Abstract
This study aimed to develop and characterize bio-nanocomposite coatings by incorporating titanium nanoparticles (TiO2 NPs) (30–50 nm) (10 mg/L), which have antimicrobial effects, and rosmarinic acid (RA) (0.005 mg/mL), which has strong antioxidant and antimicrobial activities, into the chitosan matrix using the solvent casting method. The prepared bio-nanocomposite coatings were characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM-EDX), and atomic force microscopy (AFM). In the XRD analysis, the crystal structure of the bio-nanocomposite coating material was evaluated, but the absence of the expected TiO2 NPs diffraction peak in the coating containing TiO2 NPs was discussed in detail. The TiO2 NPs decreased the crystallinity, compared to the control film, while rosmarinic acid increased the order of the molecular matrix. FT-IR analysis showed the presences of O–H, C=O, and C–O bonds in the coating materials, and the changes in the positions and intensities of the bands observed in the FTIR spectra of the bio-nanocomposite coatings (CHT and CHTRA) proved that TiO2 NPs and RA were successfully integrated into the chitosan matrix. The broadening and flattening of the bands belonging to OH groups (3288–3356 cm−1) indicated that the hydrogen bonds in the chitosan matrix were strengthened during the formation of the bio-nanocomposite structure. The bands representing the C=O stretching vibrations at 1659 cm−1 (amide I) and the N–H bending vibrations at 1558 cm−1 (amide II) indicated protein-based features in the structure of chitosan and confirmed the existence of the bio-nanocomposite structure. The SEM-EDX analysis showed that TiO2 NPs were distributed homogeneously on the chitosan surface, but there was aggregation in places. The AFM images revealed that when TiO2 NPs and RA were added to the chitosan matrix, the surface topography became more homogeneous, and a topographic pattern was formed in the range of 0–20.4 nm. Therefore, it is concluded that these bio-nanocomposite coatings can be used in antimicrobial surfaces and food packaging areas and should be optimized with different antioxidant and nanoparticle combinations in the future.
1. Introduction
Bio-nanocomposites are polymers consisting of a biopolymer matrix containing materials (nanoparticles) with a size of at least nanometers (100 nm or less) [1,2]. Bio-nanocomposites are materials with properties such as low cost, flexibility, biodegradability, and environmental friendliness, and their properties can be further improved using reinforcing components [1]. Bio-nanocomposites are a new class of materials developed using nanoparticles [3,4]. Although biopolymers have disadvantages, such as poor mechanical and barrier properties [5,6], they are gaining importance, due to the increasing number of environmentally conscious consumers, rising oil prices, and global warming [7]. Nanoparticles are materials with at least one dimension smaller than 100 nm that exhibit unique properties (physical and chemical) thanks to their high surface/volume ratio [1]. These properties may vary depending on the size, shape, and chemical structure of the particle [2,3]. Although some nanoparticles have a high aspect ratio and surface area, these properties may not be valid for all nanoparticles. Nanoparticles with a high surface area and nanosize can significantly contribute to the mechanical, optical, and chemical properties of nanocomposite materials [6]. The incorporation of suitable nanomaterials can improve the mechanical and thermal performances of packaging while at the same time preventing microbial growth [8].
Chitosan is a biodegradable and biocompatible biopolymer [9,10]. Chitosan is a high-molecular-weight cationic polysaccharide [11] obtained from the deacetylation of chitin and is found in biological sources (crustaceans, fungi, insects, etc.) [12,13,14]. It exhibits antimicrobial activity, selectively permeates gases, is non-toxic, has excellent film-forming properties, and possesses adjustable chemical and physical properties [15,16,17]. Because of these properties, it is often used in food packaging [15,16]. These properties make chitosan a promising material for food packaging [11]. It has been reported that the antimicrobial properties of chitosan are due to its interaction with the cell membranes of certain target microorganisms and disruption of membrane integrity. It has also been found that the antimicrobial activity of chitosan is highly dependent on the target microorganism and the molecular weight of chitosan [18]. What makes chitosan biodegradable is the presence of hydrolase enzymes that are widespread in nature and the enzymatic degradation of chitosan [19]. This makes chitosan both antimicrobial and also biodegradable in the environment, suggesting that these two properties are not mutually exclusive. The limited mechanical strength and the inadequate barrier properties of pure chitosan underline the need for the further development of this polymer [20]. To overcome these problems, bio-nanocomposites have been developed by incorporating various nanoparticles and biological agents into a chitosan matrix [21,22]. Nanocomposite coatings obtained by adding chitosan coatings, which are produced from various nanoparticles, are used to maintain product quality and extend shelf life [19,23].
Metals and metal oxide nanoparticles (such as copper oxide, silver, zinc oxide, and titanium dioxide) can be used in the preparation of biopolymeric packaging, owing to their stability and antimicrobial properties [22].
Titanium dioxide nanoparticles (TiO2 NPs) are a metal oxide with high antimicrobial activity, antifungal, and chemically stable, low toxicity and high biocompatibility [24]. TiO2 is a remarkable material because of itschemical stability, low toxicity, low cost, and potential use in packaging [21,22]. Although there are studies in literature in which nanocomposite or bio-nanocomposite films are developed by adding TiO2 nanoparticles [16,22,25,26,27], there are few studies on nanocomposite films and coatings prepared by adding TiO2 nanoparticles to a chitosan matrix [21,28,29]. However, these studies have focused only on the effects of TiO2, and there is little information on the integration of alternative biological agents for the versatile development of bio-nanocomposite matrices.
Rosmarinic acid is a naturally water-soluble phenolic compound with numerous biological activities, such as antiviral, antibacterial, antioxidant, and anti-inflammatory activities [30,31], and studies have shown that it can be employed to produce nanocomposite films [16,20]. Abdollahi et al. [20] reported that integrating rosmarinic acid and nanoclay into bio-nanocomposite films significantly improved the tensile strength and elongation of the films. However, there is no study on the incorporation of RA and TiO2 NPs into chitosan matrix.
The aim of this study was to combine TiO2 NPs and RA in a chitosan matrix and characterize the bio-nanocomposite coatings using XRD, FT-IR, SEM-EDX, and AFM methods.
2. Materials and Methods
2.1. Materials
Chitosan (medium molecular weight, 75–85% deacetylated), glacial acetic acid (99.8%), glycerol (99.5%), and rosmarinic acid (≥98%, from Rosemarinus officinalis L.) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). TiO2 NPs (30–50 nm) were purchased from a commercial product company.
2.2. Methods
2.2.1. Preparation of the Coating and Nanocomposite Coatings
In this study, three different coating materials, shown in Table 1, were prepared using a solution casting method and a modification of the method of Nowzari et al. [32] and Al-Naamani et al. [33]. During the preparation of the coating solutions, the concentrations of TiO2 NPs and RA were determined based on similar studies in literature. A concentration of 10 mg/L for TiO2 NPs was chosen to ensure a homogeneous distribution in the matrix while increasing the antimicrobial activity [21,22]. Likewise, a concentration of 0.005 mg/mL for RA represents the level at which the bioactive properties were optimized in previous studies [20,30]. These concentrations were preferred to ensure the homogeneous distribution of nanoparticles and biological molecules in the chitosan matrix and to maintain structural stability. Low concentrations were chosen to reduce the possible toxic effects of nanoparticles or biological agents on the coatings [16,34]. A 1% (v/v) acetic acid solution was used to dissolve chitosan and prepare the 2% chitosan solution. Then, it was stirred in a heated magnetic stirrer (DAIHAN, MSH-20, Wonju, Korea) at 50 °C for 24 h to complete the solubilization process. After 24 h of mixing, 1% (v/v) glycerol was added as a plasticizer. Then, the solution was stirred for 6 h. The TiO2 NPs (10 mg/L) (30–50 nm) and/or RA (0.005 mg/mL) were prepared using ultrasound (BANDELIN, Germany). These solutions were slowly added to the coating solutions at 24,000 rpm using an ultraturax (IKA T25, Staufen, Germany). (Total mixing time was 20 min.) After this treatment, these solutions were sonicated in an ultrasonic bath (Bandelin electronic GmbH & Co. KG, Berlin, Germany) at a frequency of 35 kHz for 20 min. The bio-nanocomposite coating solutions were then treated with UV light (15 W/m2 = 365 nm) for 10 min [21].

Table 1.
Different coating material combinations.
The composite and bio-nanocomposite coatings were poured into petri dishes. Then, they were dried at room temperature for 48 h. The filmed images are shown in Figure 1. The coatings (CH, CHT, and CHTRA) were characterized using XRD, FT-IR, SEM-EDX, and AFM.

Figure 1.
Film-formed CH and bio-nanocomposite coatings.
2.2.2. X-Ray Diffraction (XRD) Analysis
XRD analysis is widely used to determine the crystal structure, lattice parameters, grain size, and crystal defects of materials [35]. The crystalline structure of the bio-nanocomposite coatings was characterized using XRD. The measurements were taken at 40 kV, 40 mA, 3 min−1 scan rates, and a 2θ angle. The XRD pattern was recorded in the range 5–60°. XRD images were obtained using an X-ray diffraction (XRD) instrument (XRD-Panalytical Empyrean, London, UK) [36].
2.2.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
The FT-IR analyses were performed to characterize the coating materials. The spectra of the samples that were prepared for characterization were recorded at a scanning speed of 4 cm−1, with wavelengths in the range of 400 cm−1–4000 cm−1. Thus, the FT-IR spectra of the bio-nanocomposite coatings were obtained.
2.2.4. Scanning Electron Microscopy—Energy Dispersive X-Ray Spectroscopy (SEM-EDX)
SEM analyses were performed to determine the morphological structure in detail to achieve the surface characterization of the composite and bio-nanocomposite coating samples. Before the measurement, the samples were coated using a 20 nm thick layer of gold and analyzed using the 300 Model Ziess SEM. The SEM-EDS analysis was performed using an SEM-EDX device (Sigma 300 Model Ziess, Oberkochen, Germany).
2.2.5. Atomic Force Microscopy (AFM) Analysis
AFM analysis was performed to identify the morphological structure in detail for the surface characterization of the coating samples. The analysis was performed using an AFM device (HITACHI 5100N, Tokyo, Japan).
3. Results and Discussion
3.1. X-Ray Diffraction (XRD) Analysis
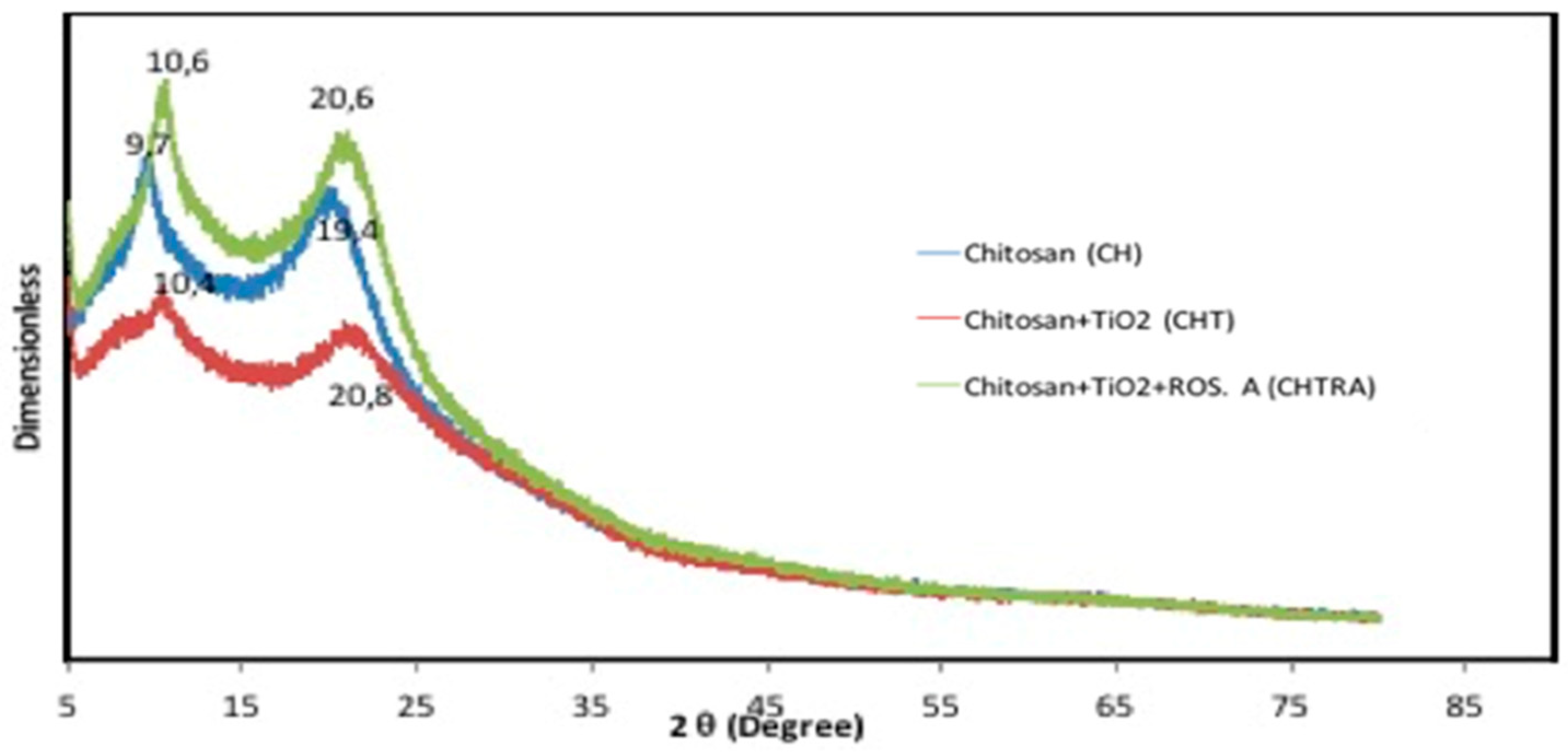
The crystalline structure of the bio-nanocomposite coatings was investigated by X-ray diffraction. The XRD patterns of bio-nanocomposite coatings (CHT and CHTRA) prepared with chitosan, TiO2 NPs, and RA are shown in Figure 2. Since TiO2 NPs were purchased from a company that sells commercial products, no XRD analysis was performed. As shown in Figure 2, the presence of two peaks indicates a semi-crystalline structure: 9.7° and 19.4° refer to the hydrated crystal structure and the amorphous state, respectively [37]. It was found that the crystallographic structure of the CH coating changed when TiO2 NPs and RA were used. In the CH coating pattern, a shift to lower angles (10.4° and 20.8°) was observed in the peaks of the bio-nanocomposite coating to which TiO2 NPs were added. All peaks were observed with a shift to higher angles in the bio-nanocomposite coating (CHTRA) to which TiO2 NPs and RA were added, and the peaks were determined to be 10.6° and 20.6°. This situation was described as a change in the broadening and decreasing intensity of the peak due to the strong interaction between the components in the bio-nanocomposite coating and the decrease in hydrogen bonds between the TiO2 NPs and the chitosan matrix.

Figure 2.
XRD graph of the chitosan coating (CH), chitosan/TiO2 (CHT), and the chitosan/TiO2/rosmarinic acid (CHTRA) bio-nanocomposite coatings.
The incorporation of TiO2 NPs decreased the crystallinity of the CH coating. On the other hand, the addition of RA increased the crystallinity of CH. The broad peak of CHT may be due to the cross-linking reaction between TiO2 NPs and chitosan. This may have disturbed the crystalline structure of the chitosan coating [38]. Furthermore, the peak associated with CHTRA was broader than the pure chitosan profile, indicating the formation of this nanocomposite structure [39]. The results showed that TiO2 NPs and RA were successfully attached to the chitosan matrix. It was observed that CHT and CHTRA can change the intermolecular structure of the coating [34]. In addition, XRD results showed that the addition of TiO2 NPs and RA to the chitosan coating caused changes in the crystalline structure of chitosan. However, it is noteworthy that the diffraction peaks of TiO2 nanoparticles were not evident in the coating spectrum. The reasons for this are thought to be that the TiO2 concentration (10 mg/L) was not high enough to create a detectable peak in XRD analysis [40] or that the TiO2 NPs were in the amorphous phase during the addition stage to the chitosan matrix.
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
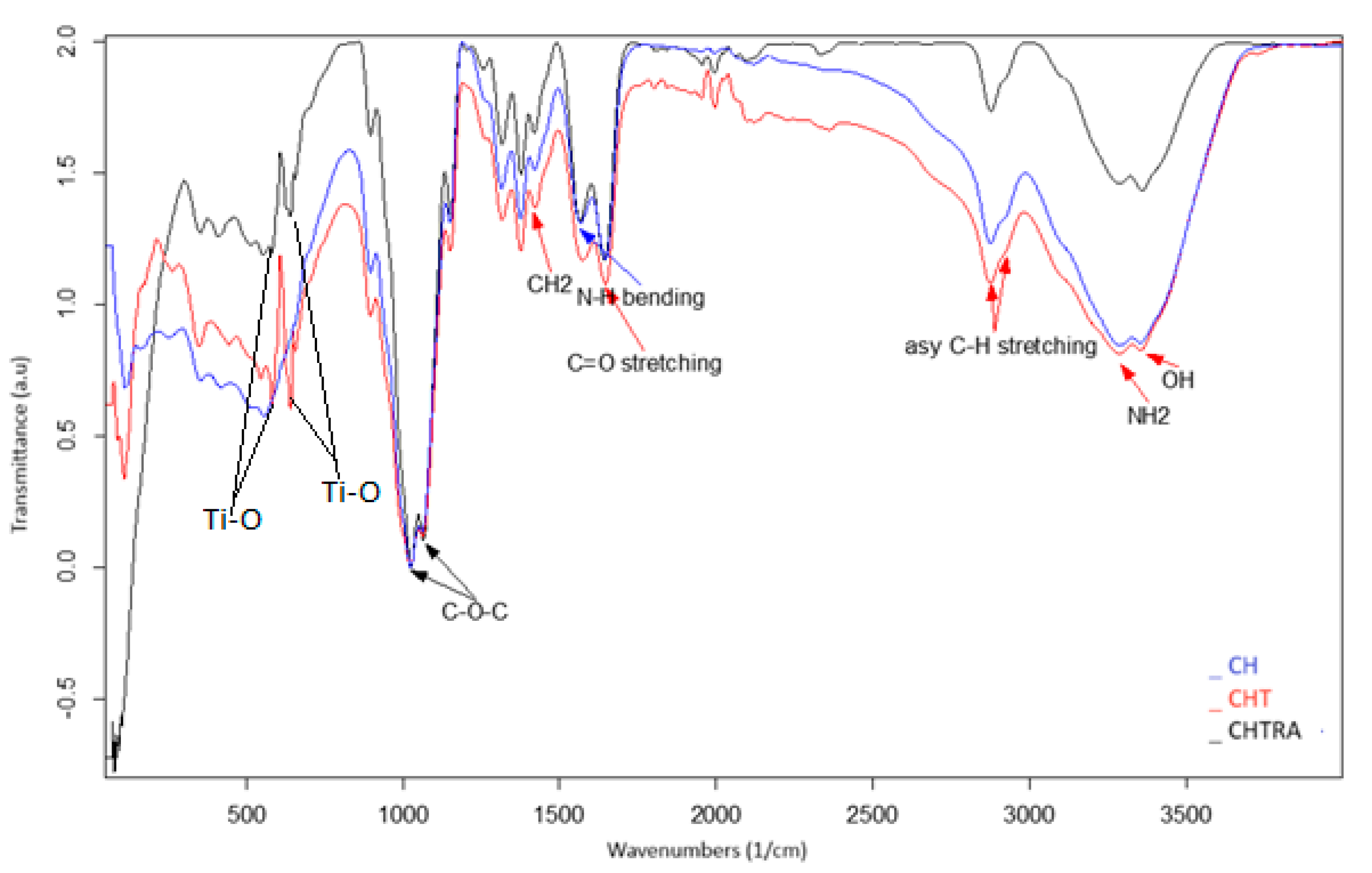
The interactions between CH, TiO2 NPs, and RA were determined using FT-IR spectroscopy. Although the spectra of chitosan (CH) and bio-nanocomposite coatings (CHT and CHTRA) showed similarities, there were some differences that were consistent with the results of other nanocomposite studies [34,37]. The FT-IR spectra of CH coatings, CHT, and CHTRA bio-nanocomposite coatings are shown in Figure 3. For CH coatings (Figure 3), the characteristic peaks were at about 3365 and 3284 cm−1, corresponding to the stretching vibrations of –OH and –NH2 groups, respectively. The bands at 2935 and 2847 cm−1 were attributed to the asymmetric stretching of C–H groups [41]. These strong bands became wider and flatter in the nanocomposite structures. Similar results were also obtained by Harudin et al. [42], who reported that chitosan was characterized by broad and dense bands at 3450–3200 cm−1 and that it had a C–H tension band (hydrogen-bonded OH stretching coincided with the N-H tension bands) at 2783 cm−1. In the FT-IR spectrum, Ti-O peaks in CHT and CHTRA bio-nanocomposite coatings were observed in the same range of 600–400 cm−1 in another study using TiO2 NPs [43]. As can be seen in Figure 3, the important bands in all coatings were approximately at 1659 cm−1 (amide I) (C=O stretching) and at 1558 cm−1 (N–H bending), which are strong bonds. Similar results were observed in a study conducted by Lin et al. [21] on chitosan-based nanocomposites. Luo et al. [44] reported that the amide I and amide II bands were shifted to 1635.52 and 1558.23 cm−1, respectively, due to electrostatic interaction. On the other hand, weak bonds were observed at 1419 cm−1 (CH2), 1393 cm−1 (CH3), 1236 cm−1 (NH2), and 1026 and 1061 cm−1 (C–O–C). The presence of the bands at 1026 cm−1 in all coating samples indicated a possible interaction between the plasticizer and the film. In her study on a chitosan/clay nanocomposite, Rahvalı [45] reported similar FT-IR/ATR spectra for the chitosan films as in the present study. The researcher found that the characteristic absorption peak at 3300 cm−1 was attributed to the hydroxyl group (–OH), and the peak at 2900 cm−1 belonged to the N–C stretching group. On the other hand, they reported that the peak in the 1650 cm−1 region corresponded to the amide group, and the peak in the 1400–1500 cm−1 region corresponded to the C–H bending vibrations. It was also reported that the peak in the 1400–1500 cm−1 region belonged to CH bending vibrations, the peak in the 1350 cm−1 region belonged to –CH3 and pyranose C=O–C, and the peak at 1100–900 cm−1 belonged to C–OH stretching–bending vibrations. It was also observed that the addition of clay at different rates increased the intensity of the characteristic peaks.

Figure 3.
FT-IR spectra of the chitosan coating and bio-nanocomposite coating (CH, CHT, CHTRA) groups.
The region showing carbohydrate fingerprints was reported to be in the range of 800 to 1200 cm−1 [46] with a sharp peak, possibly due to the vibration of the C–O–C bonds in the presence of chitosan at 1026 cm−1 [47]. The bands at 1200 and 1500 cm−1 were reported to correspond to the deformation vibrations of hydrogen atoms, i.e., groups such as CCH, HCH, COH, and HCO. Spectral analysis of carbohydrates showed that there were five frequency ranges, and the range between 3600 and 2800 cm−1 corresponded to the OH and CH flexure vibrations. These (–OH) vibrations were very strong and broad bands with strong, intramolecular, and intermolecular hydrogen bonds [48].
3.3. SEM-EDX Analysis
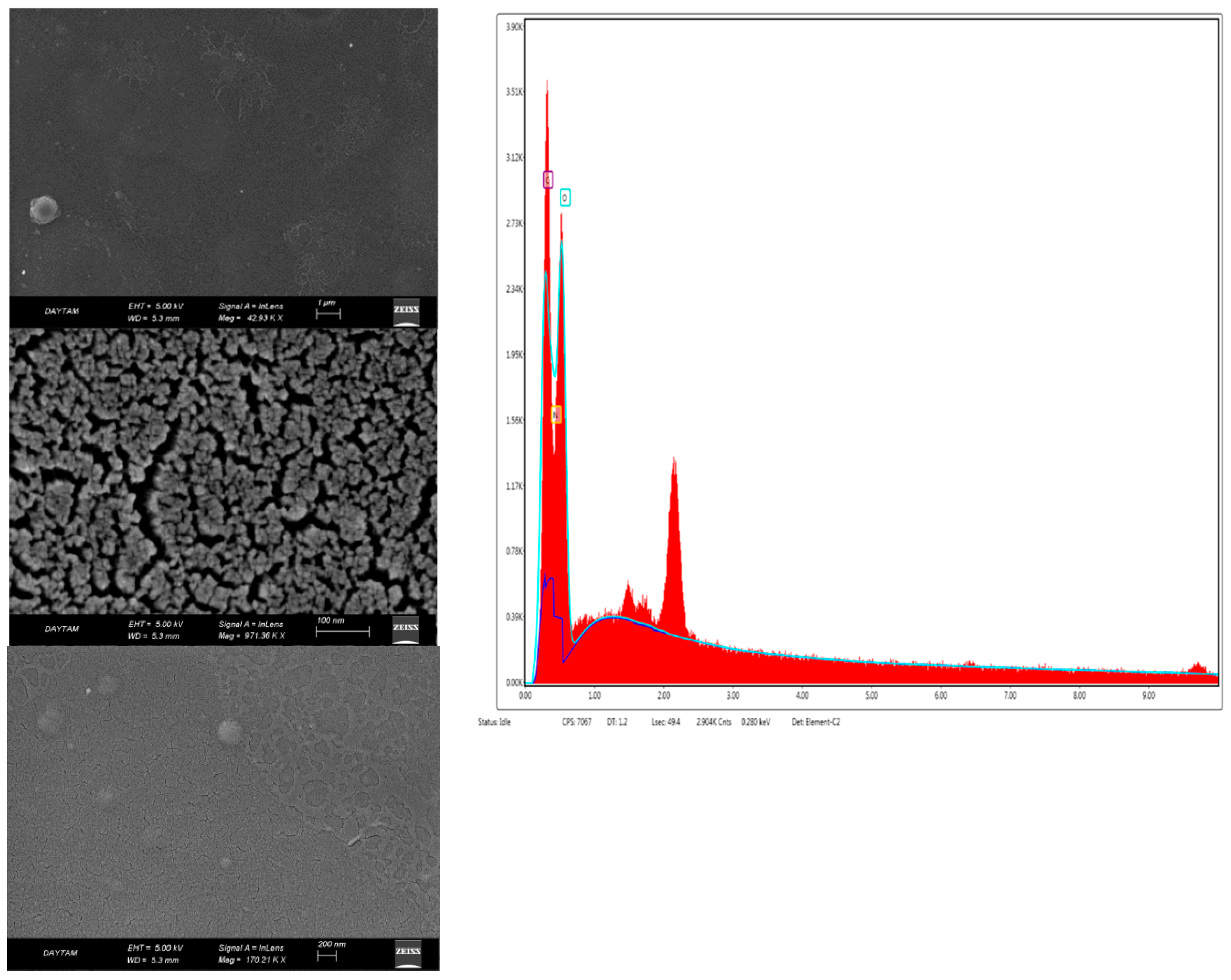
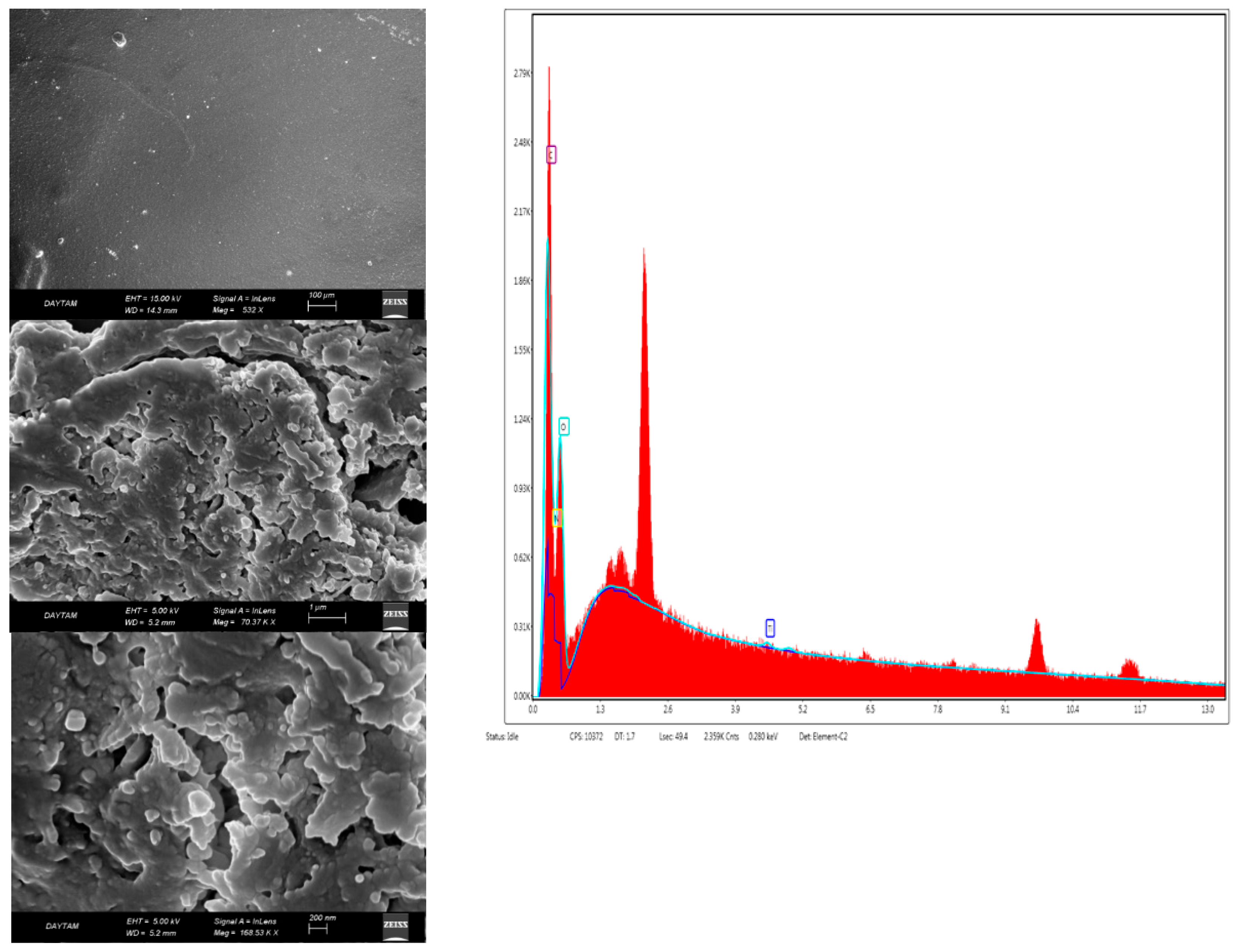
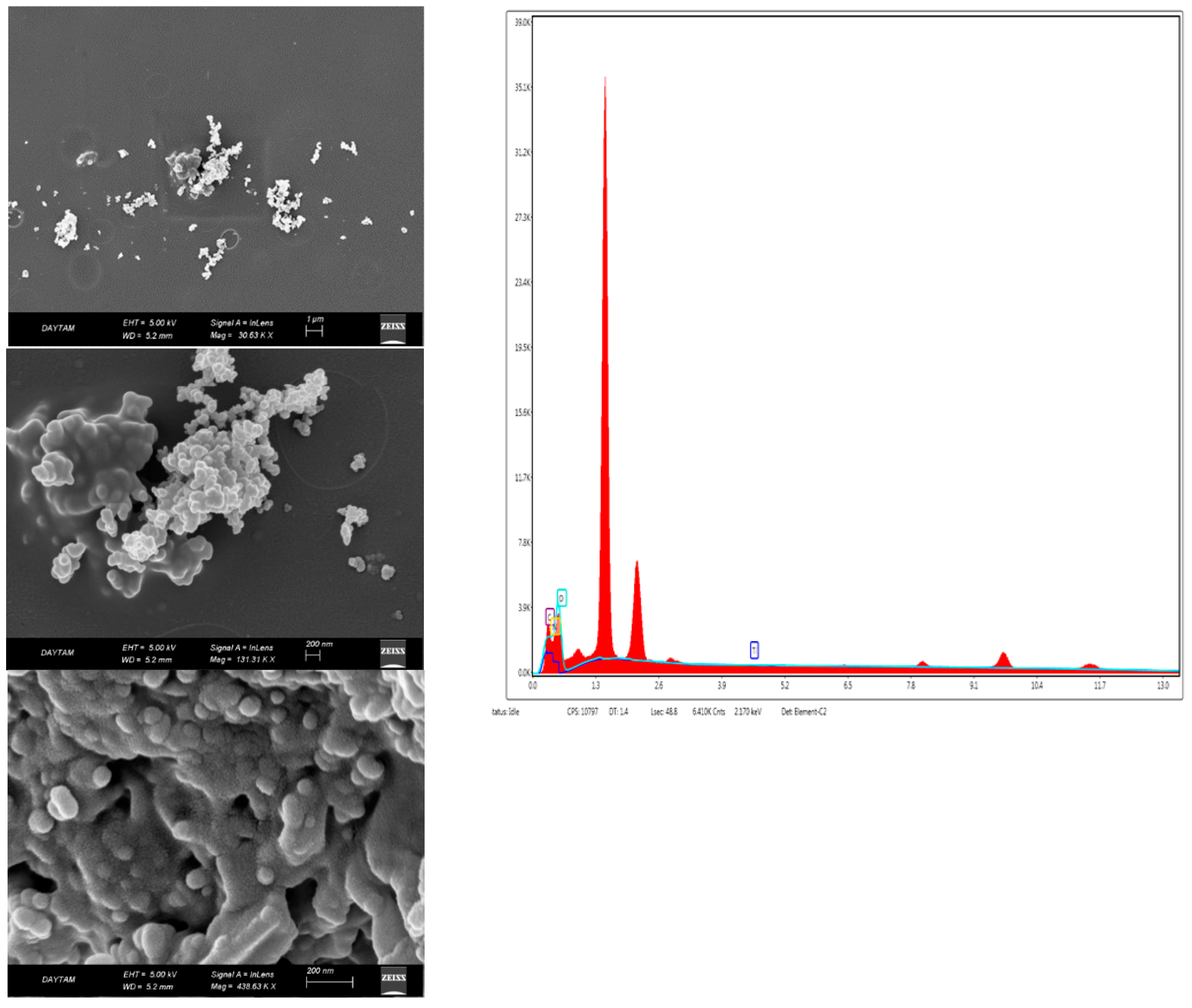
Scanning electron microscope images (SEM) provided information about the surface roughness of bio-nanocomposite coatings and the homogeneity of the coating surface. Figure 4 shows the morphology of the chitosan coating, and Figure 5 and Figure 6 show the morphology of nanocomposites in the scanning electron micrographs. The appearance of the tree-like fractal structure of the nanocomposite (Figure 5) structure showed similarity to those reported in previous studies [49]. The SEM images of nanostructured titanium dioxide dispersed on chitosan were also similar to those of other studies [50]. The morphological structure changed when TiO2 NPs and RA were added to chitosan. In addition, the irregular shape in the image of the bio-nanocomposite coating (Figure 5) revealed the presence of aggregates of titanium dioxide on chitosan. The high magnification EDXs (Figure 5 and Figure 6) confirmed that the aggregates belonged to the TiO2 NPs and that the dimensions of the nanoparticles were between 50 nm and 100 nm. The micrographs showed that the nanoparticles were well distributed on the chitosan surface but formed agglomerates. This was mainly due to the attractive forces between the nanoparticles. The results confirmed the formation of the chitosan/TiO2 bio-nanocomposite prepared by the solvent casting method. Due to the aggregation and dispersion of the TiO2 NPs in the TiO2 NPs and RA bio-nanocomposite coatings, the surfaces had a rough and bumpy appearance. This was in agreement with the AFM images. According to the investigation by Xing et al. [40], the SEM image showed that the TiO2 NPs were agglomerated in the chitosan-based coating films.

Figure 4.
SEM-EDX photographs of the chitosan coating (CH).

Figure 5.
SEM-EDX photographs of the chitosan + TiO2 NPs bio-nanocomposite coating (CHT).

Figure 6.
SEM photographs of the chitosan + TiO2 NPs + RA bio-nanocomposite coating (CHTRA).
3.4. Atomic Force Microscopy (AFM) Analysis
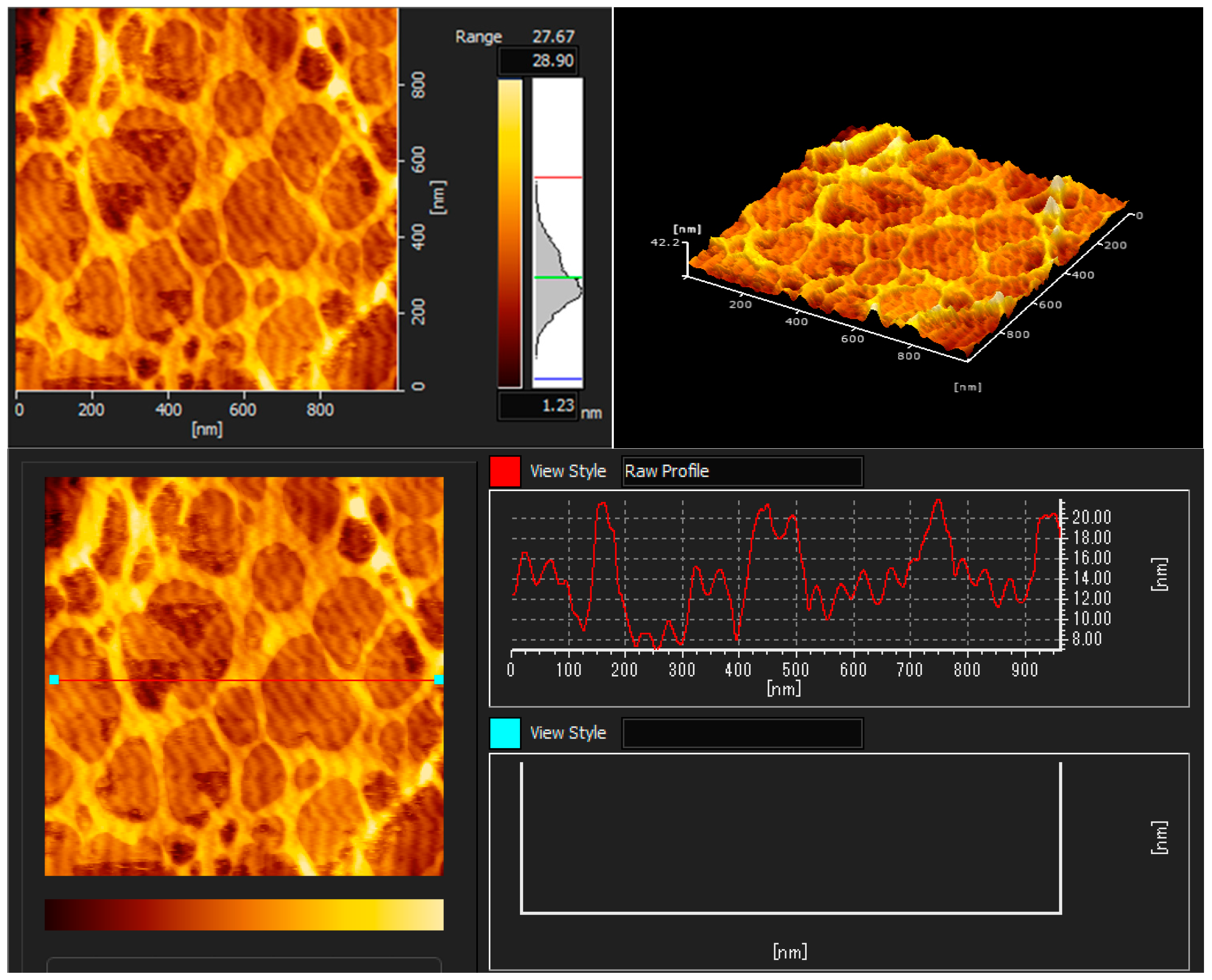
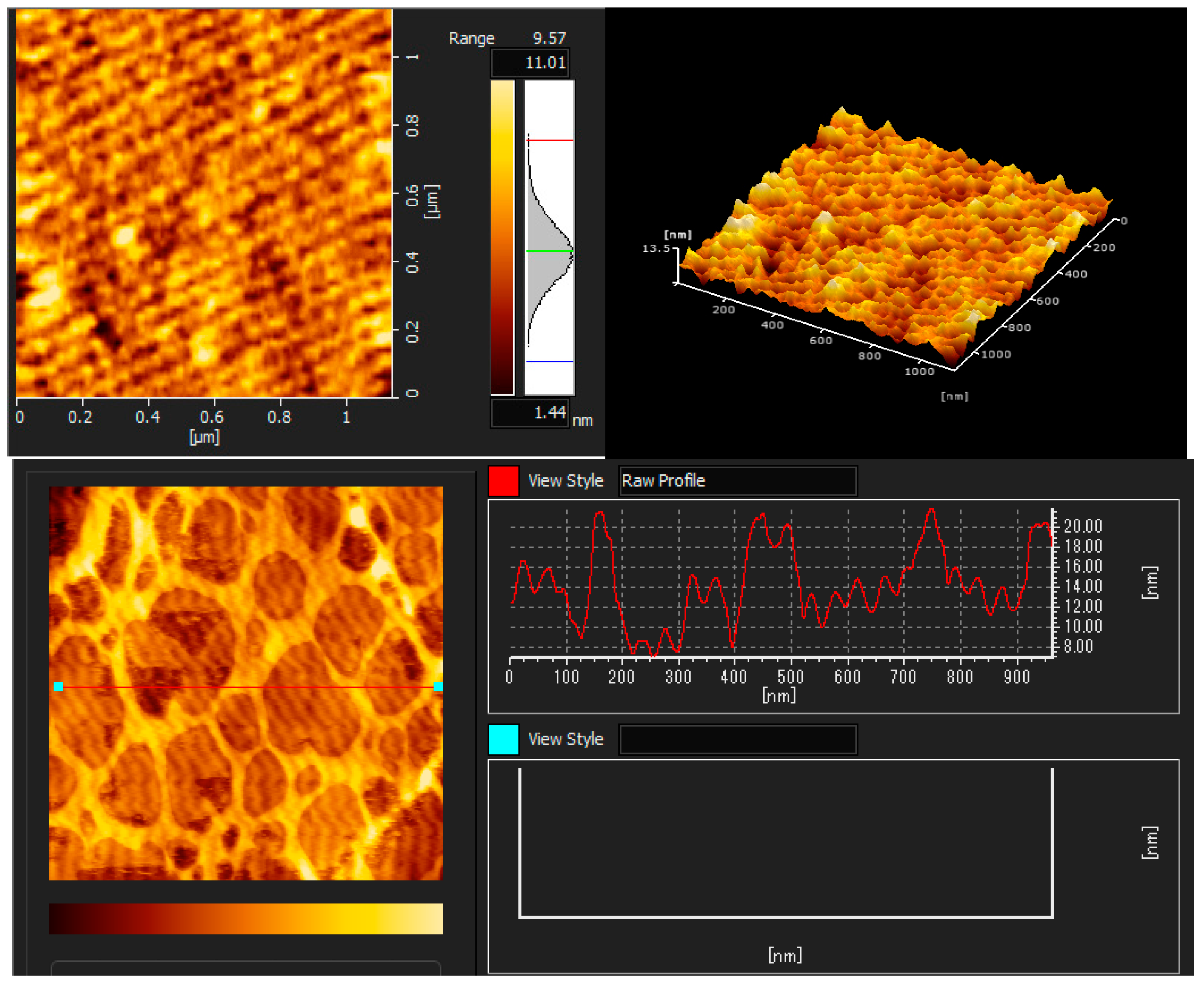
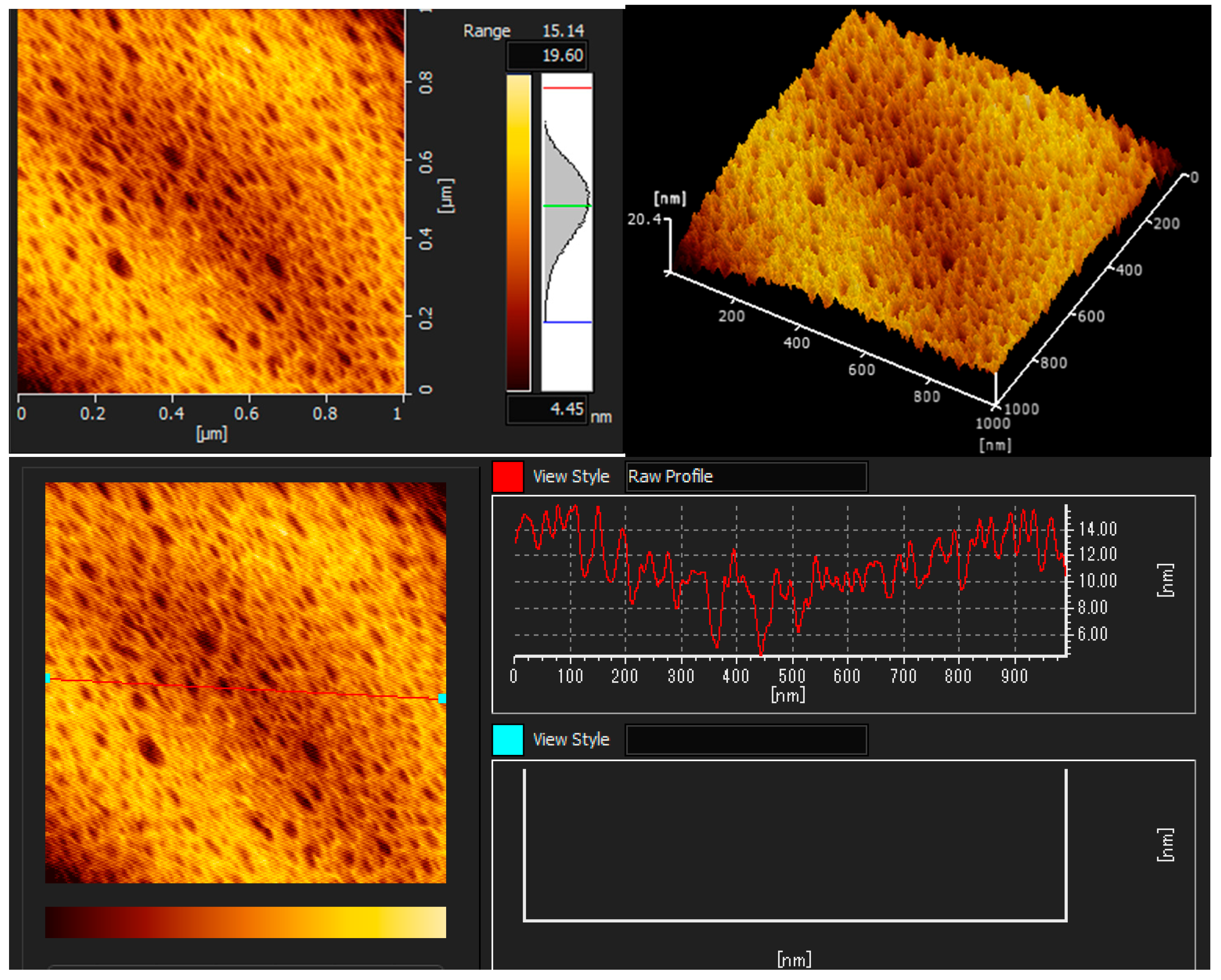
The AFM images illustrated the coatings’ topography, as shown in Figure 3, Figure 4 and Figure 5. The topographic structure can be detected by various parameters that exist to quantify a surface’s root mean square (RMS) roughness. The RMS roughness value can be calculated from the cross-sectional profile or a surface area [51]. The RMS roughness acquired by CH, CHT, and CHTRA composite and bio-nanocomposite coatings were 42.2 nm, 13.5 nm, and 20.4 nm, respectively. As shown in Figure 7, the pure chitosan coating was non-homogeneous. Figure 8 and Figure 9 show that the TiO2 and rosmarinic acid particles in the nanocomposite coatings formed a homogeneous surface. Figure 7 shows the pure chitosan coating, Figure 8 shows the nanoparticles embedded in the chitosan matrix, and Figure 8 shows the three-dimensional views of the chitosan-TiO2 nanocomposites. The nanoscale topography of the chitosan nanocomposite coating revealed a surface with irregular pores and the formation of almost a hill-valley structure. The heights of the topographic patterns of the nanoparticles ranged from 0 to 20.4 nm.

Figure 7.
AFM images of the chitosan coating (CH).

Figure 8.
AFM images of the chitosan + TiO2 NPs bio-nanocomposite coating (CHT).

Figure 9.
AFM images of the chitosan + TiO2 NPs+ RA bio-nanocomposite coating (CHTRA).
The three-dimensional (3D) images clearly showed that the number and height of the peaks on the chitosan nanocomposite surfaces were greater and higher, compared to those on the chitosan coating surface. Studies have shown that various physico-chemical properties of films, such as the adsorption, wettability, friction, or electrical contact properties, vary depending on the surface morphology of films [52]. In this study, the comparison of the AFM images revealed that the surface roughness, adsorption capacity, and hydrophilicity of the nanocomposite coatings were greater than those of the chitosan coating.
4. Conclusions
In this study, bio-nanocomposite coatings were developed by adding TiO2 and RA to a chitosan matrix using solvent casting. The prepared coatings were extensively characterized by FT-IR, AFM, SEM, and XRD. Key findings of the study were that TiO2 NPs and RA were successfully incorporated into the chitosan matrix, as confirmed by XRD analysis. It was found that the addition of TiO2 NPs changed the crystal structure of chitosan and provided a more homogeneous structure in the bio-nanocomposite coatings. The presences of O–H, C=O, and C–O–C bonds in the coatings were shown in the FT-IR spectra. Changes in the positions and densities of these bonds revealed that TiO2 and RA formed them because of their strong interactions with chitosan. The SEM images showed that TiO2 NPs were homogeneously distributed on the chitosan surface and that RA increased this homogeneity. AFM analysis showed that the surface roughness of the bio-nanocomposite coatings increased, and a more uniform topography was obtained. According to the results of this study, it was concluded that TiO2 NPs and RA had a synergistic effect. In addition, it was believed that they could be particularly effective in applications such as food packaging, due to their antimicrobial activity and oxidation resistance. The most important innovation of this study is that the TiO2 NPs and RA were combined in the same bio-nanocomposite matrix. This approach could be important for improving the properties of chitosan-based coatings. In future studies, the use of different antioxidant and nanoparticle combinations in polymer-based nanocomposites and bio-nanocomposite films and coatings can be investigated. In addition, evaluating the effects of long-term storage by applying it to different food products can contribute to the wide application of these materials.
Author Contributions
Conceptualization, P.K. and M.K.; Methodology, P.K.; Validation, P.K. and M.K.; Formal analysis, P.K.; İnvestigation, P.K.; Resources, P.K.; Data curation, P.K. and M.K.; Writing—review and editing, P.K. and M.K.; Visualization, P.K.; Supervision, M.K.; Project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We would like to thank Atatürk University, Eastern Anatolia High Technology Application and Research Center (DAYTAM) for giving us the opportunity to perform our study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Kumar Das, A. Recent advancements in nanocomposite coating manufactured by laser cladding and alloying Technique: A critical review. Mater Today Proc. 2022, 57, 1852–1857. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Rahmani, S.; Maroufkhani, M.; Mohammadzadeh-Komuleh, S.; Khoubi-Arani, Z. Polymer nanocomposites for biomedical applications. In Fundamentals of Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 175–215. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in food packaging applications and their risk assessment for health. Electron. Physician 2016, 8, 2531–2538. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.A.B.A.A.; Bakar, A.A.; Khalil, H.A.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Perveen, S.; Zafar, S.; Iqbal, N. Applications of bionanocomposites in agriculture. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 485–504. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]
- Sobhana, S.S.L.; Sundaraseelan, J.; Sekar, S.; Sastry, T.P.; Mandal, A.B. Gelatin-Chitosan composite capped gold nanoparticles: A matrix for the growth of hydroxyapatite. J. Nanopart. Res. 2009, 11, 333–340. [Google Scholar] [CrossRef]
- Sedaghat, S. Synthesis and characterization of new biocompatible copolymer: Chitosan-graft-polyaniline. Int. Nano Lett. 2014, 4, 2–6. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT 2021, 140, 110809. [Google Scholar] [CrossRef]
- Ahmad, M.; Zhang, B.; Manzoor, K.; Ahmad, S.; Ikram, S. Chitin and chitosan-based bionanocomposites. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 145–156. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Drouiche, N.; Lounici, H.; Pauss, A.; Mameri, N. Effect of shrimp chitosan coatings as affected by chitosan extraction processes on postharvest quality of strawberry. Food Meas. 2013, 7, 215–221. [Google Scholar] [CrossRef]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Sani, M.A.; Soleimani, M. Nanocomposite active packaging based on chitosan biopolymer loaded with nano-liposomal essential oil: Its characterizations and effects on microbial, and chemical properties of refrigerated chicken breast fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Maleki, G.; Woltering, E.J.; Mozafari, M.R. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Lin, B.; Luo, Y.; Teng, Z.; Zhang, B.; Zhou, B.; Wang, Q. Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage. LWT 2015, 63, 1206–1213. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films. Adv. Colloid Interface Sci. 2022, 300, 102593. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of Antimicrobial Properties of Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Noviagel, I.; Heryanto, H.; Putri, S.E.; Rauf, I.; Tahir, D. Tapioca-starch-based bionanocomposites with fructose and titanium dioxide for food packaging and fertilization applications. Int. J. Biol. Macromol. 2024, 273, 132803. [Google Scholar] [CrossRef] [PubMed]
- Candra, A.; Tsai, H.-C.; Saragi, I.R.; Hu, C.-C.; Yu, W.-T.; Krishnamoorthi, R.; Hong, Z.-X.; Lai, J.-Y. Fabrication and characterization of hybrid eco-friendly high methoxyl pectin/gelatin/TiO2/curcumin (PGTC) nanocomposite biofilms for salmon fillet packaging. Int. J. Biol. Macromol. 2023, 232, 123423. [Google Scholar] [CrossRef]
- Geeta; Shivani; Devi, N.; Shayoraj; Bansal, N.; Sharma, S.; Dubey, S.K.; Kumar, S. Novel chitosan-based smart bio-nanocomposite films incorporating TiO2 nanoparticles for white bread preservation. Int. J. Biol. Macromol. 2024, 267, 131367. [Google Scholar] [CrossRef]
- Singha, S.K.; Hoque, S.M.; Das, H.; Alim, M.A. Evaluation of chitosan-Ag/TiO2 nanocomposite for the enhancement of shelf life of chili and banana fruits. Heliyon 2023, 9, e21752. [Google Scholar] [CrossRef]
- Ivanov, M.; Kostić, M.; Stojković, D.; Soković, M. Rosmarinic acid–Modes of antimicrobial and antibiofilm activities of common plant polyphenol. S. Afr. J. Bot. 2022, 146, 521–527. [Google Scholar] [CrossRef]
- Kim, T.-H.; Bormate, K.J.; Custodio, R.J.P.; Cheong, J.H.; Lee, B.K.; Kim, H.J.; Jung, Y.-S. Involvement of the adenosine A1 receptor in the hypnotic effect of rosmarinic acid. Biomed. Pharmacother. 2022, 146, 112483. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan-gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-Ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Al-Taweel, S.S.; Saud, H.R.; Kadhum, A.A.H.; Takriff, M.S. The influence of titanium dioxide nanofiller ratio on morphology and surface properties of TiO2/chitosan nanocomposite. Results Phys. 2019, 13, 102296. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rokhade, A.P.; Agnihotri, S.A.; Patil, S.A.; Mallikarjuna, N.N.; Kulkarni, P.V.; Aminabhavi, T.M. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr. Polym. 2006, 65, 243–252. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin-TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Santos, K.O.; Barbosa, R.C.; da Silva Buriti, J.; Bezerra Junior, A.G.; de Sousa, W.J.B.; de Barros, S.M.C.; de Oliveira, R.J.; Fook, M.V.L. Thermal, chemical, biological and mechanical properties of chitosan films with powder of eggshell membrane for biomedical applications. J. Therm. Anal. Calorim. 2019, 136, 725–735. [Google Scholar] [CrossRef]
- Haerudin, H.; Pramono, A.W.; Kusuma, D.S.; Jenie, A.; Voelcker, N.H.; Gibson, C. Preparation and characterization of chitosan/montmorillonite (MMT) nanocomposite systems. Int. J. Technol. 2010, 1, 65–73. [Google Scholar]
- Abisharani, J.M.; Dineshkumar, R.; Devikala, S.; Arthanareeswari, M.; Ganesan, S. Influence of 2,4-Diamino-6-Phenyl-1-3-5-triazine on bio synthesized TiO2 dye-sensitized solar cell fabricated using poly (ethylene glycol) polymer electrolyte. Mater. Res. Express 2020, 7, 025507. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Cheng, W.H.; Wang, Q. Preparation, characterization and evaluation of selenite-loaded chitosan/TPP nanoparticles with or without zein coating. Carbohydr. Polym. 2010, 82, 942–951. [Google Scholar] [CrossRef]
- Rahvalı, F. Kitosan/Farklı Tip Cloisite ile Nanokompozit Filmlerin Hazırlanması ve Karaterizasyonu. Master’s Thesis, Çanakkale Onsekiz Mart University, Çanakkale, Türkiye, 2016. [Google Scholar]
- Paradkar, M.M.; Sivakesava, S.; Irudayaraj, J. Discrimination and classification of adulterants in maple syrup with the use of infrared spectroscopic techniques. J. Sci. Food Agric. 2002, 82, 497–504. [Google Scholar] [CrossRef]
- Breda, C.A.; Morgado, D.L.; Assis, O.B.G.; Duarte, M.C.T. Processing and characterization of chitosan films with incorporation of ethanolic extract from “pequi” peels. Macromol. Res. 2017, 25, 1049–1056. [Google Scholar] [CrossRef]
- Vanloot, P.; Dupuy, N.; Guiliano, M.; Artaud, J. Characterisation and authentication of A. senegal and A. seyal exudates by infrared spectroscopy and chemometrics. Food Chem. 2012, 135, 2554–2560. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A “green” chitosan-silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology 2008, 19, 015603. [Google Scholar] [CrossRef]
- Agafonov, A.; Davydova, O.; Krayev, A.; Ivanova, O.; Evdokimova, O.; Gerasimova, T.; Baranchikov, A.; Kozik, V.; Ivanov, V. Unexpected Effects of Activator Molecules’ Polarity on the Electroreological Activity of Titanium Dioxide Nanopowders. J. Phys. Chem. B 2017, 121, 6732–6738. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Dragostin, O.M.; Samal, S.K.; Lupascu, F.; Pânzariu, A.; Dubruel, P.; Lupascu, D.; Tuchilus, C.; Vasile, C.; Profire, L. Development and characterization of novel films based on sulfonamide-chitosan derivatives for potential wound dressing. Int. J. Mol. Sci. 2015, 16, 29843–29855. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).