Research and Development of pH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite
Abstract
1. Introduction
- Long biodegradation time to control drug release [16];
- Slow drug release due to porous material structure [17];
- Adsorbs both positively and negatively charged molecules [18];
- Maintains mechanical and physical properties even when internal factors such as temperature and pH change [19];
- Synthetic hydroxyapatite retains the composition, crystal structure, and size that it has in target tissues (e.g., bone or teeth) [20].
2. Materials and Methods
2.1. Synthesis Techniques
2.2. Materials Analysis
- Phosphate buffer solution pH 5.0: 2.72 g potassium dihydrophosphate dissolved in 800.0 mL water. Adjust to pH 5.0 potentiometrically with 1 M potassium hydroxide solution and bring the volume of the solution with water to 1000.0 mL.
- Phosphate buffer solution pH 6.5: dissolve 1.79 g dihydrophosphate, 1.36 g potassium dihydrophosphate and 7.02 g sodium chloride in water and bring the volume of the solution to 1000.0 mL with water.
- Phosphate buffer solution pH 7.4: 393.4 mL of 0.1 M sodium hydroxide solution is mixed with 250.0 mL of 0.2 M potassium dihydrophosphate solution.
3. Results
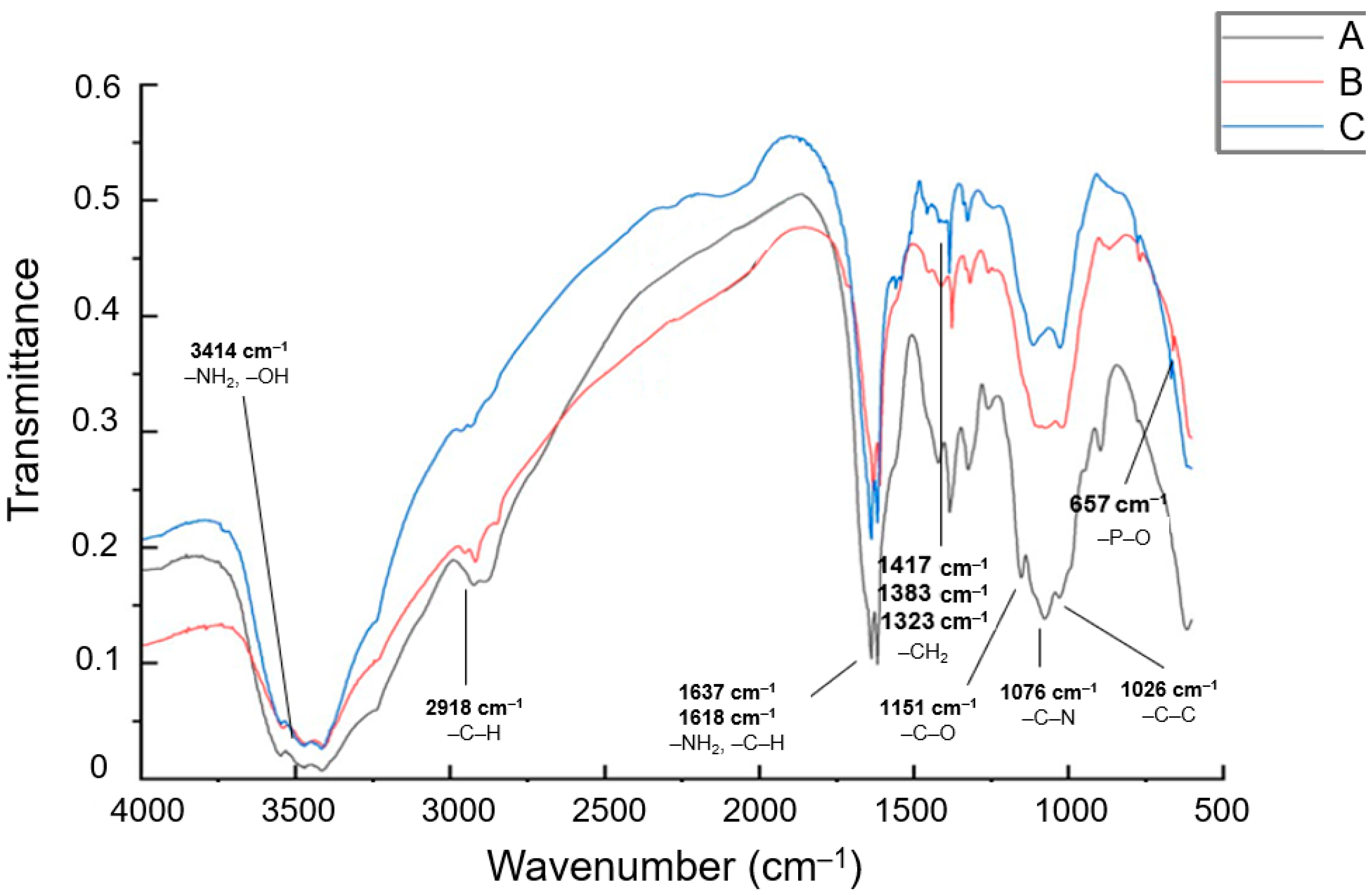
3.1. IR Spectroscopy Analysis of Particle Structure
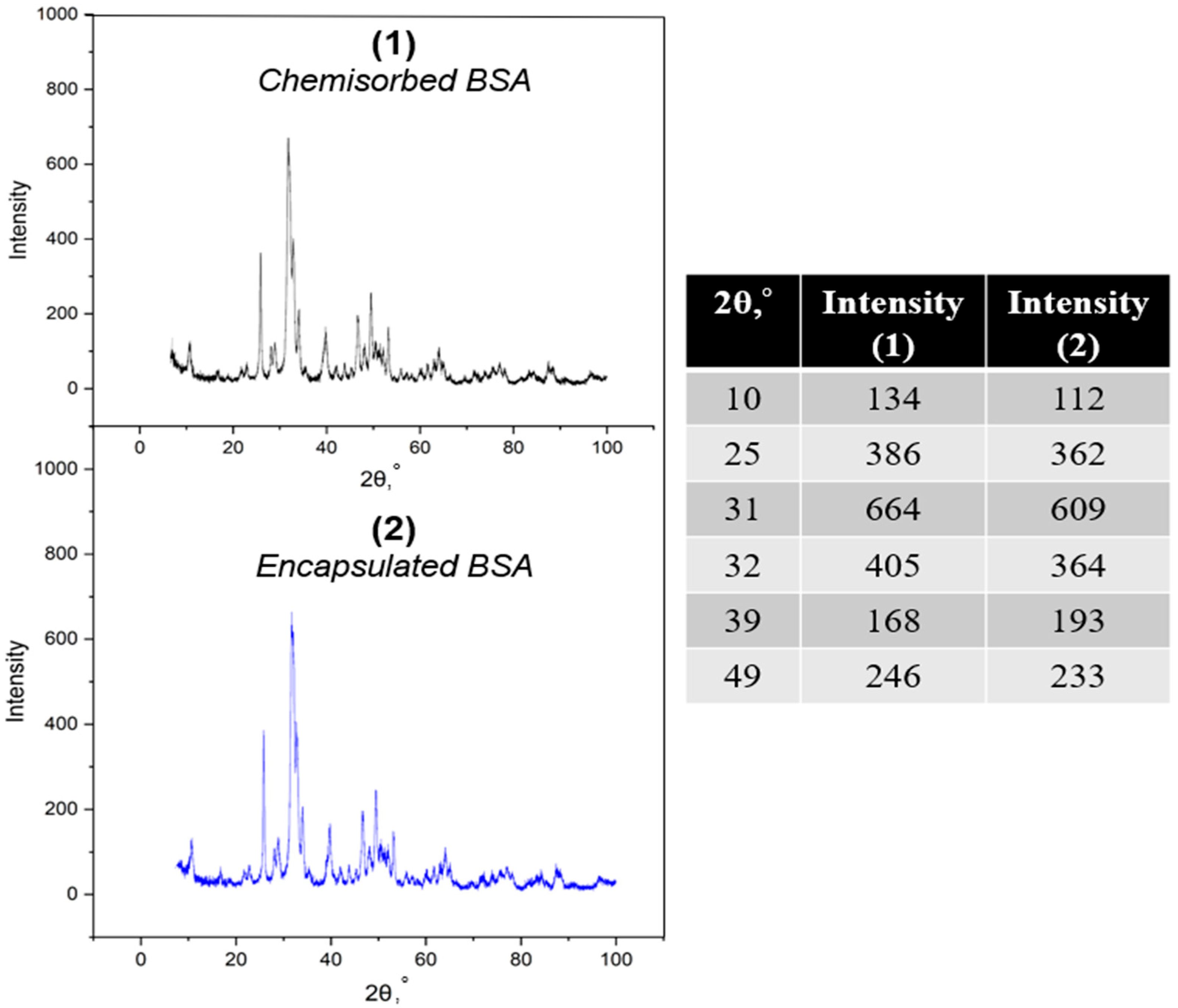
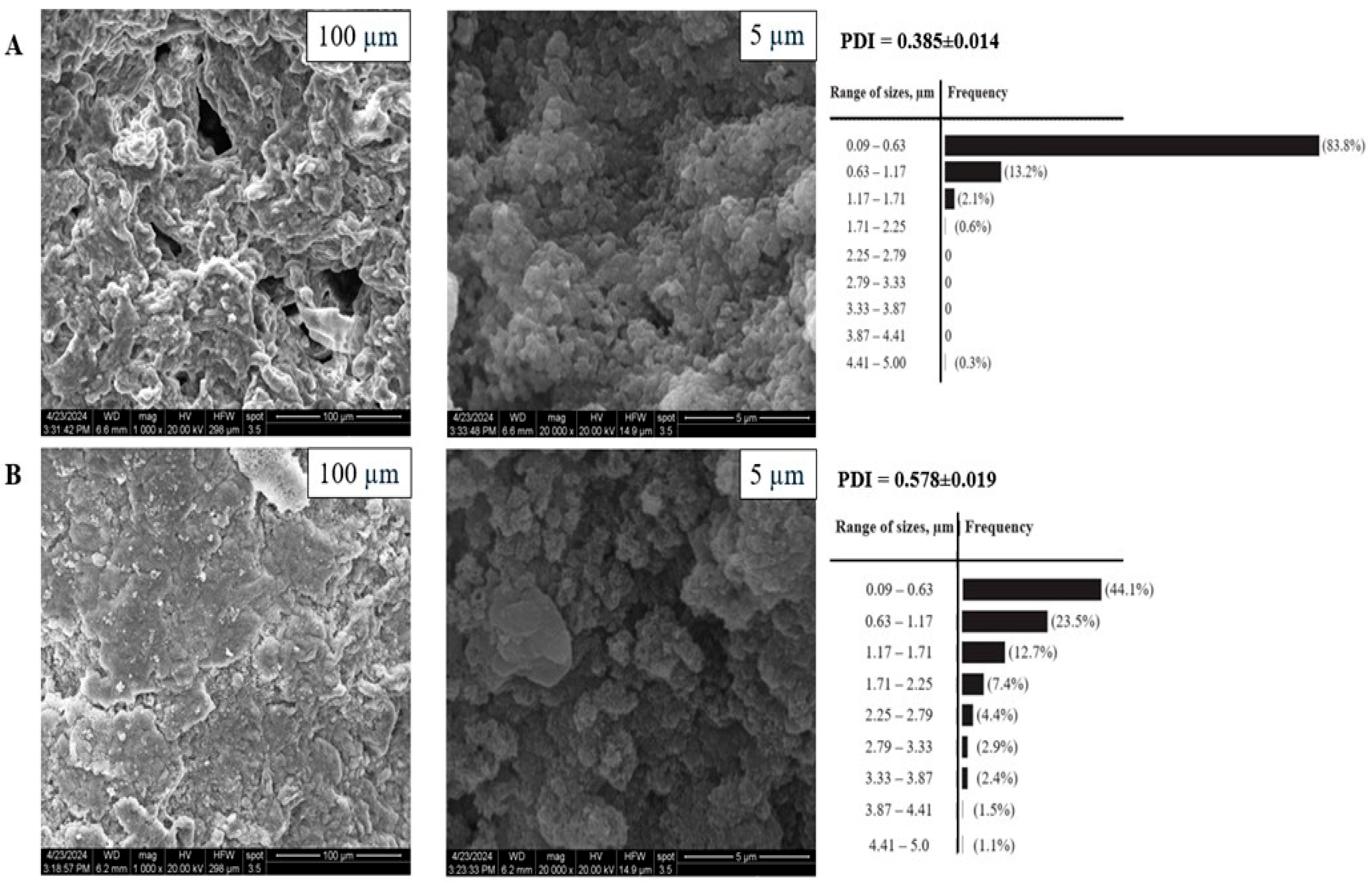
3.2. X-Ray and SEM Analysis of Particle Morphology
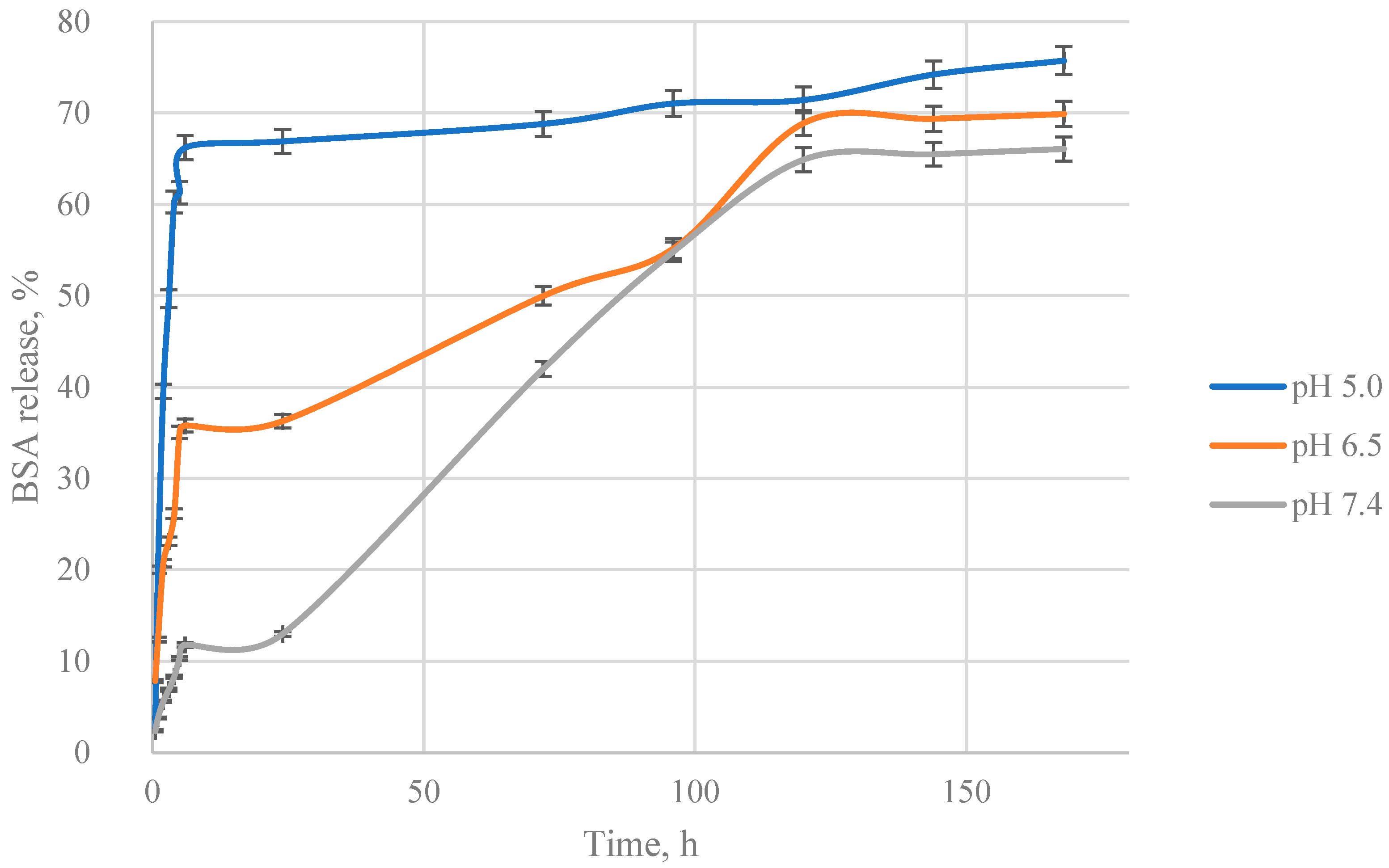
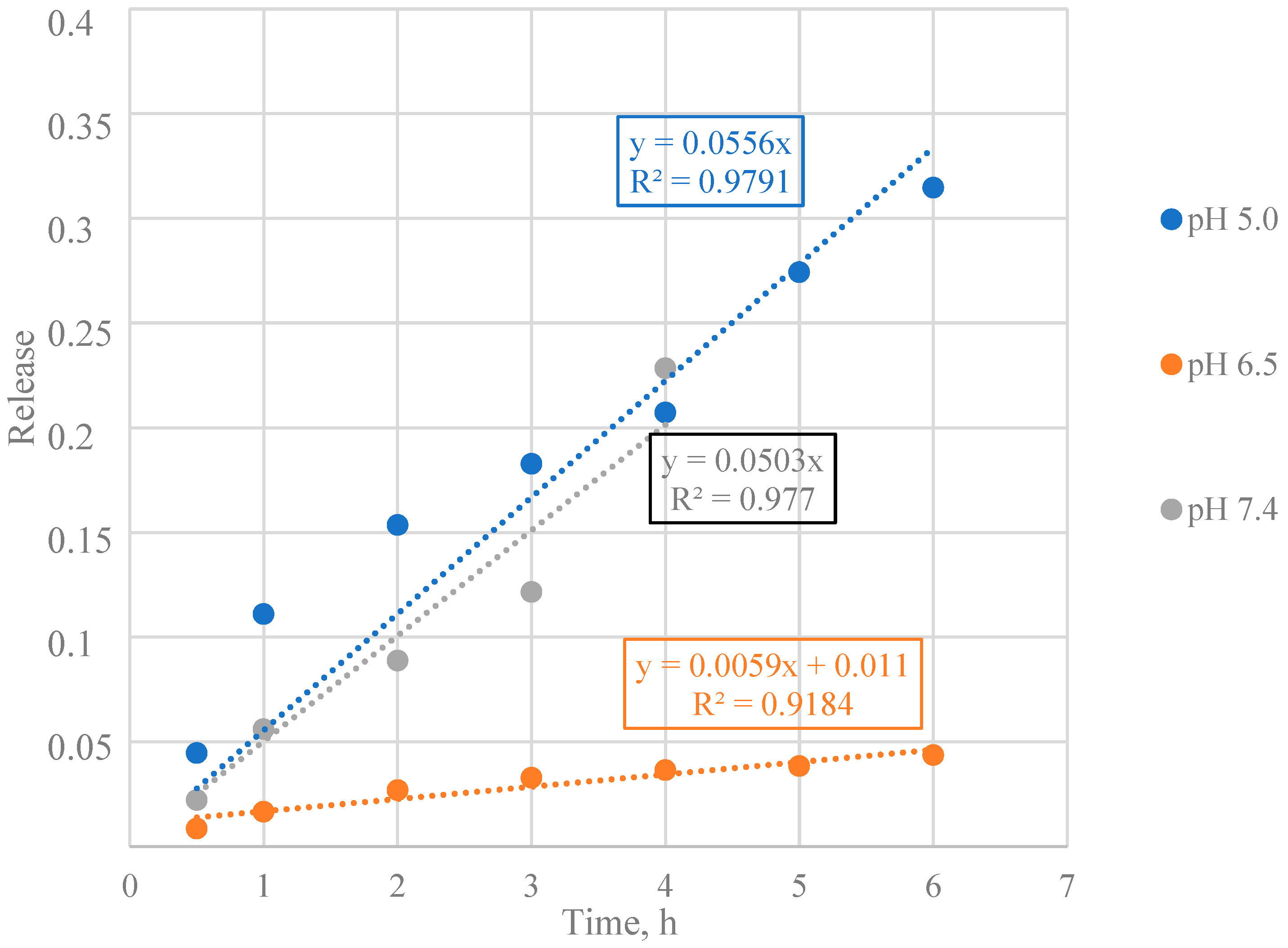
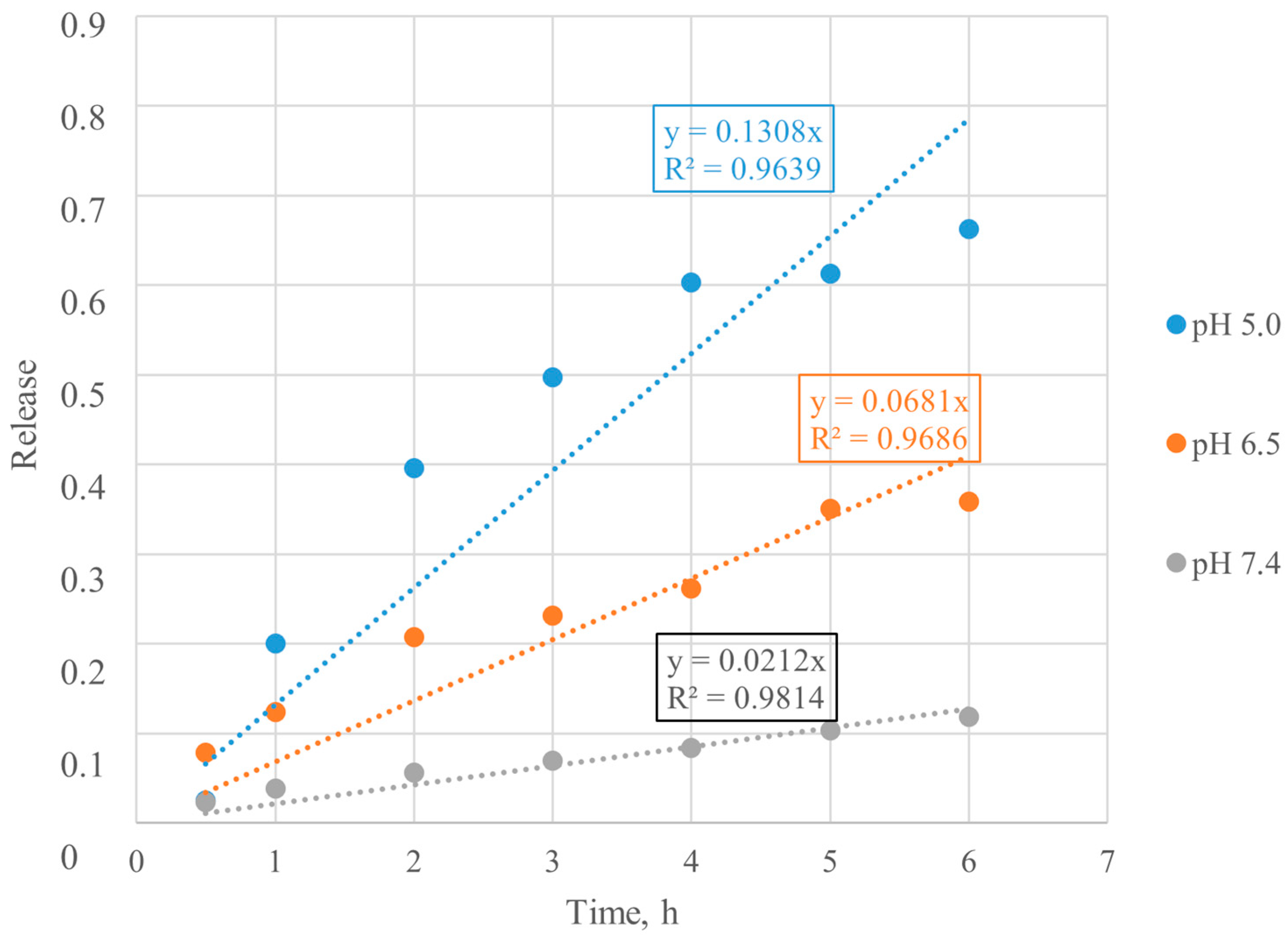
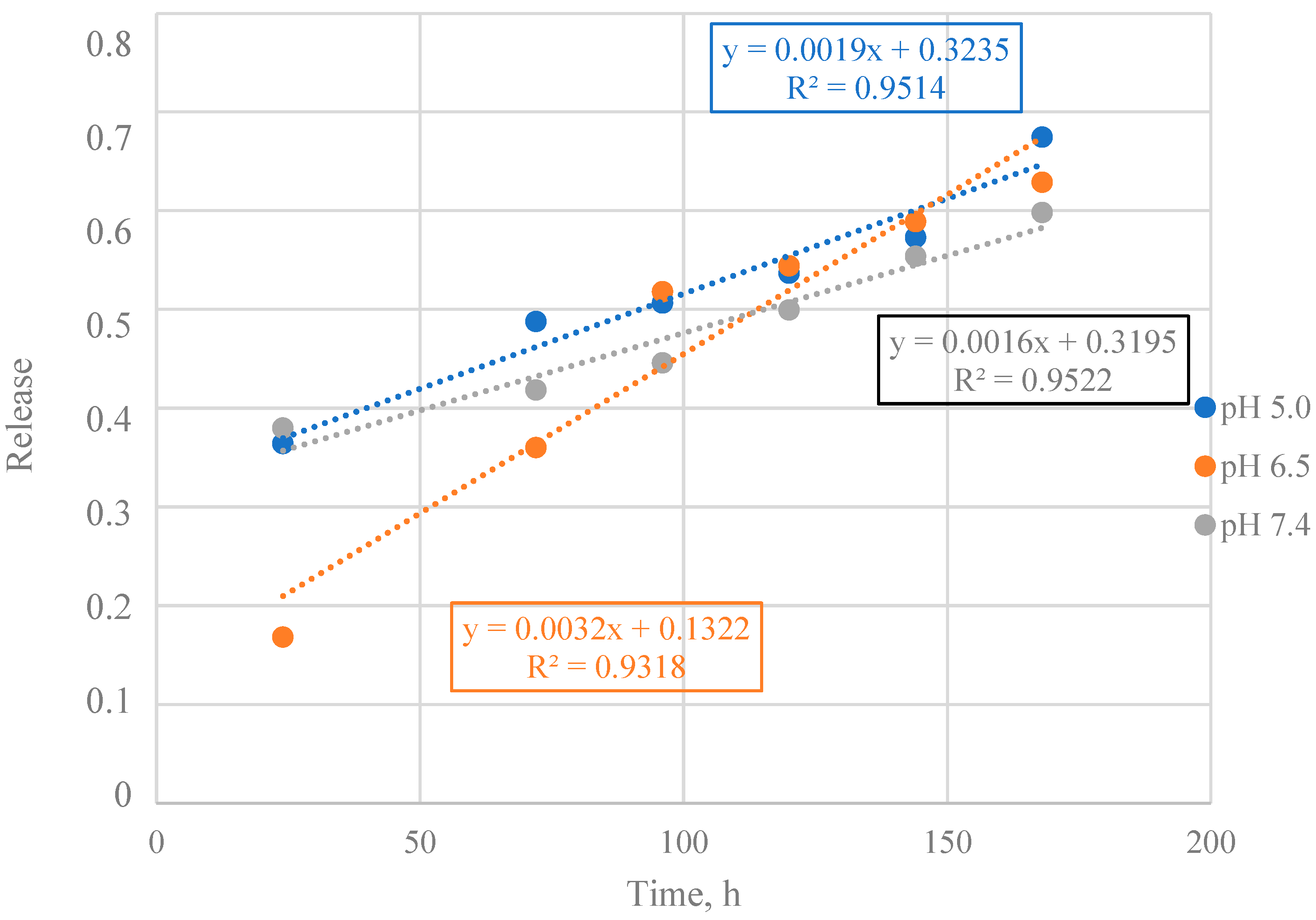
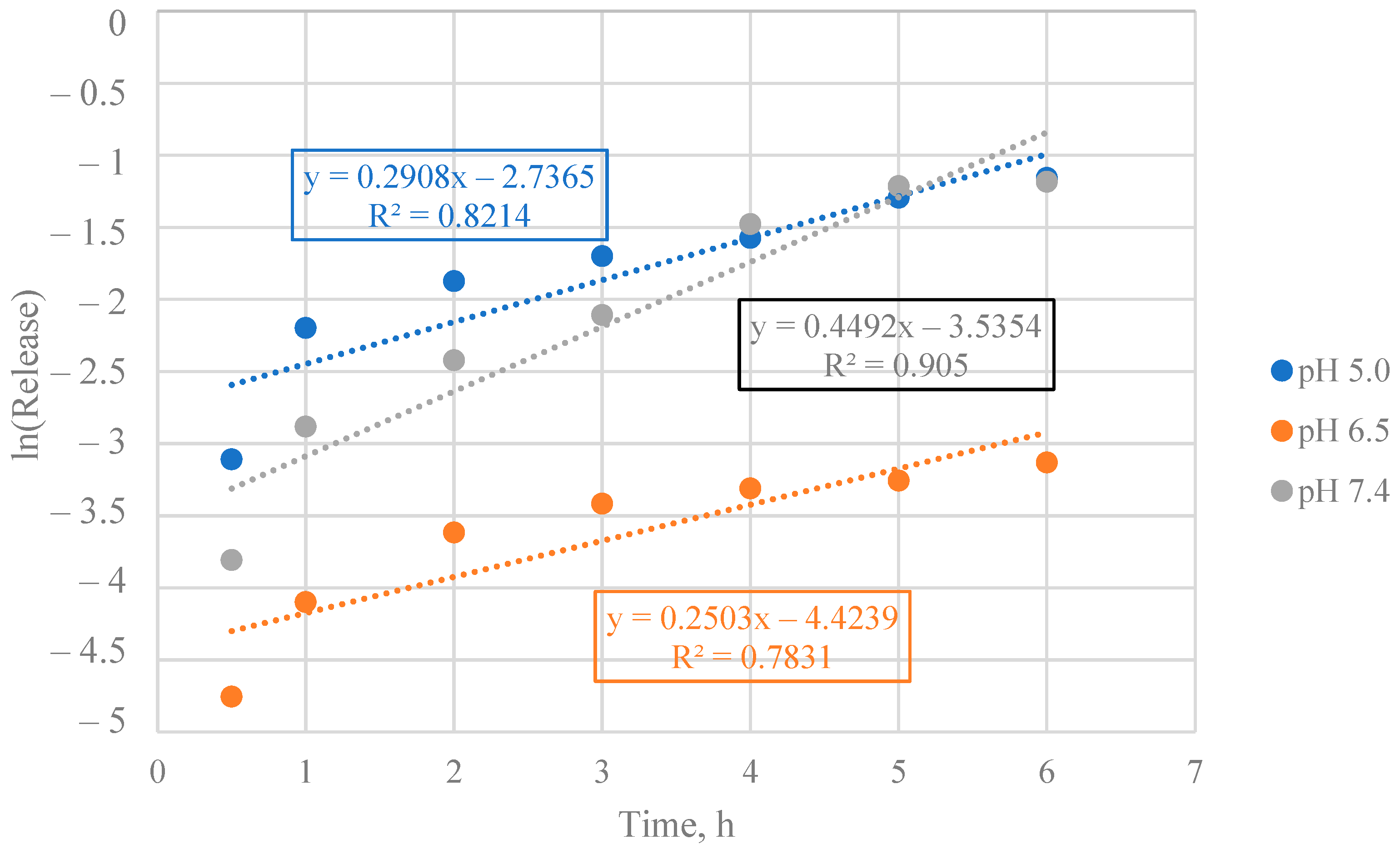
3.3. Mathematical Model Selection of BSA Release from Particles
- (1)
- Construction of a dot plot of the drug concentration dependence in the test liquid on time or the released substance fraction on time.
- (2)
- Initial estimation and selection of the mathematical model. For example, if the dependence is close to linear, then linear approximation by the first-order model (zero order release) is first used.
- (3)
- Calculation of the adjusted determination coefficient (R2). The closer R2 is to unity, the higher the reliability of approximation and the more the mathematical model reflects the real experiment.
- (4)
- Use the model coefficients to describe the release mechanisms.
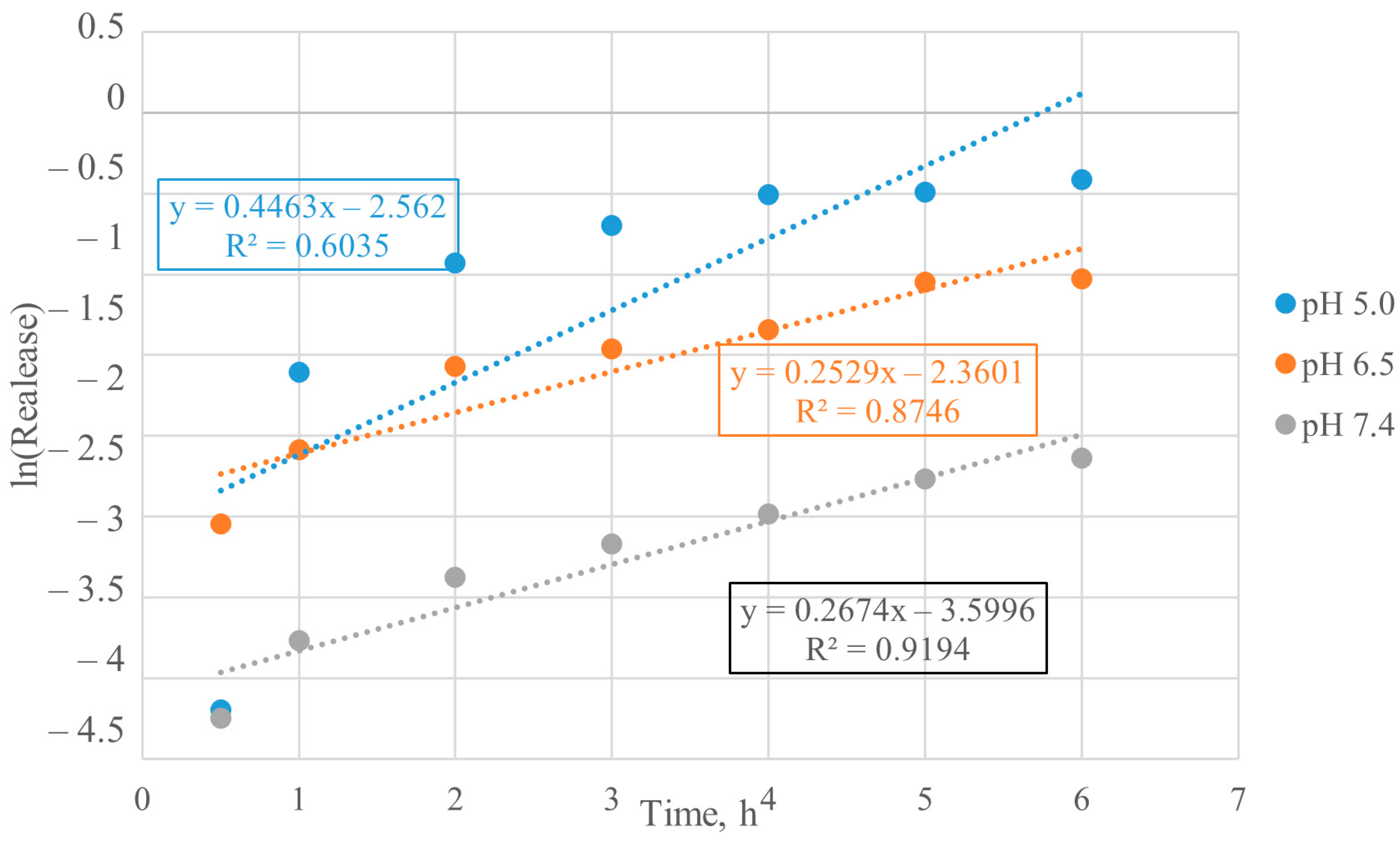
3.4. Mathematical Model Chosen for BSA Release from Particles
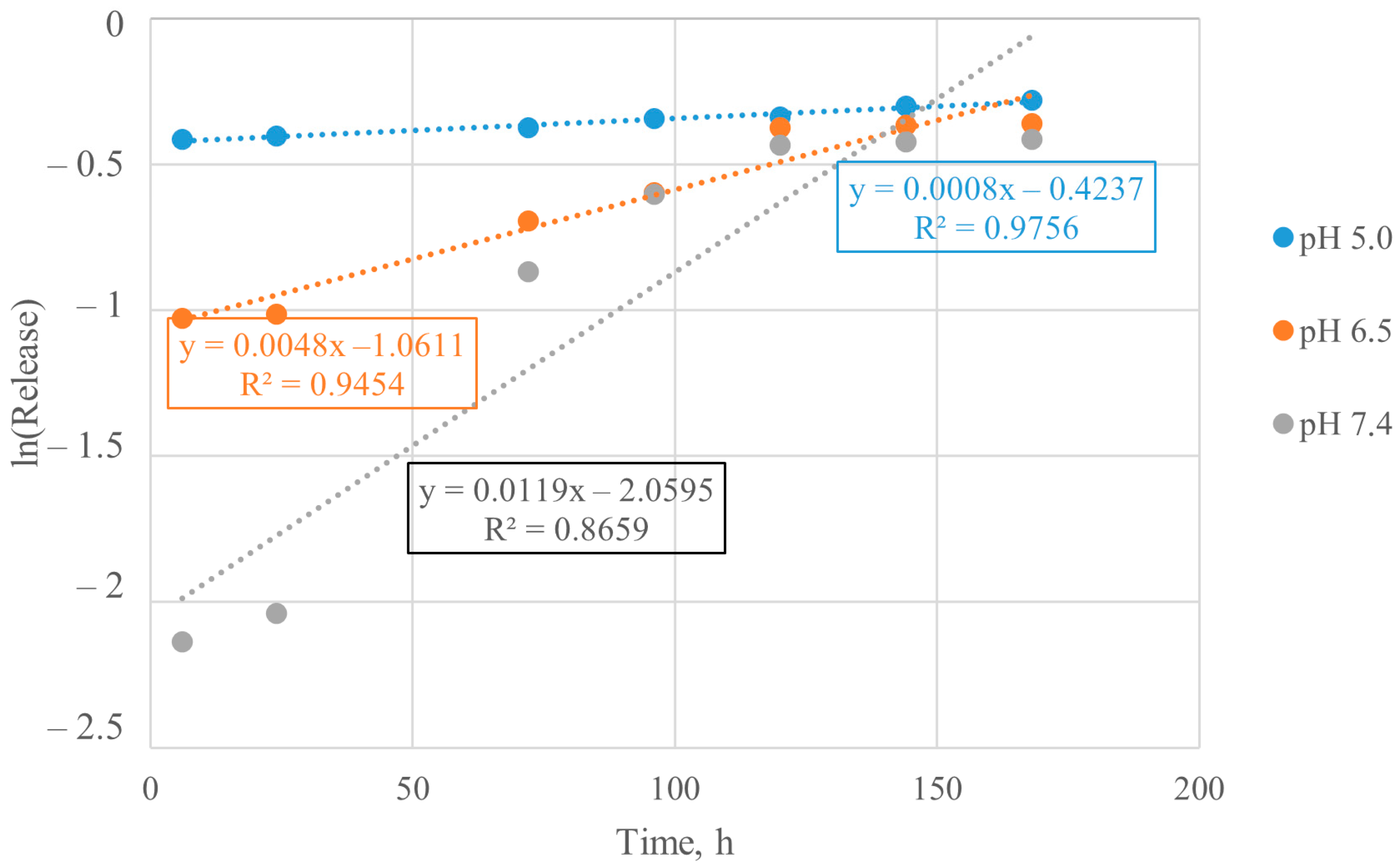
3.5. Calculation of Kinetic Parameters of BSA Release from Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadwal, A.; Baldi, A.; Kumar-Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Zhao, C. Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Stefanache, A.; Lungu, I.I.; Anton, N.; Damir, D.; Gutu, C.; Olaru, I.; Condratovici, A.P.; Covrig, M.D.; Constantin, M.; Calin, G.; et al. Chitosan Nanoparticle-Based Drug Delivery Systems: Advances, Challenges, and Future Perspectives. Polymers 2025, 17, 1453. [Google Scholar] [CrossRef]
- Thakur, S.; Godela, R.; Mandava, K.; Kolure, R. Advances in nanocarrier technology for drug encapsulation: A comprehensive overview. Discov. Mater. 2025, 5, 124. [Google Scholar] [CrossRef]
- Zhao, B.; Zhong-Ming, L.; Song, X.; Xu, H.; Gao, Y.; Xu, J.-Z.; Yin, H.-M.; Xu, L. Promoting osteoblast proliferation on polymer bone substitutes with bone-like structure by combining hydroxyapatite and bioactive glass. Mater. Sci. Eng. 2019, 96, 1–9. [Google Scholar]
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent advances of chitosan-based polymers in biomedical applications and environmental protection. J. Polym. Res. 2022, 29, 251–259. [Google Scholar] [CrossRef]
- Mohammed, A.; Abourehab, S.; Pramanik, S.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J. The transformation of single-crystal calcium phosphate ribbon-like fibres to hydroxyapatite spheres assembled from nanorods. Nanotechnology 2008, 23, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.R.; Venkatesh, R. A study of hydroxyapatite fibers prepared via sol-gel route. Mater. Lett. 2004, 58, 320–323. [Google Scholar] [CrossRef]
- Zhang, H.; Darvell, B.W. Synthesis and characterization of hydroxyapatite whiskers by hydrothermal homogeneous precipitation using acetamide. Acta Biomater. 2010, 6, 155–189. [Google Scholar] [CrossRef]
- Sahni, G.; Gopinath, P.; Jeevanandam, P. A novel thermal decomposition approach to synthesize hydroxyapatite-silver nanocomposites and their antibacterial action against GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2013, 103, 441–447. [Google Scholar] [CrossRef]
- Aizawa, M.; Hanazawa, T.; Itatani, K.; Howell, F.S.; Kishioka, A. Characterization of hydroxyapatite powders prepared by ultrasonic spray-pyrolysis technique. J. Mater. Sci. 1999, 34, 266–287. [Google Scholar] [CrossRef]
- Zhang, H.G.; Zhu, Q. Preparation of fluoride-substituted hydroxyapatite by a molten salt synthesis route. J. Mater. Sci. Mater. Med. 2006, 17, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Tabrizi, B.; Honarmandi, P.; Ebrahimi-Kahrizsangi, R. Synthesis of nanosize single-crystal hydroxyapatite via mechanochemical method. Mater. Lett. 2009, 63, 543–546. [Google Scholar] [CrossRef]
- Lin, K.; Pan, J.; Chen, Y.; Cheng, R.; Xu, X. Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J. Hazard. Mater. 2009, 161, 231–240. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Hou, C.-H.; Hou, S.-M.; Hsueh, Y.-S.; Lin, J.; Wu, H.-C.; Lin, F.-H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef]
- Shenoy, B.D.; Udupa, N.; Nagarajkumari, Y. Implantable drug delivery systems for centchroman. Indian J. Pharm. Sci. 1997, 59, 246–250. [Google Scholar]
- Walduck, A.K.; Opdebeeck, J.P.; Benson, H.E.; Prankerd, R.P. Biodegradable implants for the delivery of veterinary vaccines: Design, manufacture and antibody responses in sheep. J. Control. Release 1998, 51, 269–280. [Google Scholar] [CrossRef]
- Chen, L.; Mccrate, J.; Lee, J.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Zagvozkin, M.D.; Chernikova, E.E.; Brusevich, A.; Kurzina, I.A.; Ulasevich, S.A. Development of pH-sensitive delivery systems for bovine serum albumin based on chitosan by chemisorption method. Tomsk. State Univ. J. Chem. 2024, 139–151. [Google Scholar] [CrossRef]
- Chernikova, E.E.; Zagvozkin, M.D.; Kurzina, I.A.; Brusevich, A.; Ulasevich, S.A. Preparation and study of pH-sensitive delivery systems for encapsulated protein molecules based on chitosan. Tomsk. State Univ. J. Chem. 2024, 126–138. [Google Scholar]
- Sun, H.; Qiu, X.; Li, X.; Wang, H. Eco-friendly, pH-sensitive curcumin-loaded sodium alginate/hydroxyapatite/quaternary ammonium chitosan microspheres with enhanced antibacterial and antioxidant activities for fruit preservation. Int. J. Biol. Macromol. 2024, 279, 135297. [Google Scholar] [CrossRef]
- Wu, M.Y.; Kao, I.F.; Fu, C.Y.; Yen, S.K. Effects of Adding chitosan on drug entrapment efficiency and release duration for paclitaxel-loaded hydroxyapatite—Gelatin composite microspheres. Pharmaceutics 2023, 15, 2025. [Google Scholar] [PubMed]
- Devine, D.M.; Hoctor, E.; Hayes, J.S.; Sheehan, E.; Evans, C.H. Extended release of proteins following encapsulation in hydroxyapatite/chitosan composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. 2018, 84, 281–289. [Google Scholar]
- Sha, Z.; Wu, Y.; Zheng, Y.; Yang, K.; Gong, X.; Xuan, L.; Li, X.; Chen, X. Advances in pH-responsive drug delivery systems for periodontitis treatment. Drug Deliv. 2025, 32, 2522109. [Google Scholar] [CrossRef] [PubMed]










| Synthesis Method | Advantages | Morphology and Size | Reference |
|---|---|---|---|
| Co-precipitation | Simplest and most efficient chemical method. Synthesis at room temperature. Particle size can easily be controlled by adjusting pH and ionic strength of reaction media. Structures have wide range in size and morphology. | Irregular, sphere, rod, needle, tube, fiber, filament, wire, whisker, strip, platelet, flower. Size range: 3 nm–1000 μm. | [6] |
| Sol-gel synthesis | Molecular-level mixing of reactants improves the chemical homogeneity of pure and hybrid synthesized nanostructures at low temperature. | Irregular, sphere, rod, needle, tube, filament, whisker, platelet. Size range: 3 nm–1000 μm. | [7] |
| Hydrothermal synthesis | Enhanced solubility of precursors. Controlled growth dynamics. | Irregular, sphere, rod, needle, tube, fiber, wire, whisker, feathery structures. | [8] |
| Thermal decomposition | Good size control, narrow size variation, and good crystallinity. | Irregular, flake, plate, sheet, formless. Size range: 5 nm–200 μm. | [9] |
| Pyrolysis | Rod-like nanoparticles and single phase with high crystallinity and good stoichiometry. | Nanorods embedded to micron form. Size range: 10 nm–1000 μm. | [10] |
| Solid state | Well-crystallized structure. | Irregular, filament, rod, needle, whisker. Size range: 5 nm–1000 μm. | [11] |
| Mechano-chemical | No calcination is required. | Irregular, sphere, rod, needle, whisker. Size range: 5 nm–200 μm. | [12] |
| n | Release Mechanism | Release Rate as a Function of Time | Release Mechanism |
|---|---|---|---|
| n < 0.5 | Quasi–Fickian diffusion | tn | Diffusion from a non-swelling matrix |
| 0.5 | Fickian diffusion | t0.5 | Diffusion from a non-swelling matrix |
| 0.5 < n < 1.0 | Anomalous transport (non-Fickian) | tn−1 | Diffusion and/or relaxation |
| 1.0 | Case II (non-Fickian diffusion) | Time-independent | Zero order |
| n > 1.0 | Supercase II | tn−1 | Relaxation (swelling) or erosion |
| System | pH Value | n Value | Release Mechanism |
|---|---|---|---|
| Encapsulated BSA | 5.0 | 0.3596 | Diffusion from a non-swelling matrix |
| 6.5 | 0.7541 | Diffusion and/or relaxation | |
| 7.4 | 0.4465 | Diffusion from a non-swelling matrix | |
| Chemisorbed BSA | 5.0 | 0.3204 | Diffusion from a non-swelling matrix |
| 6.5 | 0.3154 | Diffusion from a non-swelling matrix | |
| 7.4 | 0.5690 | Diffusion and/or relaxation |
| System | pH Value | Rate Constant | Order |
|---|---|---|---|
| Encapsulated BSA | 5.0 | 0.0786 | 1.899 |
| 6.5 | 0.0575 | 0.194 | |
| 7.4 | 0.0533 | 1.539 | |
| Chemisorbed BSA | 5.0 | 0.0588 | 1.943 |
| 6.5 | 0.0520 | 2.298 | |
| 7.4 | 0.0634 | 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernikova, E.E.; Zagvozkin, M.D.; Buzaev, A.A.; Kurzina, I.A.; Ulasevitch, S.A. Research and Development of pH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite. J. Compos. Sci. 2025, 9, 525. https://doi.org/10.3390/jcs9100525
Chernikova EE, Zagvozkin MD, Buzaev AA, Kurzina IA, Ulasevitch SA. Research and Development of pH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite. Journal of Composites Science. 2025; 9(10):525. https://doi.org/10.3390/jcs9100525
Chicago/Turabian StyleChernikova, Elina E., Maxim D. Zagvozkin, Aleksander A. Buzaev, Irina A. Kurzina, and Svetlana A. Ulasevitch. 2025. "Research and Development of pH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite" Journal of Composites Science 9, no. 10: 525. https://doi.org/10.3390/jcs9100525
APA StyleChernikova, E. E., Zagvozkin, M. D., Buzaev, A. A., Kurzina, I. A., & Ulasevitch, S. A. (2025). Research and Development of pH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite. Journal of Composites Science, 9(10), 525. https://doi.org/10.3390/jcs9100525








