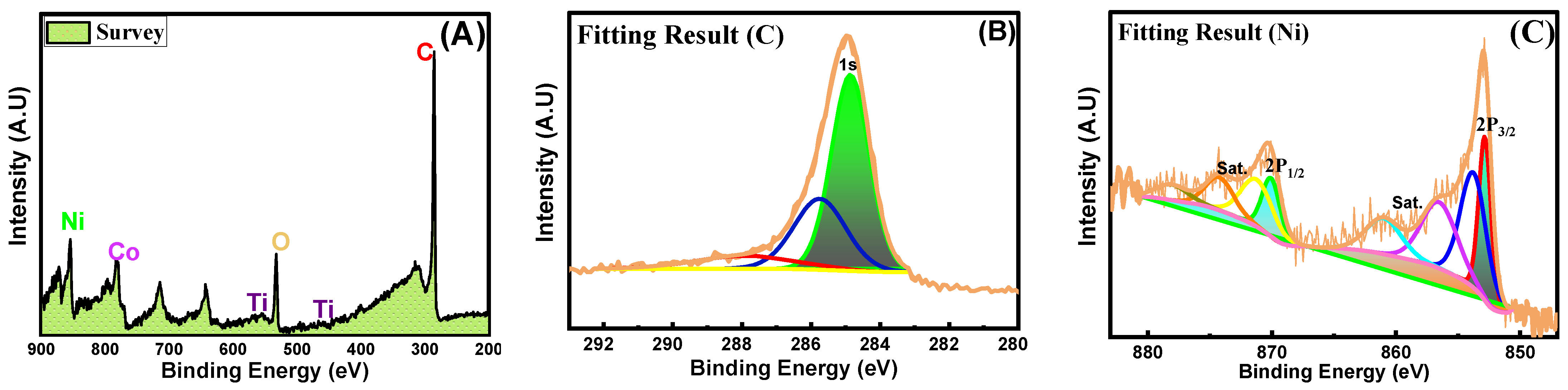
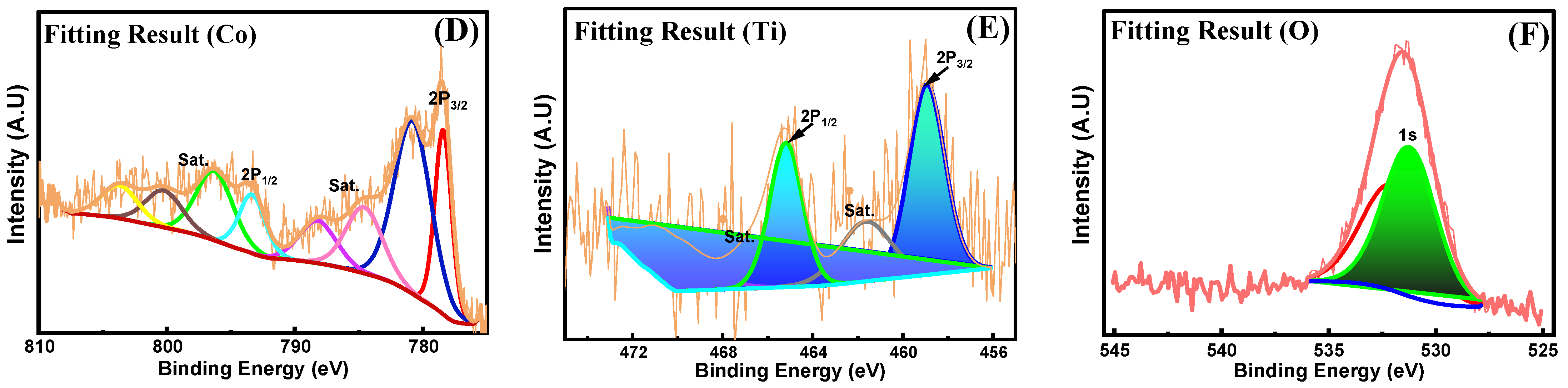
| Catalyst | Ni (%) | Co (%) | TiO2 (%) |
|---|---|---|---|
| T1 | 12 | 7 | 1 |
| T2 | 12 | 5 | 3 |
| Samples | (hkl) |
Peak Position (Degree) |
FWHM (Degree) |
D (nm) (Sherrer Equation) | Surface Area (m2/g) |
|---|---|---|---|---|---|
| T1 | (101) (TiO2) | 25.14 | 0.78 | 10.90 | 65.24 ± 0.052 |
| (112) (TiO2) | 37.75 | 0.84 | 10.43 | ||
| (111) (Ni) | 44.24 | 1.07 | 8.31 | ||
| T2 | (101) (TiO2) | 25.16 | 1.09 | 7.80 | 62.78 ± 0.063 |
| (112) (TiO2) | 37.35 | 0.86 | 9.79 | ||
| (111) (Ni) | 44.30 | 0.95 | 9.06 |
| Fuel Type | Conc. | TiO2 3% (T2) | TiO2 1% (T1) | ||||
|---|---|---|---|---|---|---|---|
| (JPE) mA/cm2 | (JP) mA/cm2 | (V) Peak Potential | (JPE) mA/cm2 | (JP) mA/cm2 | (V) Peak Potential | ||
| Ethanol | 0.0 M | 38.40 | 8.60 | 0.268 | 24.3 | 9.52 | 0.376 |
| 0.5 M | 74.21 | 42.11 | 0.539 | 30.08 | 3.77 | 0.483 | |
| 1.0 M | 63.00 | 39.33 | 0.539 | 48.18 | 6.00 | 0.465 | |
| 2.0 M | 38.01 | 21.87 | 0.499 | 57.8 | 10.51 | 0.469 | |
| 3.0 M | 53.3 | 10.16 | 0.466 | ||||
| Methanol | 0.0 M | 38.60 | 12.25 | 0.306 | 24.4 | 9.44 | 0.362 |
| 0.5 M | 60.39 | 41.87 | 0.576 | 27.55 | 7.96 | 0.352 | |
| 1.0 M | 55.80 | 37.32 | 0.549 | 35.63 | 10.09 | 0.344 | |
| 2.0 M | 51.03 | 32.43 | 0.533 | 38.69 | 10.86 | 0.372 | |
| 3.0 M | 37.72 | 9.91 | 0.344 | ||||
| Concentration | Methanol | Ethanol | |||||
|---|---|---|---|---|---|---|---|
| Rs (Ω) | Rct (Ω) | Cdl (mF) | Rs (Ω) | Rct (Ω) | Cdl (mF) | ||
| Ni12Co7(TiO2)1 | 0.5 M | 40.68 ± 0.21 | 31.5 ± 0.16 | 12 ± 0.036 | 34.30 ± 0.17 | 25.82 ± 0.13 | 12 ± 0.036 |
| 1 M | 36.72 ± 0.18 | 26.67 ± 0.13 | 10.95 ± 0.03 | 32.71 ± 0.16 | 20.61 ± 0.10 | 11.3 ± 0.033 | |
| 2 M | 33.10 ± 0.17 | 22.35 ± 0.11 | 9.20 ± 0.28 | 27.23 ± 0.14 | 17.03 ± 0.09 | 10.9 ± 0.033 | |
| Ni12Co5(TiO2)3 | 0.5 M | 25.09 ± 0.13 | 23.12 ± 0.11 | 10.78 ± 0.03 | 23.42 ± 0.12 | 21.67 ± 0.11 | 10 ± 0.030 |
| 1 M | 28.94 ± 0.14 | 27.37 ± 0.13 | 11 ± 0.033 | 32.34 ± 0.16 | 27.92 ± 0.14 | 10.5 ± 0.032 | |
| 2 M | 30.65 ± 0.15 | 28.41 ± 0.14 | 12 ± 0.036 | 37.86 ± 0.19 | 43.22 ± 0.22 | 12 ± 0.036 | |
| Catalyst | Preparation Method | Electrolyte | E Onset (mV) | Current (mA/cm2) | Refs. |
|---|---|---|---|---|---|
| Co1–Ni4/Graphene | Sol-Gel | 1 M Methanol + 1 M KOH | ~0.2 | 22.5 | [20] |
| Ni1Co3/Graphene | Electrochemical co-deposition | 1 M Methanol + 1 M KOH | ~0.2 | 15.82 | [21] |
| Ni-Co/RGO | Sol-Gel | 1 M Ethanol + 1 M NaOH | ~0.4 | 30 | [22] |
| NiCo2O4 nanocatalyst | nanofibers | 1 M Methanol + 0.1 M NaOH | ~0.3 | 21 | [65] |
| Ni NPs/TNTs/Ti | DC electrodeposition | 0.5 M NaOH + 0.5 M methanol | ~0.6 | 2.56 | [66] |
| Ni/TiO2/Ti | nanotubes | 0.5 M NaOH + 0.1 M methanol | ~0.35 | 4.5 | [67] |
| Ni-P-TiO2 composite | coating | 0.5 M Ethanol + 1 M KOH | ~0.42 | 23.3 | [68] |
| Pt/C | Nanoparticles | 1 M Methanol + 1 KOH | ~0.4 | 100 | [69] |
| Ni12Co5(TiO2)3/CNFs | Nanofiber | 0.5 M Methanol + 1 M KOH | ~0.4 | 60.39 | In this study |
| Nanofiber | 0.5 M Ethanol + 1 M KOH | ~0.37 | 74.21 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, W.M.; Mohamed, M.A.; Abdel-Hamed, M.O.; Abdel-Hady, E.E. Enhanced Electrocatalytic Performance of Nickel-Cobalt-Titanium Dioxide-Embedded Carbon Nanofibers for Direct Alcohol Fuel Cells. J. Compos. Sci. 2025, 9, 125. https://doi.org/10.3390/jcs9030125
Mohammed WM, Mohamed MA, Abdel-Hamed MO, Abdel-Hady EE. Enhanced Electrocatalytic Performance of Nickel-Cobalt-Titanium Dioxide-Embedded Carbon Nanofibers for Direct Alcohol Fuel Cells. Journal of Composites Science. 2025; 9(3):125. https://doi.org/10.3390/jcs9030125
Chicago/Turabian StyleMohammed, Wael M., Mahmoud A. Mohamed, Mohamed O. Abdel-Hamed, and Esam E. Abdel-Hady. 2025. "Enhanced Electrocatalytic Performance of Nickel-Cobalt-Titanium Dioxide-Embedded Carbon Nanofibers for Direct Alcohol Fuel Cells" Journal of Composites Science 9, no. 3: 125. https://doi.org/10.3390/jcs9030125
APA StyleMohammed, W. M., Mohamed, M. A., Abdel-Hamed, M. O., & Abdel-Hady, E. E. (2025). Enhanced Electrocatalytic Performance of Nickel-Cobalt-Titanium Dioxide-Embedded Carbon Nanofibers for Direct Alcohol Fuel Cells. Journal of Composites Science, 9(3), 125. https://doi.org/10.3390/jcs9030125






