The Role of Graphite-like Materials in Modifying the Technological Properties of Rubber Composites
Abstract
1. Introduction
2. Experiment
2.1. Graphite-like Materials
- A graphite oxide (GO) sample was obtained using the modified Hummers method [22]. High-purity artificial graphite (99.99%), typically used for the preparation of nipples for metallurgical graphitized electrodes, with a mass of 7.5 g and a particle size of less than 250 μm, was used for synthesis. This graphite powder was placed into a flask with 3.0 g of NaNO3 and 115 mL of concentrated H2SO4 (with a sulfuric acid content of 96.0%). The obtained mixture was stirred magnetically for 10 min at 0 °C (in an ice bath). Then, 15 g of anhydrous KMnO4 was added, and the mixture was kept at 0 °C for 20 min. After that, the mixture was heated to 35 °C and stored for 35 min. The mixture was poured into a flask containing ice (230 g) and allowed to sit for 15 min at room temperature (25 ± 2 °C) without any heating or cooling. Then, 210 mL of H2O2 (with a hydrogen peroxide mass fraction of 32%) was added to the reaction mixture and kept for 15 min at room temperature. The mixture was then filtered and thoroughly washed with deionized water. The filter cake (graphite oxide powder) was dried in air at 80 °C for 48 h.
- Sample rGO#1 was obtained using the programmable heating of GO described above. The method for obtaining rGO was first reported in [23,24], where the heating of the GO sample at a rate of 15 °C/min was utilized. A GO sample (3 g) was placed into a stainless-steel barrel (250 mL) and then inserted into a laboratory furnace. The sample was heated from room temperature to 350 °C at a heating rate of 15 K/min. After reaching 350 °C, the sample was exposed for 55 min, and then the furnace was cooled down.
- Sample rGO#2 was obtained using the programmable heating of GO described above (the rGO#1 sample), but a different type of graphite oxide was used. Briefly, this sample of graphite oxide was synthesized using the same modified Hummers’ method described for the rGO#1 sample, but with a reduced amount of H2O2 (21 mL). The volume of hydrogen peroxide was 10 times lower compared to that used for the synthesis of rGO#1, which led to a decrease in the oxygen content of the material.
- Thermally expanded graphite (TEG) was obtained by programmable heating of commercial intercalated graphite (EG-350-50, Khimicheskie Sistemy, Moscow, Russia) in a muffle furnace. The sample was placed into an Al2O3 crucible and heated from 25 °C to 400 °C at a rate of 20 °C/min. After reaching 400 °C, the furnace was switched off, and the sample was allowed to cool inside.
- Graphite nanoplatelets (GNPs) were obtained by sonication (22 kHz) of a reduced graphite oxide (rGO#1) sample in isopropanol. The ultrasonic bath operated at a specific power of 1.6 W/cm3 for 6 h.
2.2. Rubber Composites and Their Characteristics
- − the time necessary for increase in minimum torque to 2 units ts2, min;
- − the time to reach the optimum vulcanization t90, min;
- − the rate of vulcanization Rh, dNm/min.
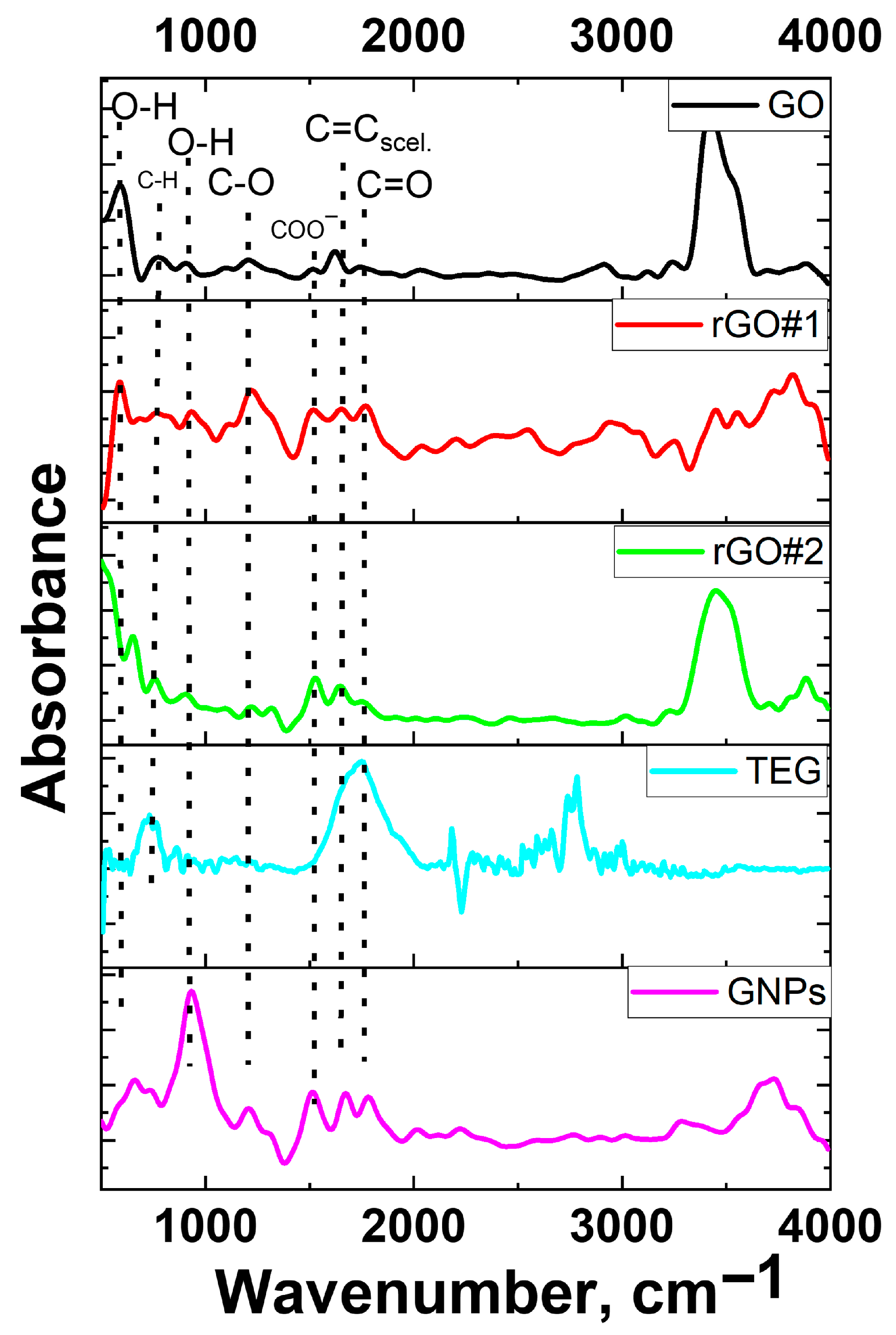
2.3. Characterization of Graphite-like Materials
3. Results and Discussion
3.1. Characterization of Graphite-like Materials
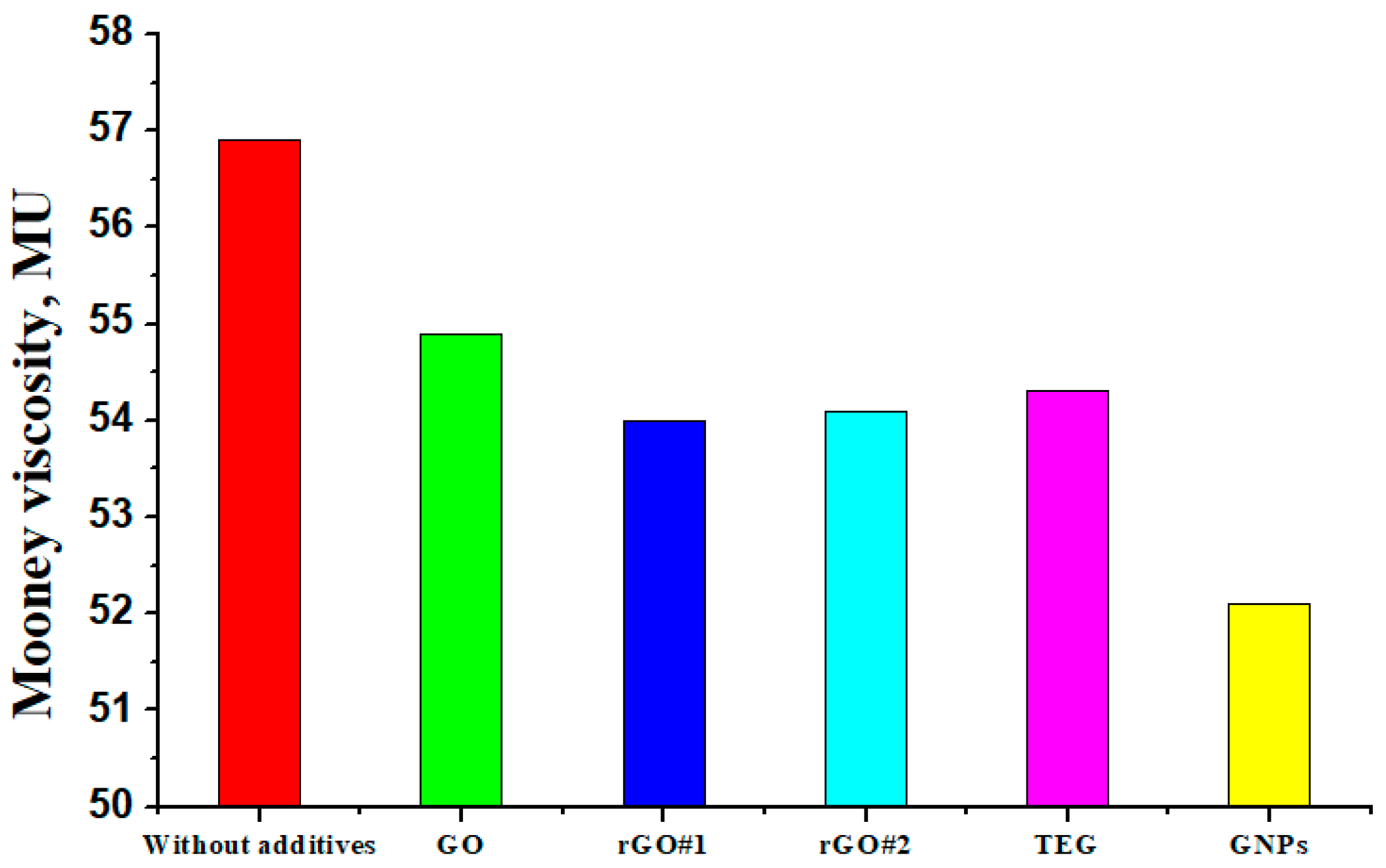
3.2. Viscosity
3.3. Relaxation Characteristics
3.4. Vulcanization Kinetics
3.5. Crosslinking Density
3.6. Elastic Strength
3.7. Heat Aging Resistance of Rubbers
- Before vulcanization. These are physical interactions of different types, such as simple interlocking and surface area changes, as well as Van der Waals interactions.
- After vulcanization. Since vulcanization involves changes in the crosslinking density and reaction kinetics, it appears that additives also participate in these reactions, leading to the formation of new bonds or just their reorganization/modification. This, in turn, affects the change in mechanical properties and the aging process.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDX | Energy dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| GNPs | Graphite nanoplatelets |
| GO | Graphite oxide |
| rGO | Reduced graphite oxide |
| SEM | Scanning electron microscopy |
| TEG | Thermally expanded graphite |
| TEM | Transmission electron microscopy |
References
- Bakošová, D.; Bakošová, A. Testing of Rubber Composites Reinforced with Carbon Nanotubes. Polymers 2022, 14, 3039. [Google Scholar] [CrossRef]
- De Falco, A.; Goyanes, S.; Rubiolo, G.H.; Mondragon, I.; Marzocca, A. Carbon nanotubes as reinforcement of styrene–butadiene rubber. Appl. Surf. Sci. 2007, 254, 262–265. [Google Scholar] [CrossRef]
- Melo, D.S.; Hiranobe, C.T.; Tolosa, G.R.; Malmonge, J.A.; Cena, C.R.; Job, A.E.; Santos, R.J.; Silva, M.J. Analyzing the Reinforcement of Multiwalled Carbon Nanotubes in Vulcanized Natural Rubber Nanocomposites Using the Lorenz–Park Method. Appl. Sci. 2024, 14, 8973. [Google Scholar] [CrossRef]
- Esmaeili, A.; Masters, I.; Hossain, M. A novel carbon nanotubes doped natural rubber nanocomposite with balanced dynamic shear properties and energy dissipation for wave energy applications. Results Mater. 2023, 17, 100358. [Google Scholar] [CrossRef]
- Gumede, J.I.; Carson, J.; Hlangothi, S.P.; Bolo, L.L. Effect of single-walled carbon nanotubes on the cure and mechanical properties of reclaimed rubber/natural rubber blends. Mater. Today Commun. 2020, 23, 100852. [Google Scholar] [CrossRef]
- Ponnamma, D.; Ramachandran, R.; Hussain, S.; Rajaraman, R.; Amarendra, G.; Varughese, K.T.; Thomas, S. Free-volume correlation with mechanical and dielectric properties of natural rubber/multi walled carbon nanotubes composites. Compos. Part A Appl. Sci. Manuf. 2015, 77, 164–171. [Google Scholar] [CrossRef]
- Ata, S.; Mizuno, T.; Nishizawa, A.; Subramaniam, C.; Futaba, D.N.; Hata, K. Influence of matching solubility parameter of polymer matrix and CNT on electrical conductivity of CNT/rubber composite. Sci. Rep. 2014, 4, 7232. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.; Klüppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene–butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, W.; Li, H.; Xie, Z.; Huang, G.; Wu, J. Fundamental researches on graphene/rubber nanocomposites. Adv. Ind. Eng. Polym. Res. 2019, 2, 32–41. [Google Scholar] [CrossRef]
- Berki, P.; László, K.; Tung, N.T.; Karger-Kocsis, J. Natural rubber/graphene oxide nanocomposites via melt and latex compounding: Comparison at very low graphene oxide content. J. Reinf. Plast. Compos. 2017, 36, 808–817. [Google Scholar] [CrossRef]
- Le, H.H.; Hait, S.; Das, A.; Wießner, S.; Stöckelhuber, K.W.; Böhme, F.; Reuter, U.; Naskar, K.; Heinrich, G.; Radusch, H.J. Self-healing properties of carbon nanotube filled natural rubber/bromobutyl rubber blends. Express Polym. Lett. 2017, 11, 230–242. [Google Scholar] [CrossRef]
- Cambraia, L.V.; Cotta, T.A.B.; Pereira, G.C.; Oliveira, M.P.; Palhares, J.B.; de Rezende, D.B.; Silva, G.G.; Nunes, E.H.M. Effect of Carbon Nanofillers on the Properties of Rubber Composites with High Levels of Carbon Black and Silica. J. Appl. Polym. Sci. 2025, 142, e57183. [Google Scholar] [CrossRef]
- Pirityi, D.Z.; Bárány, T.; Pölöskei, K. Hybrid reinforcement of styrene-butadiene rubber nanocomposites with carbon black, silica, and graphene. J. Appl. Polym. Sci. 2022, 139, e52766. [Google Scholar] [CrossRef]
- Xie, Z.T.; Fu, X.; Wei, L.Y.; Luo, M.C.; Liu, Y.H.; Ling, F.W.; Huang, C.; Huang, G.; Wu, J. New evidence disclosed for the engineered strong interfacial interaction of graphene/rubber nanocomposites. Polymer 2017, 118, 30–39. [Google Scholar] [CrossRef]
- Liu, X.; Kuang, W.; Guo, B. Preparation of rubber/graphene oxide composites with in-situ interfacial design. Polymer 2015, 56, 553–562. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Huang, J.; Cao, M.; Du, G. Expanded Graphite/Paraffin/Silicone Rubber as High Temperature Form-stabilized Phase Change Materials for Thermal Energy Storage and Thermal Interface Materials. Materials 2020, 13, 894. [Google Scholar] [CrossRef]
- Hatui, G.; Malas, A.; Bhattacharya, P.; Dhibar, S.; Kundu, M.K.; Das, C.K. Effect of expanded graphite and PEI-co-Silicon Rubber on the thermo mechanical, morphological as well as rheological properties of in situ composites based on poly (ether imide) and liquid crystalline polymer. J. Alloys Compd. 2015, 619, 709–718. [Google Scholar] [CrossRef]
- Azam, S.; Kumar, V.; Park, S.-S. Electro-bio-mechanical behavior of graphite nanoplatelets–silicone rubber-based composites for wearable electronic systems. J. Appl. Polym. Sci. 2024, 141, e55387. [Google Scholar] [CrossRef]
- Kigozi, M.; Koech, R.K.; Kingsley, O.; Ojeaga, I.; Tebandeke, E.; Kasozi, G.N.; Onwualu, A.P. Synthesis and characterization of graphene oxide from locally mined graphite flakes and its supercapacitor applications. Results Mater. 2020, 7, 100113. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, R.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974. [Google Scholar] [CrossRef]
- Gantayat, S.; Prusty, G.; Rout, D.; Swain, S.K. Expanded graphite as a filler for epoxy matrix composites to improve their thermal, mechanical and electrical properties. Carbon 2016, 98, 734. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Aguilar-Bolados, H.; Lopez-Manchado, M.A.; Brasero, J.; Avilés, F.; Yazdani-Pedram, M. Effect of the morphology of thermally reduced graphite oxide on the mechanical and electrical properties of natural rubber nanocomposites. Compos. Part B Eng. 2016, 87, 350–356. [Google Scholar] [CrossRef]
- Bannov, A.G.; Ukhina, A.V.; Maksimovskii, E.A.; Prosanov, I.Y.; Shestakov, A.A.; Lapekin, N.I.; Lazarenko, N.S.; Kurmashov, P.B.; Popov, M.V. Highly Porous Expanded Graphite: Thermal Shock vs. Programmable Heating. Materials 2021, 14, 7687. [Google Scholar] [CrossRef]
- Lucking, A.D.; Pan, L.; Narayanan, D.L.; Clifford, C.E.B. Effect of expanded graphite lattice in exfoliated graphite nanofibers on hydrogen storage. J. Phys. Chem. B 2005, 109, 12710–12717. [Google Scholar] [CrossRef]
- Asghar, H.M.a.; Hussain, S.N.; Sattar, H.; Brown, N.W.; Roberts, E.P.L. Potential Graphite Materials for the Synthesis of GICs. Chem. Eng. Commun. 2015, 202, 508–512. [Google Scholar] [CrossRef]
- Zhu, X.L.; Yuan, Z.Y.; Jiang, L.; Zhang, K.; Wang, Z.R.; Luo, H.W.; Gu, Y.; Cao, J.W.; Qin, X.L.; Zhang, P. Computational analysis of vibrational spectrum and hydrogen bonds of ice XVII. New J. Phys. 2019, 21, 043054. [Google Scholar] [CrossRef]
- Margoshes, M.; Fassel, V.A. The infrared spectra of aromatic compounds: I. The out-of-plane C-H bending vibrations in the region 625–900 cm−1. Spectrochim. Acta 1955, 7, 14–24. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N. The Oleic Acid Composition Effect on the Carboxymethyl Cellulose Based Biopolymer Electrolyte. J. Cryst. Process Technol. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Redington, R.L.; Lin, K.C. On the oh stretching and the low-frequency vibrations of carboxylic acid cyclic dimers. J. Chem. Phys. 1971, 54, 4111–4119. [Google Scholar] [CrossRef]
- Laberge, M.; Sharp, K.A.; Vanderkooi, J.M. Effect of charge interactions on the carboxylate vibrational stretching frequency in c-type cytochromes investigated by continuum electrostatic calculations and FTIR spectroscopy. Biophys. Chem. 1998, 71, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Elderderi, S.; Leman-Loubière, C.; Wils, L.; Henry, S.; Bertrand, D.; Byrne, H.J.; Chourpa, I.; Enguehard-Gueiffier, C.; Munnier, E.; Elbashir, A.A.; et al. ATR-IR spectroscopy for rapid quantification of water content in deep eutectic solvents. J. Mol. Liq. 2020, 311, 113361. [Google Scholar] [CrossRef]
- Amer, M.S.; Schadler, L.S. Stress concentration phenomenon in graphite/epoxy composites: Tension/compression effects. Compos. Sci. Technol. 1997, 57, 1129–1137. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Yang, L.; Liu, B.; Xie, S.; Qi, R.; Zhan, Y.; Xia, H. Graphene-Based Hybrid Fillers for Rubber Composites. Molecules 2024, 29, 1009. [Google Scholar] [CrossRef]




| No. | Component | Content, phr |
|---|---|---|
| 1 | CIR (cis-1,4-isoprene rubber) | 50.00 |
| 2 | BR (cis-1,4-polybutadiene rubber) | 50.00 |
| 3 | Carbon black N339 | 44.00 |
| 4 | Oil I-40 | 2.50 |
| 5 | Durez 29,095 resin | 3.00 |
| 6 | Pine rosin | 5.00 |
| 7 | Zinol | 2.60 |
| 9 | ZnO | 3.00 |
| 10 | Stearic acid | 5.00 |
| 11 | Okerin wax | 2.50 |
| 12 | Dusatntox 6PPD (N-1,3 dimethylbutyl-N’-phenyl-p-phenylenediamine) | 2.50 |
| 13 | Acetonanil N (poly(1,2-dihydro-2,2,4-trimethylquinoline)) | 2.00 |
| 14 | Santokur CBS (N-Cyclohexyl-2-benzothiazolesulfenamide) | 1.10 |
| 15 | Sulfur Cristex OT 33 | 1.80 |
| Total | 175.0 | |
| Sample | Concentration of Elements, at. % | ||||
|---|---|---|---|---|---|
| C | O | S | Other Elements | C:O (at.) | |
| GO | 70.44 | 22.46 | 7.14 | - | 3.1 |
| rGO#1 | 85.56 | 10.95 | 1.76 | K—0.3; Mn—0.43 | 7.8 |
| rGO#2 | 80.68 | 17.31 | 0.84 | Si—0.14; K—0.17; Mn—0.68 | 4.7 |
| TEG | 95.14 | 4.33 | 0.29 | Si—0.13; Cu—0.12 | 22.0 |
| GNPs | 87.79 | 10.95 | 0.72 | K—0.31; Mn—0.24 | 8.0 |
| Rubber Composite | Initial Viscosity of Rubber Mixture, MU |
|---|---|
| Composite without carbon additives | 86.9 |
| Composite + GO | 86.5 |
| Composite + rGO#1 | 85.1 |
| Composite + rGO#2 | 88.6 |
| Composite + TEG | 86.5 |
| Composite + GNPs | 87.6 |
| Rubber Composite | The Mooney Viscosity of the Rubber Mixture Measured 1 s After Stopping the Rotor, MU |
|---|---|
| Composite without carbon additives | 23.6 |
| Composite + GO | 22.3 |
| Composite + rGO#1 | 21.8 |
| Composite + rGO#2 | 21.6 |
| Composite + TEG | 21.7 |
| Composite + GNPs | 21.1 |
| Rubber Composite | ts2, min 1 | t90, min 2 | Rate of Vulcanization, dNm/min (Rh) |
|---|---|---|---|
| Composite without carbon additives | 2.67 | 5.51 | 17.43 |
| Composite + GO | 3.23 | 8.18 | 11.16 |
| Composite + rGO#1 | 3.29 | 8.44 | 10.25 |
| Composite + rGO#2 | 3.62 | 8.69 | 10.24 |
| Composite + TEG | 3.11 | 8.00 | 8.56 |
| Composite + GNPs | 3.54 | 8.81 | 9.62 |
| Rubber Composite | Mc 1, kg/mol | ν·105, mol/cm3 | n·10−19, cm−3 |
|---|---|---|---|
| Composite without carbon additives | 12,894.10 | 7.10 | 4.27 |
| Composite + GO | 11,722.25 | 8.07 | 4.86 |
| Composite + rGO#1 | 12,285.37 | 7.45 | 4.49 |
| Composite + rGO#2 | 12,433.88 | 7.37 | 4.43 |
| Composite + TEG | 13,471.77 | 6.79 | 4.09 |
| Composite + GNPs | 13,108.57 | 6.98 | 4.20 |
| Rubber Composite | Elongation at Break, % | Tensile Strength, MPa |
|---|---|---|
| Composite without carbon additives | 418.1 | 19.39 |
| Composite + GO | 520.3 | 18.44 |
| Composite + rGO#1 | 523.6 | 17.38 |
| Composite + rGO#2 | 585.3 | 20.71 |
| Composite + TEG | 581.4 | 18.20 |
| Composite + GNPs | 484.2 | 19.96 |
| Rubber Composite | Elongation at Break, % | Tensile Strength, MPa | Change | |
|---|---|---|---|---|
| Elongation at Break, % | Tensile Strength, % | |||
| Composite without carbon additives | 425.0 | 18.39 | +1.65 | −5.16 |
| Composite + GO | 390.1 | 17.33 | −25.03 | −6.02 |
| Composite + rGO#1 | 366.6 | 16.78 | −29.98 | −3.45 |
| Composite + rGO#2 | 327.0 | 15.84 | −44.13 | −23.52 |
| Composite + TEG | 338.0 | 15.66 | −41.86 | −13.96 |
| Composite + GNPs | 323.0 | 15.36 | −33.29 | −23.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnevskii, K.V.; Kurmashov, P.B.; Golovakhin, V.; Maksimovskiy, E.A.; Jin, H.; Shashok, Z.S.; Bannov, A.G. The Role of Graphite-like Materials in Modifying the Technological Properties of Rubber Composites. J. Compos. Sci. 2025, 9, 522. https://doi.org/10.3390/jcs9100522
Vishnevskii KV, Kurmashov PB, Golovakhin V, Maksimovskiy EA, Jin H, Shashok ZS, Bannov AG. The Role of Graphite-like Materials in Modifying the Technological Properties of Rubber Composites. Journal of Composites Science. 2025; 9(10):522. https://doi.org/10.3390/jcs9100522
Chicago/Turabian StyleVishnevskii, Konstantin V., Pavel B. Kurmashov, Valeriy Golovakhin, Eugene A. Maksimovskiy, Huile Jin, Zhanna S. Shashok, and Alexander G. Bannov. 2025. "The Role of Graphite-like Materials in Modifying the Technological Properties of Rubber Composites" Journal of Composites Science 9, no. 10: 522. https://doi.org/10.3390/jcs9100522
APA StyleVishnevskii, K. V., Kurmashov, P. B., Golovakhin, V., Maksimovskiy, E. A., Jin, H., Shashok, Z. S., & Bannov, A. G. (2025). The Role of Graphite-like Materials in Modifying the Technological Properties of Rubber Composites. Journal of Composites Science, 9(10), 522. https://doi.org/10.3390/jcs9100522







