Abstract
As an important neurotransmitter, the concentration of dopamine (DA) reflects certain physiological conditions and DA-related diseases. Rapid monitoring of DA levels is of great significance in regulating body health. However, regular electrochemical DA sensors suffer from poor sensitivity, low selectivity and interference immunity, as well as a complex preparation process. Herein, we developed an accessible and cost-effective electrochemical sensor with a copper indium selenide (CuInSe2 or CIS)-modified screen-printed carbon electrode for DA discrimination. This DA sensor was developed using a facile one-step hydrothermal method without high-temperature quenching. Benefitting from the inherent merits of CIS and the conversion of Cu2+ and Cu+ during the catalytic reaction, the sensor attained both excellent sensitivity (2.511 μA·µM−1·cm−1) and selectivity among multiple substances interfering with DA. This work demonstrates the potential to improve the analytical performance of traditional electrochemical sensors.
1. Introduction
Released from the adrenal gland of the brain, dopamine (DA, 3, 4-dihydroxyphenylethylamine) acts as an imperative catecholamine neurotransmitter to immensely dominate the behavior, cognition, emotion and memory of human beings [1,2]. Abnormalities in DA levels cause depression, addiction, cognitive impairment and even various neurological disorders, such as autonomic dysfunction, schizophrenia, Parkinson’s syndrome and Alzheimer’s disease [3,4]. In this regard, the sensitive and precise monitoring of DA levels is conducive to early diagnosis of and therapy for the aforementioned diseases. Until now, various methods have been established for the determination of DA, including high-performance liquid chromatography (HPLC), surface-enhanced Raman spectroscopy (SERS), ultraviolet–visible (UV–Vis) spectroscopy, fluorescence and electrochemical means [5,6,7].
Among them, electrochemical sensing technology has aroused wide attention due to its advantages of rapid response, easy operation, high sensitivity and low cost [8]. Meanwhile, DA also possesses splendid electroactive traits for high-precision electrochemical detection [9]. However, the coexistence of ascorbic acid (AA), uric acid (UA) and other chemicals in organisms challenges the accurate detection of DA due to their similar oxidation potential [10,11,12]. Furthermore, DA concentration in the biological system has a relatively narrow range, from 10 nM to 1 µM [13].
To address the aforementioned issues, the most widely adopted approach lies in a modified electrode with highly active electrocatalytic materials. For example, Chen et al. modified a glassy carbon electrode (GCE) with Co/Mn co-doped carbon nanofibers (CNFS), achieving remarkable sensitivity and strong anti-interference ability [14]. Shi et al. embedded MnS/Co3S4 hybrids on reduced graphene oxide to modify GCE electrodes, rendering a wide linear response for DA ranging from 6.0 nM to 20.0 μM [15]. However, these DA sensors usually suffer from complex craft, dependence on precise equipment and high costs [3,8].
As a bi-metallic selenide, CuInSe2 (CIS) has been explored in photovoltaic cells, photodetectors, light-emitting diode displays, etc., on account of its excellent photoelectric conversion and high absorption coefficient [16,17,18]. In fact, CIS also demonstrates tremendous promise in the electrochemical field due to the superiority of its extraordinary conductivity, chemical stability and reproducibility, large specific surface area and easily chemically modified surfaces during electrochemical measurement [19]. CIS enables specific identification and capture of dopamine at the microscopic level through certain functional groups or biomolecules, improving the selectivity and anti-interference ability of the sensor [20,21]. Furthermore, in comparison to GCE, the screen-printed carbon electrode (SPCE) exhibits lower background signal, ease of preparation, modification and miniaturization, low cost, stability, etc. [5].
Herein, an electrochemical sensor for DA detection with high selectivity and sensitivity was developed based on the CIS-modified SPCE electrode. The simple one-pot low-temperature hydrothermal method and easily available SPCE reduce the fabrication cost of sensors. In addition, the stability, reproducibility and anti-interference ability of the sensors were investigated, as well as the effects of different CIS loading and pH conditions on the sensing properties toward DA. The catalytic mechanism involving valence changes of Cu2+/Cu+ ions and the unique reaction between DA and CIS were proposed. As far as we know, it is the first time that CIS has been adopted for electrochemical DA detection. This work facilitates the early diagnosis and warning of DA-related diseases and opens up a new paradigm for the design and optimization of electrochemical biosensors for physiological monitoring.
2. Experimental Section
2.1. Reagents
The following reagents were purchased and used as received: copper acetate monohydrate (Cu(Ac)2·H2O, ≥98.0%, Adamas, Switzerland), indium nitrate tetrahydrate ((InNO3)3·4H2O, ≥99.99%, Maclin Biochemical Technology Co., LTD, Shanghai, China), sodium selenite pentahydrate (Na2SeO3·5H2O, ≥99.0%, Adamas, Switzerland) and hydrazine hydrate (NH2NH2·H2O, ≥99.0%, Maclin Biochemical Technology Co., LTD, Shanghai, China). Anhydrous ethanol and deionized water were used to clean the samples.
2.2. Synthesis of CuInSe2 Catalyst
Specifically, 1.5 mM Cu(Ac)2·H2O, 1.5 mM I InNO3)3·4H2O, 1.5 mM NH2NH2·H2O and 3 mM Na2SeO3·5H2O were mixed in a beaker containing 25 mL of H2O with continuous magnetic stirring for 4 h. Then, the dispersion solution was transferred to a Teflon hydrothermal reactor and heated at 180 °C for 18 h. Once allowed to cool to room temperature, the product was centrifuged and washed several times with a 1:1 mixture of ethanol and deionized water and dried in an oven at 60 °C to obtain the CIS catalyst.
2.3. Fabrication of the CIS-Modified SPCE Sensor
Firstly, 2 mg CIS and 10 µL of 0.5% naphthol were added in 500 mL of a 1:1 mixture of deionized water and ethanol and then dispersed by ultrasonic oscillation. Subsequently, 9 µL of the suspensions was dropped slowly onto the work electrode of the screen-printed carbon electrode (SPCE, TC201, POTE, Qingdao Biocarbon Technology Co., LTD, Qingdao, China; counter electrode (CE): carbon; working electrode (WE): carbon; reference electrode (RE): Ag/AgCl; substrate: PET; electrode size: 12 × 34 mm; electrode thickness: 0.3 mm; WE diameter: 4 mm). Finally, the CIS-SPCE sensor was dried at room temperature.
2.4. Apparatus and Characterizations
A CHI76 electrochemical workstation (Chenhua Instruments Co., Ltd., Shanghai, China) was employed to determine the electrochemical performance. All electrochemical experiments were performed at room temperature. For cyclic voltammetry (CV), taking the 50-turn cycle as an example, the test range is from −0.2 V to 0.6 V, the scanning rate is 50 mV/s, the sample interval is 0.001 V, the quiet time is 2 s and the segment (number of turns) is 50 turns. In the 20 mV/s–200 mV/s potassium ferricyanide test, the test range was −0.4 V to 0.6 V, the scan rate was changed from 20–200 mV/s, the number of laps was 10 laps and the rest of the conditions were the same as above. For differential pulse voltammetry (DPV), the test range is set from −0.2 V to 0.6 V, mainly including the characteristic peak of dopamine (0.2 V or so), the pulse period is 0.1 s, the quiet period is 2 s, the amplitude is 50 mV and the pulse width is 0.05 s. For chronoamperometry (i-t), bias voltage E= 0.3 V, the running time of the sample is 350 s and the sample interval is 0.2 s. For electrochemical impedance spectroscopy (EIS), the amplitude is 0.15 V, the high frequency is 1 × 105 Hz, the low frequency is 0.1 Hz and the period is 1.
The microstructures were investigated by scanning electron microscope (SEM, JSM-7500, JEOL Ltd., Tokyo, Japan) and high-resolution transmission electron microscope (HRTEM, Tecnai G2 F30, Thermo Fisher, Hillsboro, OR, USA). The crystalline structure was explored by XRD (D/Max-2550PC, Rigaku Corporation, Tokyo, Japan). An X-ray photoelectron spectrometer (XPS, ESCALAB 210, VG Scientific, Eastbourne, UK) was used to probe the surface chemical state. The functional groups were analyzed via a Fourier-transform infrared spectrometer (FT-IR, Thermo Nicolet iS5, Thermo Fisher, Madison, WI, USA). The elemental and chemical composition was observed by energy dispersive spectroscopy (EDS, Hitachi HF5000, Hitachi, Tokyo, Japan) elemental mapping. The molecular vibration information was recorded by Raman microscopy (Model-RE 04, Thermo Fisher, Madison, WI, USA).
3. Results and Discussion
As illustrated in Figure 1a, CIS nanoparticles (NPs) were prepared through a one-step hydrothermal synthesis process, in which some CIS nanosheets (NSs) were generated (Figure 1b). This nanoscale structure increases the active area beneficial for trapping DA molecules and the reduction of the interfacial resistance in electrochemical reactions, thus improving the efficiency of electron transfer [22,23]. The diffraction profile of CIS in the XRD pattern (Figure 1c) is in accordance with the tetragonal chalcopyrite of (PDF#65-7027), where the peaks at 26.66°, 44.32°, 52.26°, 64.38° and 71.04° are consigned to facets (112), (220), (116) (400) and (332), respectively [24,25]. The selected-area electron diffraction pattern (Figure 1d) reveals the polycrystal structure of CIS with planes (112), (220) and (116), which agrees with the XRD results. Figure 2a depicts the HRTEM image of a selected fragment, showing a d-spacing of 0.335 nm, which belongs to the (112) facet of CIS. The EDS elemental mapping displays the uniform distribution of Cu, In, O and Se (Figure 2b,c), where the occurrence of elemental O arises from the adsorption of oxygen atoms on CIS and the subsequent establishment of Se–O bonds during the hydrothermal process.
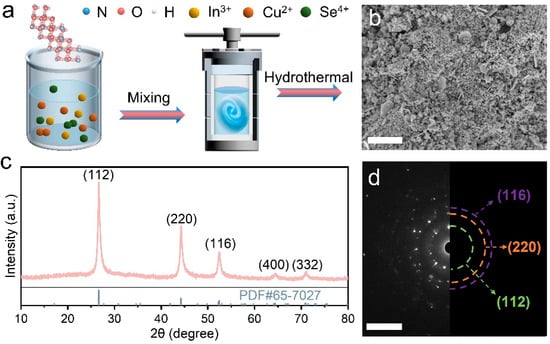
Figure 1.
Preparation of CIS. (a) Schematic of the preparation process; (b) SEM, scale bar: 2 μm; (c) XRD pattern; (d) diffraction pattern from the selected area, scale bar: 10 nm.
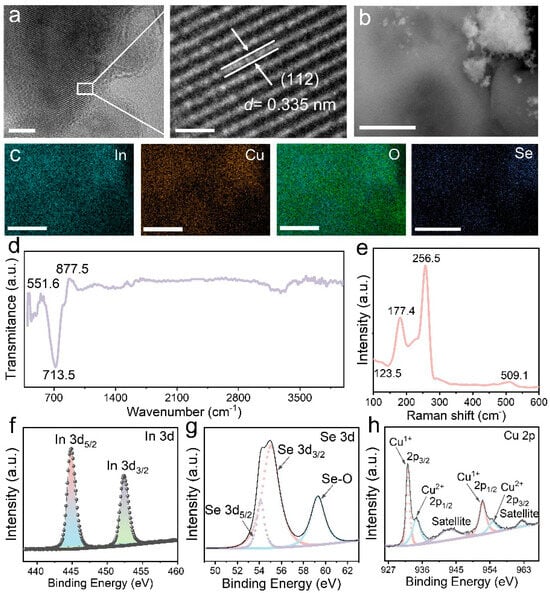
Figure 2.
Characterization of CIS. (a) TEM, scale bar: 5 nm and HR-TEM, scale bar: 1 nm; (b,c) EDS elemental maps, scale bar: 2 μm; (d) FT-IR; (e) Raman patterns of CIS. High-resolution XPS spectra of (f) In 3d, (g) Se 3d and (h) Cu 2P for CIS.
In the Fourier-transform infrared (FT-IR) spectrum (Figure 2d), the peaks near 551.6 cm−1 and 877.5 cm−1 are respectively ascribed to the stretching or bending vibration of the In–Se or Cu–Se bonds [26]. The stretching vibration at 713.5 cm−1 belongs to the Cu–Se or In–Se bonds, implying the successful preparation of CIS [27]. Figure 2e shows the Raman spectrum of the CIS, whose pleomorphic phase is clearly evidenced by three characteristic peaks at 123.5 cm−1, 177.4 cm−1 and 256.5 cm−1. The peak at 177.4 cm−1 is attributed to the B2 vibration mode, while the most intense peak at 56.5 cm−1 corresponds to the A1 mode of the CIS [16,28,29]. Another weak peak located at 509.1 cm−1 probably arises from the vibration of the Se–O bond. The XPS survey spectrum (Figure S1) reveals the existence of four major elements, Cu, In, Se and O. The high-resolution In 3d spectrum manifests two peaks at 444.8 eV (3d5/2) and 452.4 eV (3d3/2), which are in accordance with the In3+ state (Figure 2f) [16]. As depicted in Figure 2g, the divided Se 3d of CIS exhibits three peaks at 54.1 eV for Se 3d5/2 and 55.0 eV for Se 3d3/2, while an individual peak at 59.2 eV can be regarded as SeOx due to the surface oxidation [17,30]. The Cu 2p spectrum (Figure 2h) of CIS can be decomposed into peaks at 932.1/934.4 eV and 952.2/954.6 eV, assigned to the 2p3/2 and 2p1/2 of Cu+ and Cu2+ 2p3/2 and 2p1/2, respectively, which confirmed the coexistence of mixed states for Cu [20,31]. The two satellite peaks situated at 942.6 eV and 962.6 eV possibly result from the overlapping of Cu and Se [32].
To evaluate the stability of SPCE when subjected to external mechanical disturbances, the amperometric responses of the DA sensor under different bending deformations were recorded. As shown in Figure 3a–f, the amperometric responses remain essentially unchanged irrespective of bending direction and angles of the CIS-modified electrode, suggesting good mechanical stability. Then, CV tests were performed on bare SPCE and a CIS-modified electrode to verify the effect of CIS modification on the electrocatalytic performance of SPCE, and thus its feasibility as an electrochemical sensor with respect to DA detection. Obviously, bare SPCE demonstrates a negligible response to DA, with no redox peak appearing, whereas CIS-SPCE exhibits two pronounced characteristic peaks with significantly intense peak currents. The peak current (Ipa) of DA with CIS-SPCE is about 14.4 times higher than that with bare SPCE, and the oxidation and reduction potentials are observed at 0.23 V and 0.021 V (Figure 3g), respectively.
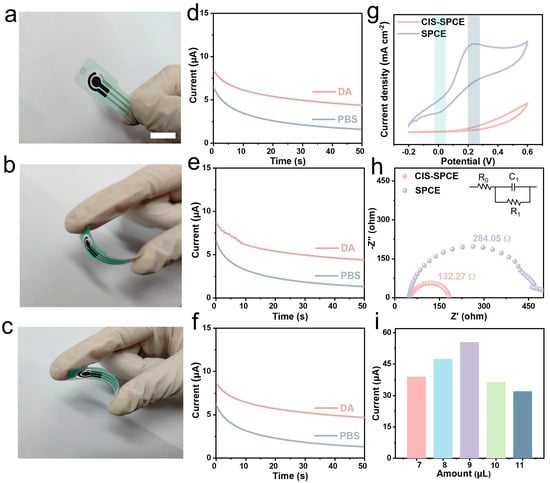
Figure 3.
Comparison of electrochemical properties of bare SPCE and CIS-SPCE. (a) Before bending, (b) outward bending, (c) inward bending and (d–f) the corresponding amperometric responses of CIS-SPCE in 0.1 M PBS with and without 0.3 mM DA, respectively. (g) CV and (h) EIS curves of bare SPCE and CIS-modified SPCE in 0.1 M KCl solution containing 1.0 mM K3Fe(CN)6. (i) DPV responses of CIS-SPCE to different electrocatalytic dosages in 0.5 mM DA.
Electrochemical impedance spectroscopy (EIS) was tested to informatively evaluate the possible electron transfer dynamics in 0.1 M KCl solution containing 1.0 mM K3Fe(CN)6. As displayed in Figure 3h, R0 corresponds to the series resistance of the electrolyte, while R1 and C1 represent the interface transfer resistance and the capacitor, respectively. CIS-SPCE (184.27 Ω) shows a much smaller semicircle arc than bare SPCE (284.05 Ω), signifying a weaker interfacial charge movement limitation, and thus improved electrochemical activity [6,9,33]. This phenomenon can be attributed to the large surface area and good conductivity of the CIS nanocatalysts and is in good agreement with the results of CV (Figure 3g) and SEM (Figure 1b) analysis. DPV was employed to seek the optimal dosage of CIS for SPCE modification by altering the amount of CIS from 7 μL to 11 μL and recording the peak currents. Interestingly, the optimal dosage of 9 μL gives rise to a maximum current (Figure 3i). This is because that sparse catalyst cannot catalyze the DA molecule, while excessive catalyst forms a thick catalytic layer that hinders the reaction [34]. The amount of catalyst was kept constant in following experiments.
Next, the sensing performances of CIS-SPCE in DA detection were evaluated, such as sensitivity, reproducibility, stability and selectivity. Figure 4a,b display the quantitative electrochemical response of CIS-SPCE on exposure to various DA concentrations by DPV and amperometric methods. The two strategies demonstrate the high sensitivity (up to 2.511 μA·µM−1·cm−1 by DPV method) and good linear relationship in the ranges of 100–1000 µM and 0.04–1000 µM (Figure S2, with the regression coefficient of R2 = 0.9935 and 0.9969, respectively, and the detection limit low to 96 nM (S/N = 3, amperometric method). These results demonstrate that the CIS-modified SPCE electrode is a high-performance DA sensor. The analytical performance of our electrode is compared with other electrodes for DA determination in Table 1 [5,7,12,35,36,37,38,39,40,41,42,43,44,45].
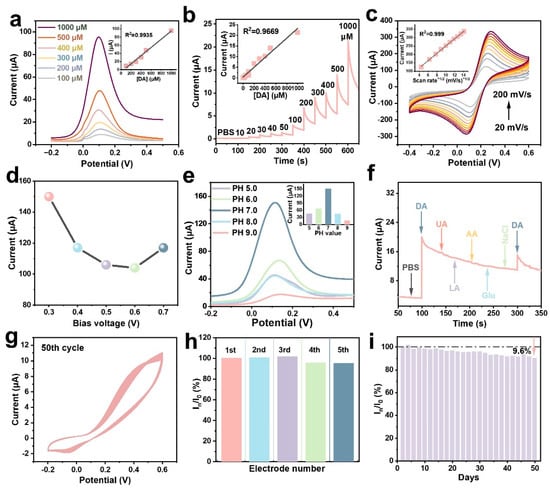
Figure 4.
Electrochemical performance analysis of CIS-SPCE for DA detection. (a) DPV and (b) amperometric responses of CIS-SPCE to different concentrations of DA (0.1–1 mM for DA test and 0–1 mM, respectively). Inset are the corresponding calibration plots of current response versus DA concentration. (c) CV curves of the CIS-SPCE at various scan rates (20–200 mV/s) in PBS with 0.5 mM DA. Inset is the linear relationship curve of the anodic peak current versus the scan rates. Effects of (d) bias, and (e) PH values of CIS-SPCE on detection of DA (PBS with 1 mM DA) (inset: peak current versus PH value). (f) Selectivity test of CIS-SPCE to 1 mM DA, 0.1 mM UA, 10 mM AA, LA, Glu and NaCl under a constant voltage of 0.3 V. (g) CV curves of CIS-SPCE in PBS with 0.5 mM DA for 50 cycles. (h) Reproducibility of five independently fabricated CIS-SPCE sensors toward 0.5 mM DA under the same conditions. (i) Repeatability during repeated daily recordings over 50 days.

Table 1.
Comparison of the prepared DA CIS-SPCE electrochemical sensor’s performance to the recent literature.
The electrode reaction kinetics of DA for CIS-SPCE were recorded through CV at various scan rates from 20 to 200 mV/s. As depicted in Figure 4c, two clear and symmetrical redox peaks can be observed, indicating the satisfactory electrochemical redox activity of CIS-SPCE on DA. The peak current increases with increasing scan rate, and is linearly related to the square root of the scan rate, implying a diffusion-controlled process [46]. Subsequently, the effects of applied potential (Figure 4d) and PH value (Figure 4e) on the sensing activity of CIS-SPCE were explored. It can be observed that the current response first decreased and then increased gradually as the bias varied from 0.3 to 0.7 V, reaching the maximum current at 0.3 V and the minimum at 0.6 V, respectively. To this end, the test current was selected at 0.3 V.
With regard to the influence of PH value on the catalytic activity of CIS-SPCE, under an acidic environment, more electrons will participate in oxidating DA with the increased pH value, resulting in a larger current [7,34]. Once under alkaline conditions, the deprotonated DA molecule will be repelled electrostatically by the surface sites, which diminishes the current. As a consequence, the utmost response is obtained with pH = 7 [34].
To explore the selectivity of CIS-SPCE, a series of common interfering substances were inspected, as exhibited in Figure 4f. The negligible current response changes for other interferences (such as 0.1 mM UA, 10 mM AA, lactic Acid (LA), glucose (Glu) and NaCl) compared to DA (1 mM) imply that the CIS-SPCE possesses outstanding selectivity for DA. The stability of CIS on the SPCE was characterized by continuous CV measurements in PBS with 0.5 mM DA. Apparently, the CV signals of the CIS-modified SPCE remained almost identical over 50 consecutive cycles (Figure 4g), confirming its excellent electrochemical stability. Figure 4h exhibits the excellent reproducibility of the fabricated CIS-SPCE by the negligible relative standard deviation (RSD) after five measurements using different electrodes. Furthermore, the long-term stability of the CIS-SPCE was explored using the same electrode across 50 days of storage. Compared to the initial response, the last test had only decreased by 9.6% (Figure 4i), suggesting superb long-term stability.
To vividly understand the process of specific recognition response of CIS to DA, the possible electrocatalytic mechanism of DA with CIS-SPCE is proposed. As presented in Figure 4, the macroscopic oxidation process could be explained as follows: DA is converted into DOQ (dopamine-O-quinone), consuming of two electrons while releasing two protonic hydrogens at the surface of electrode, which is accompanied by the conversion of O2* to water molecules and the reduction of the catalyst to its initial form (Equation (1)).
DA + O2* → DOQ + H2O
Specifically, the Cu+ ions are first released from CIS and oxidized to Cu2+, facilitating the reduction of In3+ to In2+ and of O2 to O2* (Equation (2)):
Cu+ + O2 → O2* + Cu2+
Since DA exists as a cation in biological fluids (pKa ≈ 8.9) [7,13] and cannot be directly adsorbed on the surface of CIS, it attaches to the catalyst through the intermediate medium of OH* formed by O2*, which is strongly hydrogen-bonded to DA, abiding by Equations (3)–(6) below:
O2* + H2O → HO2* + OH*
H2O2 → 2OH*
Cu2+ + OH* → Cu2+–OH*
Cu2+–OH* + DA → Cu2+–OH–DA*
Subsequently, the adsorbed DA undergoes the transfer process of H+/e−, as oxidation eventually returns to the initial state of the catalyst and produces water molecules (Equations (7) and (8)) [47], whereby a half reaction is accomplished.
Cu2+–OH–DA* → Cu+–H2O* + DA–H*
Cu+–H2O* → Cu+ + H2O
The overall reaction is an electron-consuming process, in which pH < 7, where more electrons will be involved in the redox reaction as the pH value increases, resulting in a larger response. However, in alkaline electrolyte, DA*–H will be electrostatically repelled by the surface sites, which is detrimental to the thorough reaction.
The superior catalytic capabilities of CIS-SPCE toward DA determination originate from the following aspects: (i) the admirable natural properties of the CIS catalyst, including high conductivity (from the EIS results), large specific surface area (from SEM) and good stability (Figure 4g,i); and (ii) the valence transitions between Cu2+ and Cu1+ ions involving the electron transfer (from XPS results) and the yield of abundant O2* (Figure 5). Owing to the abovementioned advantages, numerous DA cations can be effectively identified and adsorbed on the surface of CIS-SPCE, resulting in an anodic peak and allowing the use of the peak current to evaluate the DA levels.
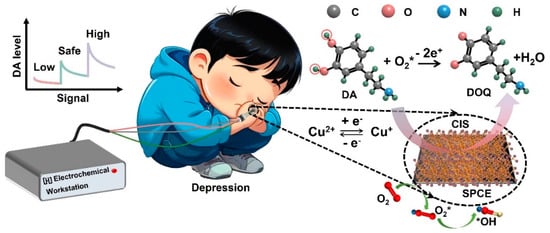
Figure 5.
Schematic illustration of possible electrochemical catalytic mechanism of DA with CIS−SPCE (“*” represent the free radical).
4. Conclusions
In summary, a highly sensitive and selective electrochemical sensor based on CIS-modified SPCE was successfully developed for determination of DA. Therein, the economically sensitive material was synthesized using a one-step hydrothermal method, while the SPCE electrode also achieved ease of fabrication and integration. Under the optimal conditions, this facile electrode exhibits a remarkable response for DA detection, with a low detection of 96 nM and a wide linear concentration range of 0.04–1000 µM. Furthermore, the CIS-SPCE electrode was observed to feature satisfactory anti-interference ability (in the existence of a possible variety of biological interferences, including AA, LA UA, glucose and NaCl), good reproducibility and high stability. This demonstrates the promise of CIS-SPCE as a high-performance electrochemical system for accurate monitoring of DA level in physiological fluids. This work not only sheds light on the fundamental underlying mechanism of interfacial electron transferring behaviors, but also paves the way for mobile clinical diagnosis and treatment of neurological diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9030123/s1, Figure S1: The XPS spectrum; Figure S2: Electrochemical analysis: Amperometric analysis; Figures S3–S6: CV measurements.
Author Contributions
Conceptualization, Y.S.; methodology, G.X., M.Y. and Y.S.; investigation, J.L. and L.D.; resources, Y.S.; data curation, J.L.; writing—original draft, J.L.; writing—review and editing, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 62074027), Sichuan Province, Hong Kong, Macao and Taiwan Science and Technology Innovation Co-operation Project (Grant No. 2024YFHZ0367) and National Undergraduate Training Program for Innovation and Entrepreneurship (Grant No.S202414389081).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, K.; Claar, L.D.; Hachisuka, A.; Bakhurin, K.I.; Nguyen, J.; Trott, J.M.; Gill, J.L.; Masmanidis, S.C. Temporally restricted dopaminergic control of reward-conditioned movements. Nat. Neurosci. 2020, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Le, Q.; Lv, Y.; Chen, X.; Cui, J.; Zhou, Y.; Cheng, D.; Ma, C.; Su, X.; Xiao, L.; et al. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res. 2021, 32, 139–156. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.-X. Graphene Quantum Dots/Multiwalled Carbon Nanotubes Composite-Based Electrochemical Sensor for Detecting Dopamine Release from Living Cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Chowdhury, S.; Nugraha, A.S.; O’May, R.; Wang, X.; Cheng, P.; Xin, R.; Osman, S.M.; Hossain, M.S.; Yamauchi, Y.; Masud, M.K.; et al. Bimetallic metal-organic framework-derived porous one-dimensional carbon materials for electrochemical sensing of dopamine. Chem. Eng. J. 2024, 492, 152124. [Google Scholar] [CrossRef]
- Niu, B.; Liu, M.; Li, X.; Guo, H.; Chen, Z. Vein-Like Ni-BTC@Ni3S4 with Sulfur Vacancy and Ni3+ Fabricated In Situ Etching Vulcanization Strategy for an Electrochemical Sensor of Dopamine. ACS Appl. Mater. Interfaces 2023, 15, 13319–13331. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Liu, T.; Wang, G.; Sun, M.; Jiang, Y.; He, H.; Wang, Y.; Zou, P.; Wang, X.; et al. A dual-template imprinted polymer electrochemical sensor based on AuNPs and nitrogen-doped graphene oxide quantum dots coated on NiS2/biomass carbon for simultaneous determination of dopamine and chlorpromazine. Chem. Eng. J. 2020, 389, 124417. [Google Scholar] [CrossRef]
- Zou, J.; Guan, J.-F.; Zhao, G.-Q.; Jiang, X.-Y.; Liu, Y.-P.; Yu, J.-G.; Li, W.-J. Construction of a highly sensitive signal electrochemical sensor based on self-assembled cobalt oxide-hydroxylated single-walled carbon nanotubes composite for detection of dopamine in bovine serum samples. J. Environ. Chem. Eng. 2021, 9, 105831. [Google Scholar] [CrossRef]
- Shukla, R.P.; Aroosh, M.; Matzafi, A.; Ben-Yoav, H. Partially Functional Electrode Modifications for Rapid Detection of Dopamine in Urine. Adv. Funct. Mater. 2021, 31, 2004146. [Google Scholar] [CrossRef]
- Dhiman, P.; Kumar, A.; Shekh, M.; Sharma, G.; Rana, G.; Vo, D.-V.N.; AlMasoud, N.; Naushad, M.; Alothman, Z.A. Robust magnetic ZnO-Fe2O3 Z-scheme hetereojunctions with in-built metal-redox for high performance photo-degradation of sulfamethoxazole and electrochemical dopamine detection. Environ. Res. 2021, 197, 111074. [Google Scholar] [CrossRef]
- Li, Z.; Liang, L.; Lin, W.; Huang, Y.; Huang, T.; Wang, W.; Ma, J.; Li, J.; Sun, L.-P.; Guan, B.-O. Optofluidic laser sensor for the detection of dopamine. Sens. Actuators B Chem. 2023, 390, 133941. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, R.; Li, X.; Dong, N.; Zhu, B.; Wang, J.; Lin, X.; Su, B. COF-Coated Microelectrode for Space-Confined Electrochemical Sensing of Dopamine in Parkinson’s Disease Model Mouse Brain. J. Am. Chem. Soc. 2023, 145, 23727–23738. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Kang, P.; Wang, S.-Q.; Liu, Z.-G.; Li, Y.-X.; Guo, Z. Ag nanoparticles anchored onto porous CuO nanobelts for the ultrasensitive electrochemical detection of dopamine in human serum. Sens. Actuators B Chem. 2021, 327, 128878. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Xing, Y.; Lv, C.; Fu, Y.; Luo, L.; Liu, J.; Xie, X.; Chen, F. Sensitive sensing platform based on Co, Mo doped electrospun nanofibers for simultaneous electrochemical detection of dopamine and uric acid. Talanta 2024, 271, 125674. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhou, C.; Wei, Y.; Chen, A.; Tang, N.; He, Q.; Deng, P. Ultrasensitive and selective electrochemical sensor for nanomolar dopamine determination based on MnS/Co3S4 hybrids embedded on electrochemically reduced graphene oxide. Microchem. J. 2023, 194, 109310. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Senapati, S.; Bisoyi, S.; Samal, S.; Naik, R. Zn doping induced optimization of optical and dielectric characteristics of CuInSe2 nanosheets for optoelectronic device applications. J. Alloys Compd. 2023, 945, 169222. [Google Scholar] [CrossRef]
- Nithiananth, S.; Silambarasan, K.; Logu, T.; Harish, S.; Ramesh, R.; Muthamizhchelvan, C.; Shimomura, M.; Archana, J.; Navaneethan, M. Transition divalent metal substitution in chalcopyrite CuInSe2 (In = Co, Ni, and Mn) counter electrode for dye-sensitized solar cell applications. Mater. Lett. 2022, 308, 130887. [Google Scholar] [CrossRef]
- Reddy, Y.B.K.; Raja, V.S. Optical, structural and electrical properties of co-evaporated CuIn2Se3.5 thin films. Mater. Lett. 2004, 58, 1839–1843. [Google Scholar] [CrossRef]
- Fuhr, A.; Yun, H.J.; Crooker, S.A.; Klimov, V.I. Spectroscopic and Magneto-Optical Signatures of Cu1+ and Cu2+ Defects in Copper Indium Sulfide Quantum Dots. ACS Nano 2020, 14, 2212–2223. [Google Scholar] [CrossRef]
- Singh, H.; Bernabe, J.; Chern, J.; Nath, M. Copper selenide as multifunctional non-enzymatic glucose and dopamine sensor. J. Mater. Res. 2021, 36, 1413–1424. [Google Scholar] [CrossRef]
- Dashtian, K.; Hajati, S.; Ghaedi, M. Ti-Based Solid-State Imprinted-Cu2O/CuInSe2 Heterojunction Photoelectrochemical platform for Highly Selective Dopamine Monitoring. Sens. Actuators B Chem. 2021, 326, 128824. [Google Scholar] [CrossRef]
- Arya Nair, J.S.; Saisree, S.; Aswathi, R.; Sandhya, K.Y. Ultra-selective and real-time detection of dopamine using molybdenum disulphide decorated graphene-based electrochemical biosensor. Sens. Actuators B Chem. 2022, 354, 131254. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Hou, P.; Hou, X.; Wang, J.; Qi, Q.; Zhu, Y.; Yang, X.; Liu, X.; Li, X.; et al. Synthesis of CdSe Nanowires and CuInSe2 Nanosheets for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 1935–1943. [Google Scholar] [CrossRef]
- Liu, F.; Zong, J.; Liang, Y.; Zhang, M.; Song, K.; Mi, L.; Feng, J.; Xiong, S.; Xi, B. Ordered Vacancies as Sodium Ion Micropumps in Cu-Deficient Copper Indium Diselenide to Enhance Sodium Storage. Adv. Mater. 2024, 36, 2403131. [Google Scholar] [CrossRef]
- Ren, S.; Feng, R.; Guo, T.; Cao, L.; Lv, R.; Liu, X.; Wang, Q.; Zheng, Z. Electroanalytical sensor based on CuInSe2/carbon sphere towards the non-invasive determination of dopamine. Electroanalysis 2023, 35, e202300203. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, S.; Jiang, X.; Ai, Y.; Xu, W.; Xie, L.; Sun, H.B.; Liang, Q. Oligo-layer graphene stabilized fully exposed Fe-sites for ultra-sensitivity electrochemical detection of dopamine. Biosens. Bioelectron. 2022, 211, 114367. [Google Scholar] [CrossRef]
- Sheng, P.; Li, W.; Tong, X.; Wang, X.; Cai, Q. Development of a high performance hollow CuInSe2 nanospheres-based photoelectrochemical cell for hydrogen evolution. J. Mater. Chem. A 2014, 2, 18974–18987. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Xie, L.; Xia, Y.; Zhan, S.; Hu, W.; Li, Y. Electronic Metal-Support Interactions Boost *OOH Intermediate Generation in Cu/In2Se3 for Electrochemical H2O2 Production. Angew. Chem. Int. Ed. 2024, 63, e202319470. [Google Scholar] [CrossRef]
- Dutková, E.; Bujňáková, Z.L.; Sphotyuk, O.; Jakubíková, J.; Cholujová, D.; Šišková, V.; Daneu, N.; Baláž, M.; Kováč, J.; Kováč, J.; et al. SDS-Stabilized CuInSe2/ZnS Multinanocomposites Prepared by Mechanochemical Synthesis for Advanced Biomedical Application. Nanomaterials 2020, 11, 69. [Google Scholar] [CrossRef]
- Umapathi, S.; Masud, J.; Coleman, H.; Nath, M. Electrochemical sensor based on CuSe for determination of dopamine. Microchim. Acta 2020, 187, 440. [Google Scholar] [CrossRef]
- Umapathi, S.; Singh, H.; Masud, J.; Nath, M. Nanostructured copper selenide as an ultrasensitive and selective non-enzymatic glucose sensor. Mater. Adv. 2021, 2, 927–932. [Google Scholar] [CrossRef]
- Saxena, A.; Liyanage, W.; Masud, J.; Kapila, S.; Nath, M. Selective electroreduction of CO2 to carbon-rich products with a simple binary copper selenide electrocatalyst. J. Mater. Chem. A 2021, 9, 7150–7161. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.; Su, Z.; Li, Y.; Wang, X.; Tian, M.; Guo, C.; Dong, T.; Chai, F. Fabrication of Cu-BTC@PW12/GO for multivariate sensing dopamine and acetaminophen as electrochemical sensor. J. Environ. Chem. Eng. 2024, 12, 114078. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, H.; Lu, K.; Li, Y.; Shi, W.; Ma, X. Electronic/ionic engineering inspired formation of carbon-encapsulated Cu3PSe4/Cu2Se heterostructured hollow nanosphere for trace level neurochemical monitoring. Appl. Surf. Sci. 2023, 613, 156142. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, X.; Ding, Y.; Luo, L.; Xu, D.; Ning, Y. Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta 2016, 146, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kim, K.-J.; Yoon, J.; Jo, J.; El-Said, W.A.; Choi, J.-W. Silver Nanoparticle Modified Electrode Covered by Graphene Oxide for the Enhanced Electrochemical Detection of Dopamine. Sensors 2017, 17, 2771. [Google Scholar] [CrossRef]
- Oh, J.-W.; Heo, J.; Kim, T.H. An electrochemically modulated single-walled carbon nanotube network for the development of a transparent flexible sensor for dopamine. Sens. Actuators B Chem. 2018, 267, 438–447. [Google Scholar] [CrossRef]
- Tan, W.; Zhu, Z.; Yang, J.; Li, H.; Li, S.; Wu, D.; Qin, Y.; Kong, Y. Synthesis of ZnO and CuO co-decorated porous carbon spheres with simultaneous accessibility to small biomelucules. Synth. Met. 2019, 258, 116193. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Jia, Z.; Huo, D.; Liu, Q.; Zhong, D.; Hu, Y.; Yang, M.; Bian, M.; Hou, C. In-situ growth of gold nanoparticles on a 3D-network consisting of a MoS2/rGO nanocomposite for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim. Acta 2019, 186, 92. [Google Scholar] [CrossRef]
- Manjula, N.; Vinothkumar, V.; Chen, S.-M.; Sangili, A. Simultaneous and sensitive detection of dopamine and uric acid based on cobalt oxide-decorated graphene oxide composite. J. Mater. Sci. Mater. Electron. 2020, 31, 12595–12607. [Google Scholar] [CrossRef]
- Mohamed, N.B.; El-Kady, M.F.; Kaner, R.B. Macroporous Graphene Frameworks for Sensing and Supercapacitor Applications. Adv. Funct. Mater. 2022, 32, 2203101. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Harraz, F.A. A novel gold-decorated porous silicon-poly(3-hexylthiophene) ternary nanocomposite as a highly sensitive and selective non-enzymatic dopamine electrochemical sensor. J. Alloys Compd. 2023, 931, 167403. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
- Kaya, H.K.; Cinar, S.; Altundal, G.; Bayramlı, Y.; Unaleroglu, C.; Kuralay, F. A novel design thia-bilane structure-based molecular imprinted electrochemical sensor for sensitive and selective dopamine determination. Sens. Actuators B Chem. 2021, 346, 130425. [Google Scholar] [CrossRef]
- Wu, R.; Yu, S.; Chen, S.; Dang, Y.; Wen, S.-H.; Tang, J.; Zhou, Y.; Zhu, J.-J. A carbon dots-enhanced laccase-based electrochemical sensor for highly sensitive detection of dopamine in human serum. Anal. Chim. Acta 2022, 1229, 340365. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT:PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Al Kiey, S.A.; Khalil, A.M.; Kamel, S. Insight into TEMPO-oxidized cellulose-based composites as electrochemical sensors for dopamine assessment. Int. J. Biol. Macromol. 2023, 239, 124302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).