Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterisation of the Biocomposite
2.2. Experimental Study and Adsorption Isotherm Models
2.2.1. Langmuir Isothermal Model
2.2.2. Freundlich Isothermal Model
2.2.3. Dubinin–Radushkevich Isothermal Model
2.3. Kinetic Models of Adsorption
2.3.1. Pseudo-First-Order Kinetic Model
2.3.2. Pseudo-Second-Order Kinetic Model
2.3.3. Elovich Kinetic Model
2.3.4. Intraparticle Diffusion Kinetics Model
2.4. Thermodynamic Study
3. Results and Discussion
3.1. SEM Analysis
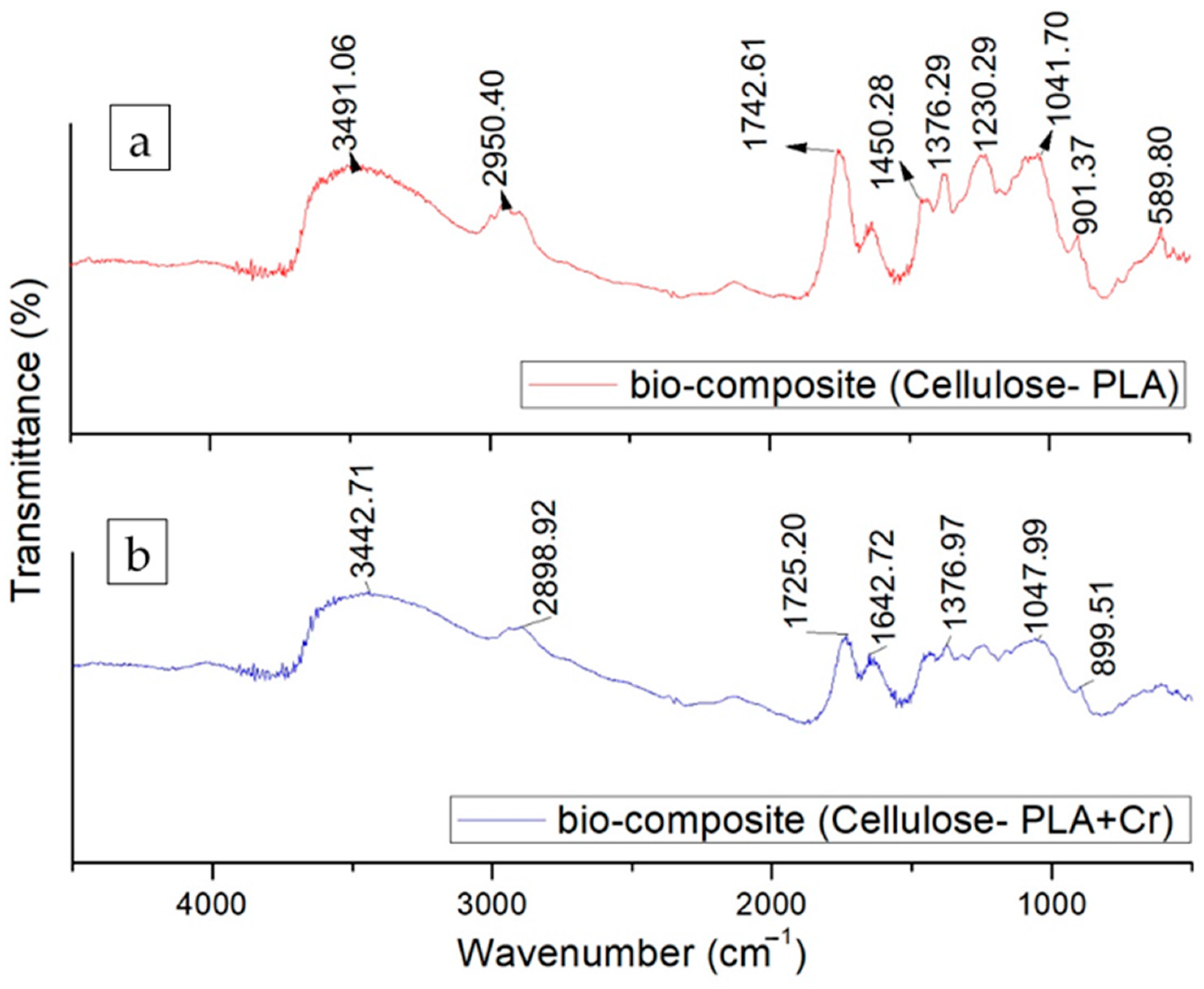
3.2. FTIR Analysis
3.3. Adsorption Kinetics Analysis
3.4. Analysis of the Adsorption Isotherms
3.5. Effect of Temperature Variations According to Thermodynamic Study
3.6. Overview of Multi-Application Biocomposites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Parameter | Unit | Description |
| Quantity adsorbed at any time, t | ||
| Quantity adsorbed at equilibrium | ||
| Pseudo-first-order rate constant of the adsorption process | ||
| b | Langmuir parameter related to the affinity of the binding sites for the pollutant | |
| Concentration of pollutant in solution at equilibrium | ||
| Maximum amount of solute in the solid phase | ||
| ((mg/g) (Lm/g) n) | Freundlich constant indicating adsorption capacity | |
| n | - | Effect of initial concentration on adsorption capacity |
| - | Dubinin–Radushkevich constant related to the amount of adsorbed gas | |
| Dubinin–Radushkevich constant related to the heat of sorption | ||
| Polanyi potential as a function of temperature | ||
| Ideal gas constant | ||
| Temperature | ||
| Pseudo-second-order rate constant of the adsorption process | ||
| Initial adsorption rate | ||
| Activation energy for chemisorption | ||
| Intraparticle diffusion rate constant | ||
| c | Thickness of the boundary layer | |
| Equilibrium constant | ||
| Equilibrium concentration |
References
- Priya, A.K.; Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Gaddam, M.K.R.; Velusamy, P.R.G.; Ravindiran, G.; Gurugubelli, T.R.; Muniasamy, S.K. Impact of climate change and anthropogenic activities on aquatic ecosystem—A review. Environ. Res. 2023, 238, 117233. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Golnaz, H. Water Pollutants and Approaches for Their Removal. Mater. Chem. Horiz. 2023, 2, 139–153. [Google Scholar] [CrossRef]
- Georgaki, M.N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Sahoo, H.; Kisku, K.; Varadwaj, K.S.K.; Acharya, P.; Naik, U.C. Mechanism of Cr(VI) reduction by an indigenous Rhizobium pusense CR02 isolated from chromite mining quarry water (CMQW) at Sukinda Valley, India. Environ. Sci. Pollut. Res. 2022, 30, 3490–3511. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Mishra, A.; Singh, R.; Thakur, I.S. Fabrication of calcite based biocomposites for catalytic removal of heavy metals from electroplating industrial effluent. Environ. Technol. Innov. 2021, 21, 101278. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.; Niu, Y.; Xu, D.; Wang, J.; Han, J.; Wang, H. Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation. Sep. Purif. Technol. 2022, 290, 120905. [Google Scholar] [CrossRef]
- Musah, M.; Yisa, J.; Suleiman, M.T.; Abdullahi, M.; Shaba, E.Y. Study of isotherm models for the adsorption of cr (vi) ion from aqueous solution onto Bombax buonopozense calyx activated carbon. Lapai J. Sci. Technol. 2018, 4, 22–32. [Google Scholar]
- Musah, M.; Azeh, Y.; Mathew, J.; Umar, M.; Abdulhamid, Z.; Muhammad, A. Adsorption Kinetics and Isotherm Models: A Review. Caliphate J. Sci. Technol. 2022, 4, 20–26. [Google Scholar] [CrossRef]
- Alaqarbeh, M. Adsorption Phenomena: Definition, Mechanisms, and Adsorption Types: Short Review. RHAZES Green Appl. Chem. 2021, 13, 43–51. [Google Scholar] [CrossRef]
- Abdul Rahim, A.R.; Mohsin, H.M.; Thanabalan, M.; Rabat, N.E.; Saman, N.; Mat, H.; Johari, K. Effective carbonaceous desiccated coconut waste adsorbent for application of heavy metal uptakes by adsorption: Equilibrium, kinetic and thermodynamics analysis. Biomass Bioenergy 2020, 142, 105805. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Paz, I.; Acevedo-Correa, D.; Espinosa-Fortich, M.; López-Badel, C. Adsorption of chrome (VI) and mercury (II) in solution using hyacinth (Eichhornia crassipes). Biotecnol. Sect. Agropecu. Agroindustrial 2021, 19, 54–65. [Google Scholar] [CrossRef]
- Manu, T.; Nazmi, A.R.; Shahri, B.; Emerson, N.; Huber, T. Biocomposites: A review of materials and perception. Mater. Today Commun. 2022, 31, 103308. [Google Scholar] [CrossRef]
- Awad, S.A.; Jawaid, M.; Ismail, A.S.; Khalaf, E.M.; Abu-Jdayil, B. Dimension stability, tensile and thermomechanical properties of bamboo/oil palm fibre reinforced bio-epoxy hybrid biocomposites. J. Mater. Res. Technol. 2024, 30, 7440–7446. [Google Scholar] [CrossRef]
- Kumar, S.; Saha, A. Utilization of coconut shell biomass residue to develop sustainable biocomposites and characterize the physical, mechanical, thermal, and water absorption properties. Biomass Convers. Biorefinery 2022, 14, 12815–12831. [Google Scholar] [CrossRef]
- Muniandy, S.K.; Sapuan, S.M.; Ilyas, R.A.; Faisal, S.; Azmi, A. Sugar Palm Lignocellulosic Fiber Reinforced Polymer Composite: A Review. J. Fibers Polym. Compos. 2022, 1, 1–19. [Google Scholar] [CrossRef]
- Ding, W.D.; Pervaiz, M.; Sain, M. Cellulose-Enabled Polylactic Acid (PLA) Nanocomposites: Recent Developments and Emerging Trends. In Functional Biopolymers; Springer Series on Polymer and Composite Materials; Thakur, V., Thakur, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 183–216. [Google Scholar]
- Zhou, L.; Ke, K.; Yang, M.B.; Yang, W. Recent progress on chemical modification of cellulose for high mechanical-performance Poly(lactic acid)/Cellulose composite: A review. Compos. Commun. 2021, 23, 100548. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M.K.; Singh, H. PLA based biocomposites for sustainable products: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 382–395. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Mahmood, Z.; Kausar, A.; Yakout, S.M.; Shair, O.H.; Iqbal, M. Biocomposites of polypyrrole, polyaniline and sodium alginate with cellulosic biomass: Adsorption-desorption, kinetics and thermodynamic studies for the removal of 2,4-dichlorophenol. Int. J. Biol. Macromol. 2020, 153, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rashid, T.U.; Debnath, T.; Haque, P.; Rahman, M.M. Application of Chitosan-Clay Biocomposite Beads for Removal of Heavy Metal and Dye from Industrial Effluent. J. Compos. Sci. 2020, 4, 16. [Google Scholar] [CrossRef]
- Li, X.; Qiu, X.; Yang, X.; Zhou, P.; Guo, Q.; Zhang, X. Multi-Modal Melt-Processing of Birefringent Cellulosic Materials for Eco-Friendly Anti-Counterfeiting. Adv. Mater. 2024, 36, 2407170. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Ortíz, Á.; Ortega-Toro, R.; Pedroza-Hernández, J. Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue. J. Compos. Sci. 2024, 8, 234. [Google Scholar] [CrossRef]
- Farch, S.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Zamouche, M.; Amrane, A.; Zhang, J.; Hadadi, A.; et al. Application of Walnut Shell Biowaste as an Inexpensive Adsorbent for Methylene Blue Dye: Isotherms, Kinetics, Thermodynamics, and Modeling. Separations 2023, 10, 60. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023, 49, 16199–16215. [Google Scholar] [CrossRef]
- Amrutha, J.G.; Girish, C.R.; Prabhu, B.; Mayer, K. Multi-component Adsorption Isotherms: Review and Modeling Studies. Environ. Process. 2023, 10, 1–52. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Kamińska, A.; Niedoba, O.; Michalkiewicz, B. CO2 Adsorption on Activated Carbons Prepared from Molasses: A Comparison of Two and Three Parametric Models. Materials 2021, 14, 7458. [Google Scholar] [CrossRef]
- Villabona-Ortíz, A.; González-Delgado, Á.D.; Tejada-Tovar, C. Evaluation of Kinetic, Equilibrium and Thermodynamics of Cationic Ion Using Agro-Industrial Residues of Plantain (Musa paradisiaca). Water 2022, 14, 1383. [Google Scholar] [CrossRef]
- Mouhamadou, S.; Dalhatou, S.; Dobe, N.; Djakba, R.; Fasanya, O.O.; Bansod, N.D.; Fita, G.; Ngayam, C.H.; Tejeogue, J.P.N.; Harouna, M. Linear and Non-linear Modelling of Kinetics and Equilibrium Data for Cr(VI) Adsorption by Activated Carbon Prepared from Piliostigma reticulatum. Chem. Africa 2023, 6, 719–731. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Lee, S.Y.; Mafize, A.A.; Kahar, N.A.M.A.; Johari, K.; Rabat, N.E. Efficient Removal of Pb(II) from Aqueous Solutions by Using Oil Palm Bio-Waste/MWCNTs Reinforced PVA Hydrogel Composites: Kinetic, Isotherm and Thermodynamic Modeling. Polymers 2020, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Kamaludin, N.H.I.; Ismail, H.; Rusli, A.; Ting, S.S. Thermal behavior and water absorption kinetics of polylactic acid/chitosan biocomposites. Iran. Polym. J. 2021, 30, 135–147. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-printed monolithic biofilters based on a polylactic acid (PLA)–hydroxyapatite (HAp) composite for heavy metal removal from an aqueous medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef] [PubMed]
- Hsini, A.; Essekri, A.; Aarab, N.; Laabd, M.; Ait Addi, A.; Lakhmiri, R.; Albourine, A. Elaboration of novel polyaniline@Almond shell biocomposite for effective removal of hexavalent chromium ions and Orange G dye from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 15245–15258. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Adekola, F.A.; Odebunmi, E.O.; Ogunlaja, A.S.; Bello, O.S. Two–three parameters isotherm modeling, kinetics with statistical validity, desorption and thermodynamic studies of adsorption of Cu(II) ions onto zerovalent iron nanoparticles. Sci. Rep. 2021, 11, 16454. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.S.; Gomathi, T.; Sudha, P.N.; Deepa, M.; Rambabu, K.; Banat, F. Biosilica/Silk Fibroin/Polyurethane biocomposite for toxic heavy metals removal from aqueous streams. Environ. Technol. Innov. 2022, 28, 102741. [Google Scholar] [CrossRef]
- Aryee, A.A.; Dovi, E.; Li, Q.; Han, R.; Li, Z.; Qu, L. Magnetic biocomposite based on peanut husk for adsorption of hexavalent chromium, Congo red and phosphate from solution: Characterization, kinetics, equilibrium, mechanism and antibacterial studies. Chemosphere 2022, 287, 132030. [Google Scholar] [CrossRef]
- Shekhawat, A.; Jugade, R.; Gomase, V.; Kahu, S.; Dhandayutham, S.; Pandey, S. Adsorptive Removal of As(III) by Cellulose-Sn(IV) Biocomposite. J. Compos. Sci. 2023, 7, 19. [Google Scholar] [CrossRef]
- Manyatshe, A.; Cele, Z.E.D.; Balogun, M.O.; Nkambule, T.T.I.; Msagati, T.A.M. Chitosan modified sugarcane bagasse biochar for the adsorption of inorganic phosphate ions from aqueous solution. J. Environ. Chem. Eng. 2022, 10, 108243. [Google Scholar] [CrossRef]
- Imgharn, A.; Ighnih, H.; Hsini, A.; Naciri, Y.; Laabd, M.; Kabli, H.; Elamine, M.; Lakhmiri, R.; Souhail, B.; Albourine, A. Synthesis and characterization of polyaniline-based biocomposites and their application for effective removal of Orange G dye using adsorption in dynamic regime. Chem. Phys. Lett. 2021, 778, 138811. [Google Scholar] [CrossRef]


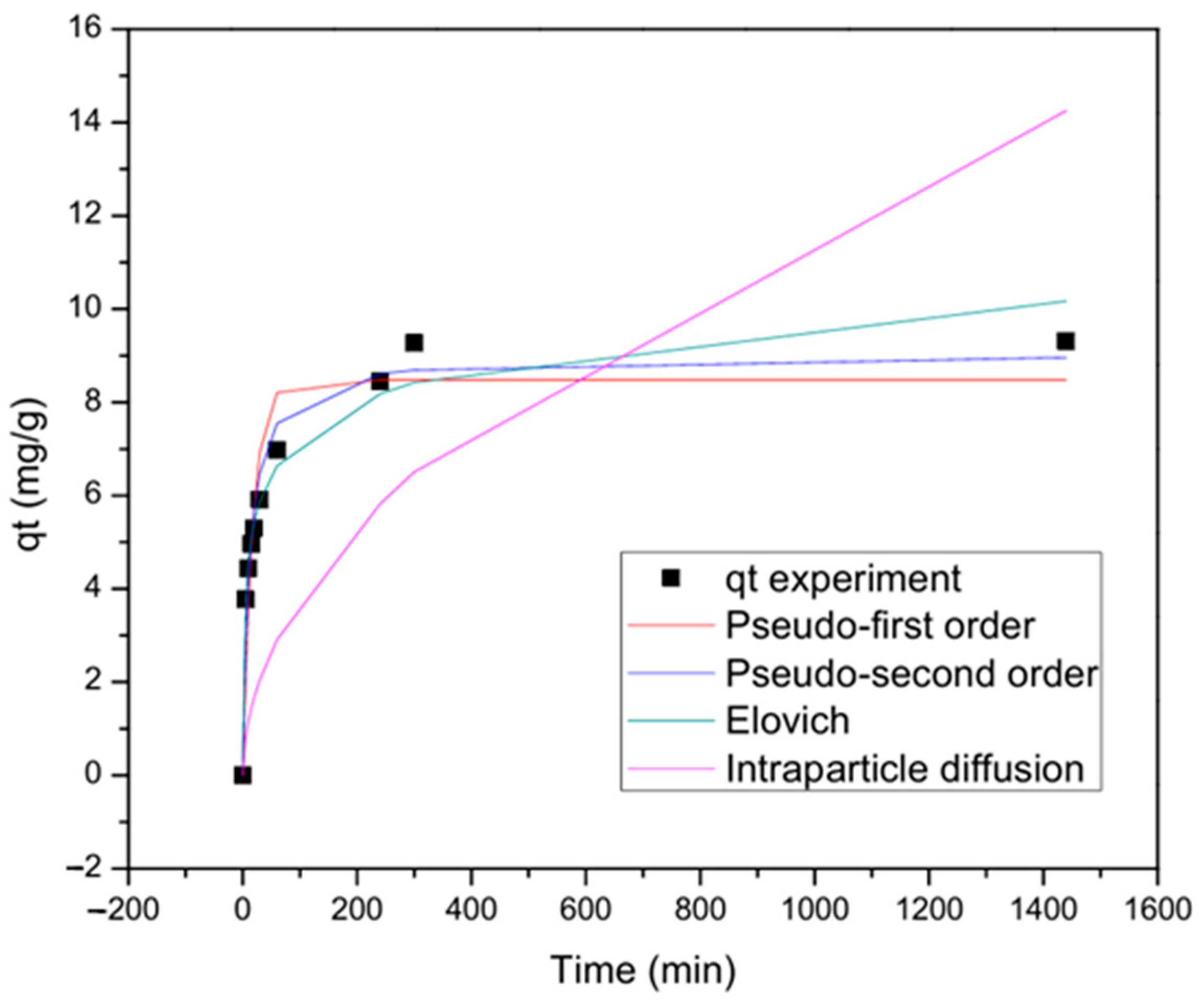
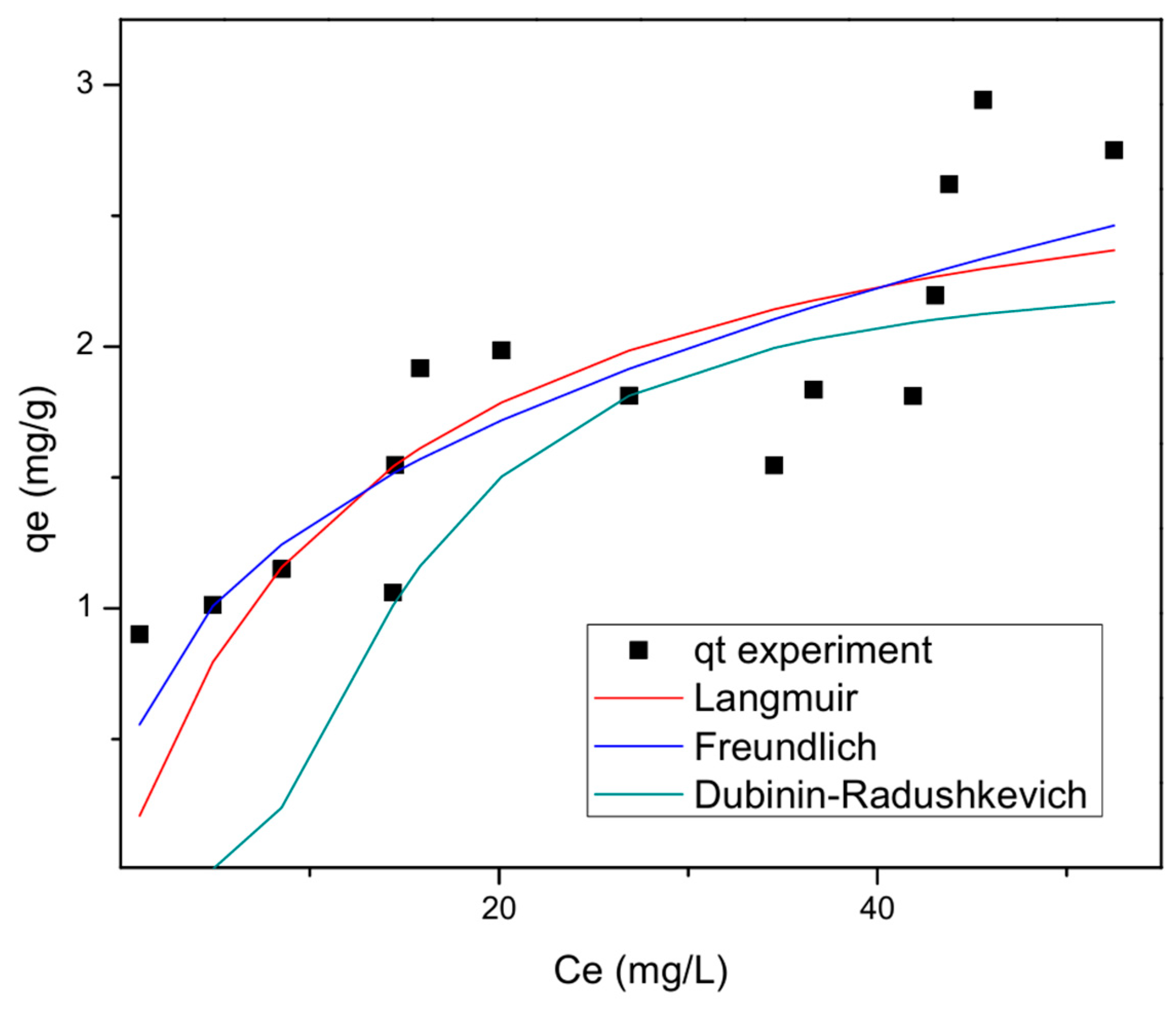
| Model | Parameter | Non-Linear Fitting |
|---|---|---|
| Pseudo-first-order | qe | 8.483 |
| k1 | 0.056 | |
| R2 | 0.885 | |
| Squared error | 7.449 | |
| Pseudo-second-order | qe | 9.031 |
| k2 | 0.009 | |
| R2 | 0.961 | |
| Squared error | 2.541 | |
| Elovich | 0.899 | |
| 7.241 | ||
| R2 | 0.973 | |
| Squared error | 1.751 | |
| Intraparticle diffusion | k3 | 0.375 |
| R2 | 0.566 | |
| Squared error | 114.85 |
| Calculated Thermodynamic Parameter | Temperature (K) | ||
|---|---|---|---|
| 295.15 | 299.15 | 301.15 | |
| (kJ/mol) | −9.007 | −9.007 | −9.007 |
| (kJ/mol·K) | −0.080 | −0.080 | −0.080 |
| (kJ/mol) | 14.578 | 14.897 | 15.057 |
| Biocomposite | Removed Pollutant | Adsorbent Dose (g) | Pollutant Concentration (mg/L) | Adsorption Capacity (mg/g) | Source |
|---|---|---|---|---|---|
| Polyhydroxy butyrate (PHB)-modified coconut husk | Methylene blue | 0.01 | 40 | 35.98 | [25] |
| Polyethyleneimine-modified magnetic peanut shells | Cr (VI) | 0.03 | 60 | 58.4 | [41] |
| Congo Red | 20 | 71.3 | |||
| Phosphate | 100 | 13.5 | |||
| Sn (IV)-modified cellulose | As (III) | 0.4 | 5 | 16.64 | [42] |
| Chitosan-modified sugarcane bagasse bio-coal | Phosphate | 0.05 | 20 | 37.2 | [43] |
| Polyaniline-modified almond biocomposite | Orange G | 0.075 | 10 | 8.92 | [44] |
| Polyaniline-modified rice husk biocomposite | 17.25 | ||||
| Banana pseudo-stem modified with polylactic acid (PLA) | Cr (VI) | 0.03 | 30, 65, 100 | 3.275 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortiz, Á.; Ortega-Toro, R. Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA). J. Compos. Sci. 2025, 9, 36. https://doi.org/10.3390/jcs9010036
Tejada-Tovar C, Villabona-Ortiz Á, Ortega-Toro R. Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA). Journal of Composites Science. 2025; 9(1):36. https://doi.org/10.3390/jcs9010036
Chicago/Turabian StyleTejada-Tovar, Candelaria, Ángel Villabona-Ortiz, and Rodrigo Ortega-Toro. 2025. "Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA)" Journal of Composites Science 9, no. 1: 36. https://doi.org/10.3390/jcs9010036
APA StyleTejada-Tovar, C., Villabona-Ortiz, Á., & Ortega-Toro, R. (2025). Kinetic, Isothermal and Thermodynamic Study on the Removal of Hexavalent Chromium with Biocomposites (Cellulose–PLA). Journal of Composites Science, 9(1), 36. https://doi.org/10.3390/jcs9010036











