Effects of Alkaline and Carboxilated Graphene Oxide (CGO) Treatment on Mechanical, Thermal, and Electrical Properties of Jute Fiber-Reinforced Epoxy Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Fiber Surface Treatment and Composite Fabrication
2.3. Characterization Techniques
2.3.1. Differential Scanning Calorimetry (DSC)
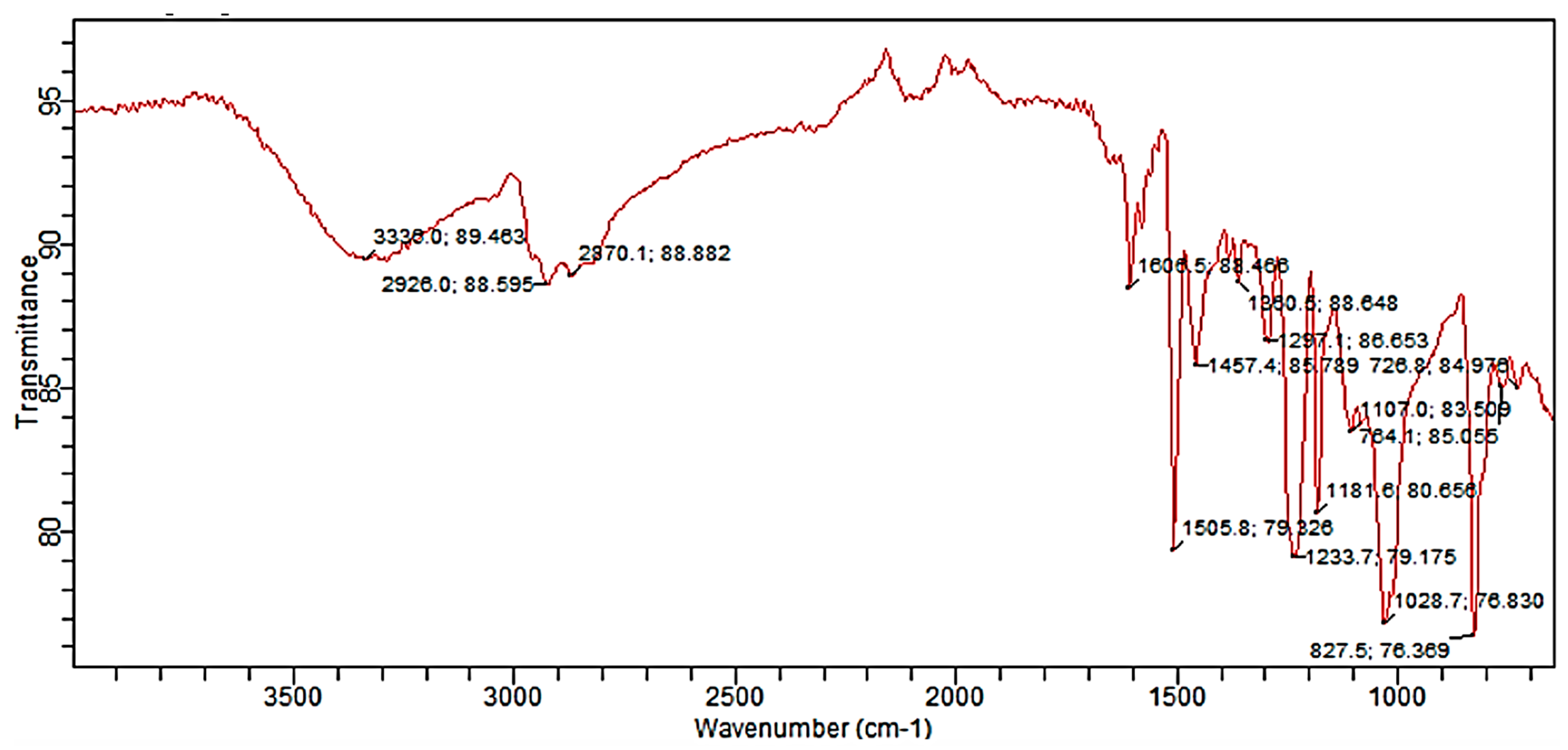
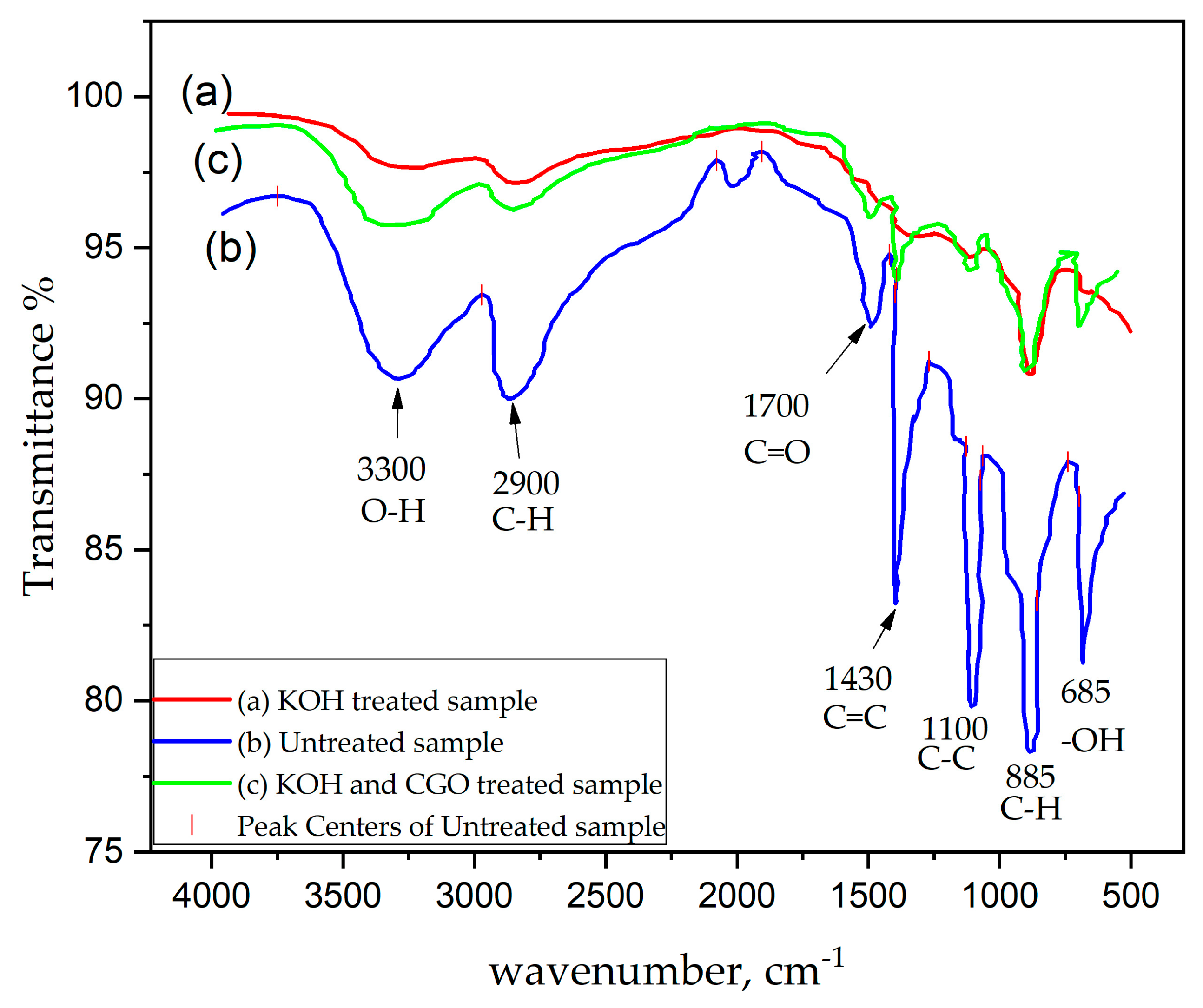
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Scanning Electron Microscopy (SEM)
2.4. Mechanical and Electrical Testing Procedure
2.4.1. Ultimate Tensile Testing
2.4.2. Flexural Test
2.4.3. Electrical Conductivity
3. Results and Discussion
3.1. Chemical Analysis
3.2. Thermal Analysis
3.3. Morphological Analysis
3.4. Tensile Strength of the Composites
3.5. Flexural Strength of the Composites
3.6. Electrical Conductivity of the Composites
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Saba, N.; Jawaid, M. Epoxy Resin Based Hybrid Polymer Composites; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Shahinur, S.; Sayeed, M.M.A.; Hasan, M.; Sayem, A.S.M.; Haider, J.; Ura, S. Current Development and Future Perspective on Natural Jute Fibers and Their Biocomposites. Polymers 2022, 14, 1445. [Google Scholar] [CrossRef]
- Luz, F.S.; Garcia Filho, F.C.; Del-Rio, M.T.C.; Nascimento, L.F.C.; Pinehro, W.A.; Monteiro, S.N. Graphene-incorporated natural fiber polymer composites: A first overview. Polymers 2020, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.H.; Islam, R.; Dulal, M.; Afroj, S.; Karim, N. The effect of surface treatments and graphene-based modifications on mechanical properties of natural jute fiber composites: A review. iScience 2022, 25, 103597. [Google Scholar] [CrossRef] [PubMed]
- Ashadujjaman; Saifullah, A.; Shah, D.U.; Zhang, M.; Akonda, M.; Karim, N.; Sarker, F. Enhancing the mechanical properties of natural jute yarn suitable for structural applications. Mater. Res. Express 2021, 8, 055503. [Google Scholar] [CrossRef]
- Anna Dilfi, K.F.; Che, Z.; Xian, G. Grafting ramie fiber with carbon nanotube and its effect on the mechanical and interfacial properties of ramie/epoxy composites. J. Nat. Fibers 2019, 16, 388–403. [Google Scholar] [CrossRef]
- Dang, C.-Y.; Shen, X.-J.; Nie, H.-J.; Yang, S.; Shen, J.-X.; Yang, X.-H.; Fu, S.-Y. Enhanced interlaminar shear strength of ramie fiber/polypropylene composites by optimal combination of graphene oxide size and content. Compos. Part B Eng. 2019, 168, 488–495. [Google Scholar] [CrossRef]
- Roy, A.; Chakraborty, S.; Kundu, S.P.; Basak, R.K.; Majumder, S.B.; Adhikari, B. Improvement in mechanical properties of jute fibres through mild alkali treatment as demonstrated by utilisation of the Weibull distribution model. Bioresour. Technol. 2012, 107, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Nayab-Ul-Hossain, A.; Sela, S.K.; Hassan, M.N.; Sarkar, A. Surface modification of ligno-cellulosic fiber(jute) to increase electrical conductivity. Mater. Lett. X 2020, 5, 100036. [Google Scholar] [CrossRef]
- Mohanty, A.; Khan, M.A.; Hinrichsen, G. Surface modification of jute and its influence on performance of biodegradable jute-fabric/Biopol composites. Compos. Sci. Technol. 2000, 60, 1115–1124. [Google Scholar] [CrossRef]
- Venkateshwaran, N.; Perumal, A.E.; Arunsundaranayagam, D. Fiber surface treatment and its effect on mechanical and visco-elastic behaviour of banana/epoxy composite. Mater. Des. 2013, 47, 151–159. [Google Scholar] [CrossRef]
- Ali, M.E.; Sultana, Z.; Uddin, M.S.; Mamun, S.A.; Haque, M.M.; Hasan, M. Effect of hydrazine post-treatment on natural fibre reinforced polymer composites. Mater. Res. Innov. 2013, 17 (Suppl. S2), s19–s26. [Google Scholar] [CrossRef]
- Saha, P.; Manna, S.; Chowdhury, S.R.; Sen, R.; Roy, D.; Adhikari, B. Enhancement of tensile strength of lignocellulosic jute fibers by alkali-steam treatment. Bioresour. Technol. 2010, 101, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Sultana, Z.; Uddin, M.S.; Al Mamun, M.S.; Haque, M.M.; Hasan, M. Effect of chemical treatment on palm fibre reinforced polypropylene composites. Int. J. Mater. Prod. Technol. 2014, 48, 3–17. [Google Scholar] [CrossRef]
- Hossain, S.; Rahman, S.; Dhar, L.; Quraishi, S.B.; Abser, N.; Rahman, F.; Rahman, M.T. Increasing the potentiality of graphene oxide by chloroacetic acid for the adsorption of lead with molecular dynamic interpretation. Curr. Res. Green Sustain. Chem. 2021, 4, 100095. [Google Scholar] [CrossRef]
- Burrola-Núñez, H.; Herrera-Franco, P.J.; Rodríguez-Félix, D.E.; Soto-Valdez, H.; Madera-Santana, T.J. Surface modification and performance of jute fibers as reinforcement on polymer matrix: An overview. J. Nat. Fibers 2019, 16, 944–960. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; D’alessio, A.; Licursi, D.; Antonetti, C.; Valentini, G.; Galia, A.; Nassi o Di Nasso, N. Midinfrared FT-IR as a tool for monitoring herbaceous biomass composition and its conversion to furfural. J. Spectrosc. 2015, 2015, 719042. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Biswas, S.; Shahinur, S.; Hasan, M.; Ahsan, Q. Physical, Mechanical and thermal properties of jute and bamboo fiber reinforced unidirectional epoxy composites. Procedia Eng. 2015, 105, 933–939. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K.; Basak, R.K.; Rana, A.K. Study of the thermal behavior of alkali-treated jute fibers. J. Appl. Polym. Sci. 2002, 85, 2594–2599. [Google Scholar] [CrossRef]
- Sinha, E.; Rout, S.K. Influence of fibre-surface treatment on structural, thermal and mechanical properties of jute fibre and its composite. Bull. Mater. Sci. 2009, 32, 65–76. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K.; Basak, R.K.; Rana, A.K. Thermal behavior of vinyl ester resin matrix composites reinforced with alkali-treated jute fibers. J. Appl. Polym. Sci. 2004, 94, 123–129. [Google Scholar] [CrossRef]
- Suwan, T.; Maichin, P.; Fan, M.; Jitsangiam, P.; Tangchirapat, W.; Chindaprasirt, P. Influence of alkalinity on self-treatment process of natural fiber and properties of its geopolymeric composites. Constr. Build. Mater. 2022, 316, 125817. [Google Scholar] [CrossRef]
- Shahinur, S.; Hasan, M.; Ahsan, Q.; Haider, J. Effect of chemical treatment on thermal properties of jute fiber used in polymer composites. J. Compos. Sci. 2020, 4, 132. [Google Scholar] [CrossRef]
- Wang, H.; Memon, H.; Hassan, E.A.M.; Miah, S.; Ali, A. Effect of jute fiber modification on mechanical properties of jute fiber composite. Materials 2019, 12, 1226. [Google Scholar] [CrossRef]
- Rajesh, G.; Prasad, A.V.R. Tensile Properties of Successive Alkali Treated Short Jute Fiber Reinforced PLA Composites. Procedia Mater. Sci. 2014, 5, 2188–2196. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Liang, W.; Wang, J.; Chen, Y. Improved mechanical properties of the graphene oxide modified bamboo-fiber-reinforced polypropylene composites. Polym. Compos. 2020, 41, 3615–3626. [Google Scholar] [CrossRef]
- Sarker, F.; Potluri, P.; Afroj, S.; Koncherry, V.; Novoselov, K.S.; Karim, N. Ultrahigh Performance of Nanoengineered Graphene-Based Natural Jute Fiber Composites. ACS Appl. Mater. Interfaces 2019, 11, 21166–21176. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.H.; Afroj, S.; Karim, N. Toward Sustainable Composites: Graphene-Modified Jute Fiber Composites with Bio-Based Epoxy Resin. Glob. Chall. 2023, 7, 2300111. [Google Scholar] [CrossRef] [PubMed]
- Godara, S.S. Effect of chemical modification of fiber surface on natural fiber composites: A review. Mater. Today Proc. 2019, 18, 3428–3434. [Google Scholar] [CrossRef]
- Singh, J.I.P.; Singh, S.; Dhawan, V. Effect of alkali treatment on mechanical properties of jute fiber-reinforced partially biodegradable green composites using epoxy resin matrix. Polym. Polym. Compos. 2020, 28, 388–397. [Google Scholar] [CrossRef]
- Al-Oqla, F.M.; Sapuan, S.; Anwer, T.; Jawaid, M.; Hoque, M. Natural fiber reinforced conductive polymer composites as functional materials: A review. Synth. Met. 2015, 206, 42–54. [Google Scholar] [CrossRef]
- Mir, A.; Zitoune, R.; Collombet, F.; Bezzazi, B. Study of mechanical and thermomechanical properties of jute/epoxy composite laminate. J. Reinf. Plast. Compos. 2010, 29, 1669–1680. [Google Scholar] [CrossRef]
- Doan, T.-T.; Brodowsky, H.; Mäder, E. Jute fibre/epoxy composites: Surface properties and interfacial adhesion. Compos. Sci. Technol. 2012, 72, 1160–1166. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, P.-G.; Jang, J.-H.; Wang, Z.; Hwang, B.-S.; DeVries, K.L. Interfacial evaluation and durability of modified Jute fibers/polypropylene (PP) composites using micromechanical test and acoustic emission. Compos. Part B Eng. 2008, 39, 1042–1061. [Google Scholar] [CrossRef]
- Rahman, M.; Mondol, M.; Hasan, M. Mechanical Properties of Chemically Treated Coir and Betel Nut Fiber Reinforced Hybrid Polypropylene Composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 438, 012025. [Google Scholar] [CrossRef]
- Pa, P.; Sasikumar, M. Viscoelastic and mechanical behaviour of reduced graphene oxide and zirconium dioxide filled jute/epoxy composites at different temperature conditions. Mater. Today Commun. 2019, 19, 252–261. [Google Scholar] [CrossRef]
- Sadangi, A.; Panda, K.K.; Kumari, K.; Srivatsava, M.; Dalai, N. Comparison study of various properties of jute reinforced composites with different nanofillers. Mater. Today Proc. 2021, 16, 1239–1243. [Google Scholar] [CrossRef]
- Alemour, B.; Badran, O.; Hassan, M.R. A Review of Using Conductive Composite. J. Aerosp. Technol. Manag. 2019, 11, e1919. [Google Scholar]
- Yang, Q.-Q.; Gao, L.-F.; Zhu, Z.-Y.; Hu, C.-X.; Huang, Z.-P.; Liu, R.-T.; Wang, Q.; Gao, F.; Zhang, H.-L. Confinement effect of natural hollow fibers enhances flexible supercapacitor electrode performance. Electrochim. Acta 2018, 260, 204–211. [Google Scholar] [CrossRef]






| Composite | Voltage (V) | Current Flow (nA) | Resistance (GΩ) | Specific Resistance (kΩm) | Conductivity (mS)m−1 |
|---|---|---|---|---|---|
| Untreated | 50 V | 44.93 | 1.11 | 2225.78 | 4.5 × 10−4 |
| 1%KOH | 53.85 | 0.93 | 1875.15 | 5.3 × 10−4 | |
| 4%KOH | 96.36 | 0.52 | 1037.74 | 1 × 10−3 | |
| 1%KOH+CGO | 926.00 | 0.05 | 107.99 | 9.3 × 10−3 | |
| 4%KOH+CGO | 2174 | 0.02 | 45.99 | 2.17 × 10−2 |
| Reference | Material Composition | Fiber Surface Treatment | Nanoparticle Additives | Tensile Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| Pa and M, 2019 [37] | Jute/Epoxy | Acetone | Graphene Oxide (GO) | 28.26 | 55.41 |
| Sadangi et al., 2021 [38] | Jute/Epoxy | Not mentioned | Graphene Oxide (GO)/Functionalized graphene | 58/59 | 18/18.8 |
| Dilfi et al., 2019 [6] | ramie/epoxy composites | Silane | CNT (Carbon Nano tube) | 77 | 84 |
| Wang et al., 2020 [27] | bamboo-fiber-reinforced polypropylene composites | NaOH | Graphene Oxide (GO) | 36 | 53 |
| Present work | Jute Fiber/Epoxy Composite | KOH (4.0 wt.%) | Carboxylated Graphene Oxide (CGO) | 80 | 89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, H.; Saha, A.; Hasan, M.; Haider, J. Effects of Alkaline and Carboxilated Graphene Oxide (CGO) Treatment on Mechanical, Thermal, and Electrical Properties of Jute Fiber-Reinforced Epoxy Composites. J. Compos. Sci. 2025, 9, 104. https://doi.org/10.3390/jcs9030104
Chowdhury H, Saha A, Hasan M, Haider J. Effects of Alkaline and Carboxilated Graphene Oxide (CGO) Treatment on Mechanical, Thermal, and Electrical Properties of Jute Fiber-Reinforced Epoxy Composites. Journal of Composites Science. 2025; 9(3):104. https://doi.org/10.3390/jcs9030104
Chicago/Turabian StyleChowdhury, Hironmoy, Atik Saha, Mahbub Hasan, and Julfikar Haider. 2025. "Effects of Alkaline and Carboxilated Graphene Oxide (CGO) Treatment on Mechanical, Thermal, and Electrical Properties of Jute Fiber-Reinforced Epoxy Composites" Journal of Composites Science 9, no. 3: 104. https://doi.org/10.3390/jcs9030104
APA StyleChowdhury, H., Saha, A., Hasan, M., & Haider, J. (2025). Effects of Alkaline and Carboxilated Graphene Oxide (CGO) Treatment on Mechanical, Thermal, and Electrical Properties of Jute Fiber-Reinforced Epoxy Composites. Journal of Composites Science, 9(3), 104. https://doi.org/10.3390/jcs9030104







