Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design and Rationale
2.3. Safe Handling Warning
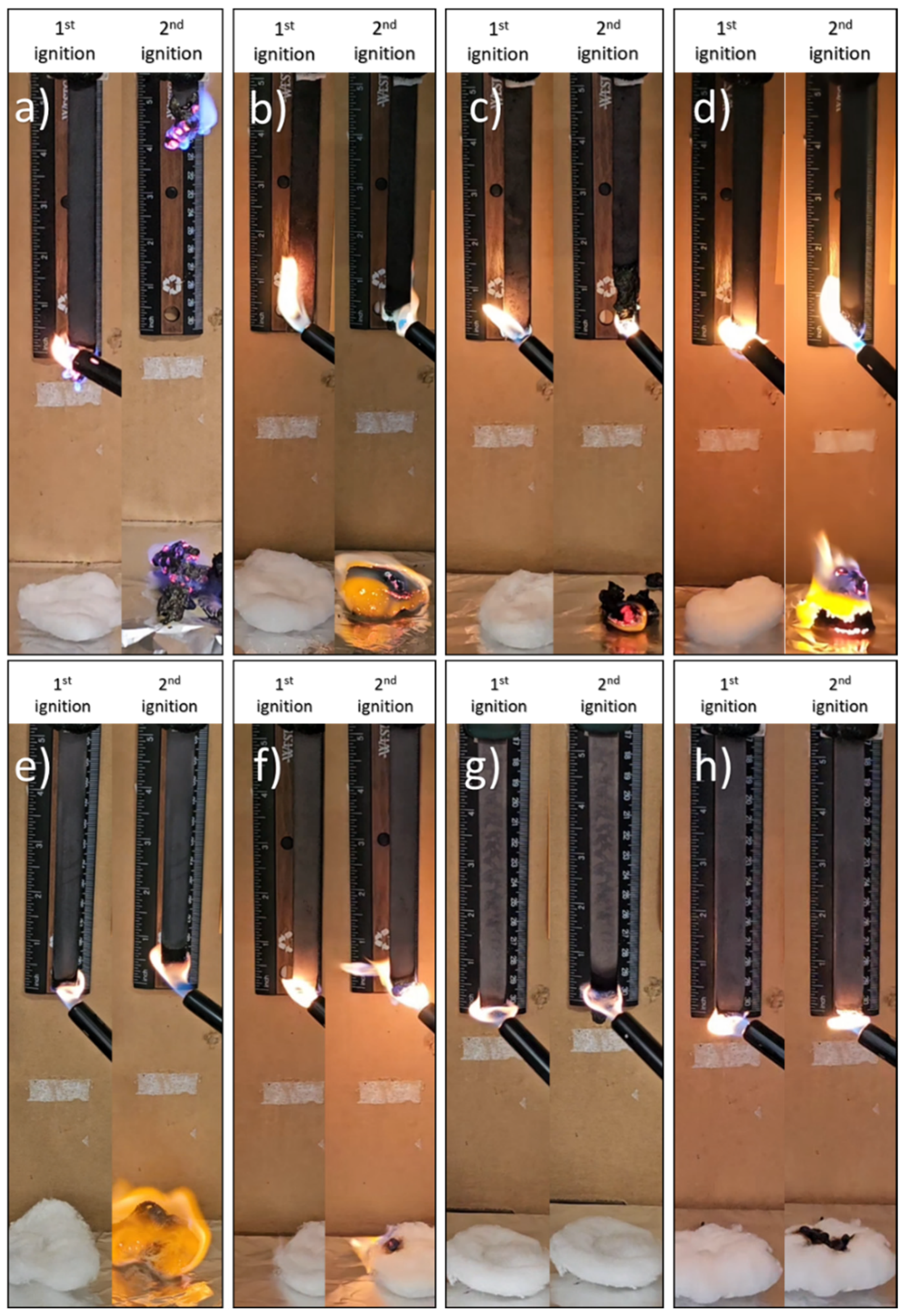
2.4. Preparation of SunBG90/FR Blends and Preparation of Samples for UL-94 Testing
2.5. UL-94HB Test and UL-94V Testing Procedure
- Duration of flaming combustion after the first burner flame application.
- Duration of flaming combustion after the second burner flame application.
- Duration of glowing combustion after the second burner flame application.
- Whether flaming drips ignited cotton placed below the specimen.
- Whether the specimen burned up to the holding clamp.
2.6. Abrasion-Resistance Testing (ASTM C1353)
2.7. Bond-Strength Testing (ASTM C482)
3. Results and Discussion
3.1. UL-94 Flame Retardancy Results
3.2. Thermal and Mechanical Properties
3.3. Abrasion Resistance (ASTM C1353)
3.4. Bond Strength (ASTM C482)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FR | flame retardant |
| HSM | high sulfur-content material |
| SunBG90 | Composite made from 90 wt. % elemental sulfur, 5 wt. % sunflower oil, and 5 wt. % brown grease. |
Appendix A
Instrumentation
References
- Allcock, H.R. Inorganic—Organic polymers. Adv. Mater. 1994, 6, 106–115. [Google Scholar] [CrossRef]
- Cullis, C.F. Thermal stability and flammability of organic polymers. Br. Polym. J. 1984, 16, 253–257. [Google Scholar] [CrossRef]
- Levchik, S.V. Introduction to flame retardancy and polymer flammability. Flame Retard. Polym. Nanocomposites 2007, 1–29. [Google Scholar]
- Cinausero, N.; Fina, A.; Hao, J.; Nazare, S.; Kandore, E.; Staggs, J.; Wang, Y.; Duquesne, S.; Hicklin, R.; Wakelyn, P. Fire Retardancy of Polymers: New Strategies and Mechanisms; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Troitzsch, J.H. The globalisation of fire testing and its impact on polymers and flame retardants. Polym. Degrad. Stab. 2005, 88, 146–149. [Google Scholar] [CrossRef]
- Suzanne, M.; Ukleja, S.; Delichatsios, M.; Zhang, J.; Karlsson, B. Fundamental flame spread and toxicity evaluation of fire retarded polymers. Fire Saf. Sci. 2014, 11, 846–859. [Google Scholar] [CrossRef]
- Purser, D. Fire safety performance of flame retardants compared with toxic and environmental hazards. In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 45–86. [Google Scholar]
- Mensah, R.A.; Shanmugam, V.; Narayanan, S.; Renner, J.S.; Babu, K.; Neisiany, R.E.; Försth, M.; Sas, G.; Das, O. A review of sustainable and environment-friendly flame retardants used in plastics. Polym. Test. 2022, 108, 107511. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Grayson, S.J.; Hirschler, M.M. Comparison of ASTM Fire Standards with International Fire Standards for Buildings and Contents. ASTM Spec. Tech. Publ. 1995, 1163, 41–62. [Google Scholar]
- Hull, T.R. Challenges in fire testing: Reaction to fire tests and assessment of fire toxicity. In Advances in Fire Retardant Materials; Elsevier: Amsterdam, The Netherlands, 2008; pp. 255–290. [Google Scholar]
- Babrauskas, V.; Fuoco, R.; Blum, A. Flame Retardant Additives in Polymers: When do the Fire Safety Benefits Outweigh the Toxicity Risks? In Polymer Green Flame Retardants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 87–118. [Google Scholar]
- Lewin, M.; Weil, E.D. Mechanisms and modes of action in flame retardancy of polymers. Fire Retard. Mater. 2001, 1, 31–68. [Google Scholar]
- Bocchini, S.; Camino, G. Halogen-containing flame retardants. Fire Retard. Polym. Mater. 2010, 2, 75–106. [Google Scholar]
- Hobbs, C.E. Recent advances in bio-based flame retardant additives for synthetic polymeric materials. Polymers 2019, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, L.; Zhang, Y.; Wang, Q.; Liang, H.; Wang, H.; Song, P. Rethinking the pathway to sustainable fire retardants. In Exploration; Wiley: Hoboken, NJ, USA, 2023; p. 20220088. [Google Scholar]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Mark, H.; Atlas, S.; Shalaby, S.; Pearce, E.M. Combustion of polymers and its retardation. In Flame-Retardant Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 1975; pp. 1–17. [Google Scholar]
- Bar, M.; Alagirusamy, R.; Das, A. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Iliescu, S.; Ilia, G. Flame Retardant Phosphorus-Chain Polymers. New Front. Chem. 2010, 76, I. [Google Scholar]
- Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.-M. Flame Retardancy of Phosphorus-Containing Polymers; The Royal Society of Chemistry: Oxford, UK, 2014. [Google Scholar]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the modes of action of phosphorus-based flame retardants in polymeric systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame retardancy of polymers: The role of specific reactions in the condensed phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Beyler, C.L.; Hirschler, M.M. Thermal decomposition of polymers. SFPE Handb. Fire Prot. Eng. 2002, 2, 111–131. [Google Scholar]
- Witkowski, A.; Stec, A.A.; Hull, T.R. Thermal decomposition of polymeric materials. SFPE Handb. Fire Prot. Eng. 2016, 167–254. [Google Scholar] [CrossRef]
- Horacek, H.; Grabner, R. Advantages of flame retardants based on nitrogen compounds. Polym. Degrad. Stab. 1996, 54, 205–215. [Google Scholar] [CrossRef]
- Klatt, M. Nitrogen-based flame retardants. In Non-Halogenated Flame Retardant Handbook; Morgan, A.B., Wilkie, C.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 143–168. [Google Scholar]
- Morgan, A.B.; Klatt, M. Nitrogen-Based Flame Retardants. In Non-Halogenated Flame Retardant Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 236–270. [Google Scholar]
- Nabipour, H.; Hu, Y. Introduction to flame retardants for polymeric materials. In Bio-Based Flame-Retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–27. [Google Scholar]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Pople, J.M.M.; Nicholls, T.P.; Pham, L.N.; Bloch, W.M.; Lisboa, L.S.; Perkins, M.V.; Gibson, C.T.; Coote, M.L.; Jia, Z.; Chalker, J.M. Electrochemical Synthesis of Poly(trisulfides). J. Am. Chem. Soc. 2023, 145, 11798–11810. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Mann, M.; Pauling, P.J.; Tonkin, S.J.; Campbell, J.A.; Chalker, J.M. Chemically Activated S-S Metathesis for Adhesive-Free Bonding of Polysulfide Surfaces. Macromol. Chem. Phys. 2022, 223, 2100333. [Google Scholar] [CrossRef]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O.N., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass-sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, sustainable, and stronger than portland cement: A composite from unseparated biomass and fossil fuel waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer-sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.; Yan, P.; Petcher, S.; Hasell, T. Incorporation of fillers to modify the mechanical performance of inverse vulcanised polymers. Polym. Chem. 2022, 13, 3930–3937. [Google Scholar] [CrossRef]
- Grimm, A.P.; Scheiger, J.M.; Roesky, P.W.; Theato, P. Inverse vulcanization of trimethoxyvinylsilane particles. Polym. Chem. 2022, 13, 5852–5860. [Google Scholar] [CrossRef]
- Dale, J.J.; Petcher, S.; Hasell, T. Dark Sulfur: Quantifying Unpolymerized Sulfur in Inverse Vulcanized Polymers. ACS Appl. Polym. Mater. 2022, 4, 3169–3173. [Google Scholar] [CrossRef]
- Zhang, B.; Dodd, L.J.; Yan, P.; Hasell, T. Mercury capture with an inverse vulcanized polymer formed from garlic oil, a bioderived comonomer. React. Funct. Polym. 2021, 161, 104865. [Google Scholar] [CrossRef]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury Sorbents Made by Inverse Vulcanization of Sustainable Triglycerides: The Plant Oil Structure Influences the Rate of Mercury Removal from Water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Chalker, J.M. Confining a spent lead sorbent in a polymer made by inverse vulcanization prevents leaching. Sustain. Mater. Technol. 2020, 26, e00222. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A Role for Terpenoid Cyclization in the Atom Economical Polymerization of Terpenoids with Sulfur to Yield Durable Composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Slann, L.M.; Lauer, M.K.; Smith, R.C. Sequential Crosslinking for Mechanical Property Development in High Sulfur Content Composites. J. Polym. Sci. 2020, 58, 2943–2950. [Google Scholar] [CrossRef]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef] [PubMed]
- Sayer, K.B.; Miller, V.L.; Merrill, Z.; Davis, A.E.; Jenkins, C.L. Allyl sulfides in garlic oil initiate the formation of renewable adhesives. Polym. Chem. 2023, 14, 3091–3098. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Dop, R.; Yan, P.; Neale, A.R.; Hardwick, L.J.; Hasell, T. Oxygen heteroatom enhanced sulfur-rich polymers synthesized by inverse vulcanization for high-performance lithium-sulfur batteries. J. Power Sources 2022, 545, 231921. [Google Scholar] [CrossRef]
- Gomez, I.; Mantione, D.; Leonet, O.; Blazquez, J.A.; Mecerreyes, D. Hybrid Sulfur-Selenium Co-polymers as Cathodic Materials for Lithium Batteries. ChemElectroChem 2018, 5, 260–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Griebel, J.J.; Dirlam, P.T.; Nguyen, N.A.; Glass, R.S.; MacKay, M.E.; Char, K.; Pyun, J. Inverse vulcanization of elemental sulfur and styrene for polymeric cathodes in Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 107–116. [Google Scholar] [CrossRef]
- Hoefling, A.; Nguyen, D.T.; Lee, Y.J.; Song, S.-W.; Theato, P. A sulfur-eugenol allyl ether copolymer: A material synthesized via inverse vulcanization from renewable resources and its application in Li-S batteries. Mater. Chem. Front. 2017, 1, 1818–1822. [Google Scholar] [CrossRef]
- Dirlam, P.T.; Park, J.; Simmonds, A.G.; Domanik, K.; Arrington, C.B.; Schaefer, J.L.; Oleshko, V.P.; Kleine, T.S.; Char, K.; Glass, R.S.; et al. Elemental Sulfur and Molybdenum Disulfide Composites for Li-S Batteries with Long Cycle Life and High-Rate Capability. ACS Appl. Mater. Interfaces 2016, 8, 13437–13448. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Liang, G.; Gu, A. Achieving ultrahigh glass transition temperature, halogen-free and phosphorus-free intrinsic flame retardancy for bismaleimide resin through building network with diallyloxydiphenyldisulfide. Polymer 2020, 203, 122769. [Google Scholar] [CrossRef]
- Jadhav, S.D. A review of non-halogenated flame retardant. Pharma Innov. 2018, 7, 380–386. [Google Scholar]
- Kang, K.-S.; Phan, A.; Olikagu, C.; Lee, T.; Loy, D.A.; Kwon, M.; Paik, H.-J.; Hong, S.J.; Bang, J.; Parker, W.O., Jr.; et al. Segmented Polyurethanes and Thermoplastic Elastomers from Elemental Sulfur with Enhanced Thermomechanical Properties and Flame Retardancy. Angew. Chem. Int. Ed. 2021, 60, 22900–22907. [Google Scholar] [CrossRef]
- Wagner, J.; Deglmann, P.; Fuchs, S.; Ciesielski, M.; Fleckenstein, C.A.; Döring, M. A flame retardant synergism of organic disulfides and phosphorous compounds. Polym. Degrad. Stab. 2016, 129, 63–76. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Sauceda-Olono, P.Y.; Borbon-Almada, A.C.; Gaxiola, M.; Smith, A.D.; Tennyson, A.G.; Smith, R.C. Thermal and Mechanical Properties of Recyclable Composites Prepared from Bio-Olefins and Industrial Waste. J. Compos. Sci. 2023, 7, 248. [Google Scholar] [CrossRef]
- Yücesoy, A.; Balçik Tamer, Y.; Berber, H. Improvement of flame retardancy and thermal stability of highly loaded low density polyethylene/magnesium hydroxide composites. J. Appl. Polym. Sci. 2023, 140, e54107. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, X.-B.; Liu, X.-L. Effect of magnesium hydroxide on the flame retardant properties of unsaturated polyester resin. Procedia Eng. 2013, 52, 336–341. [Google Scholar] [CrossRef]
- Deodhar, S.; Shanmuganathan, K.; Fan, Q.; Wilkie, C.A.; Costache, M.C.; Dembsey, N.A.; Patra, P.K. Calcium carbonate and ammonium polyphosphate-based flame retardant composition for polypropylene. J. Appl. Polym. Sci. 2011, 120, 1866–1873. [Google Scholar] [CrossRef]
- Suparanon, T.; Phusunti, N.; Phetwarotai, W. Properties and flame retardancy of polylactide composites incorporating tricresyl phosphate and modified microcrystalline cellulose from oil palm empty fruit bunch waste. Int. J. Biol. Macromol. 2023, 253, 127580. [Google Scholar] [CrossRef]
- Suparanon, T.; Phetwarotai, W. Fire-extinguishing characteristics and flame retardant mechanism of polylactide foams: Influence of tricresyl phosphate combined with natural flame retardant. Int. J. Biol. Macromol. 2020, 158, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Araby, S.; Xu, J.; Kuan, H.-C.; Wang, C.-H.; Mouritz, A.; Zhuge, Y.; Lin, R.J.-T.; Zong, T.; Ma, J. Filling natural microtubules with triphenyl phosphate for flame-retarding polymer composites. Compos. Part A Appl. Sci. Manuf. 2018, 115, 247–254. [Google Scholar] [CrossRef]
- Pan, L.; Li, G.; Su, Y.; Lian, J. Fire retardant mechanism analysis between ammonium polyphosphate and triphenyl phosphate in unsaturated polyester resin. Polym. Degrad. Stab. 2012, 97, 1801–1806. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of triphenyl phosphate, resorcinol bis (diphenyl phosphate) and bisphenol A bis (diphenyl phosphate) in polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Int. 2007, 56, 1404–1414. [Google Scholar] [CrossRef]
- Heeb, N.V.; Graf, H.; Schweizer, W.B.; Lienemann, P. Thermally-induced transformation of hexabromocyclo dodecanes and isobutoxypenta bromocyclododecanes in flame-proofed polystyrene materials. Chemosphere 2010, 80, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, J.S.; Peighambardoust, S.J.; Shenavar, A. Polystyrene-based composites and nanocomposites with reduced brominated-flame retardant. Iran. Polym. J. 2016, 25, 607–614. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Chang, P. Preparation and properties of flame-retardant polycarbonates and copolycarbonates from 3, 3′, 5, 5′-tetrabromobisphenol AF and bisphenol A. Polymer 1997, 38, 5545–5550. [Google Scholar] [CrossRef]
- Covaci, A.; Voorspoels, S.; Abdallah, M.A.-E.; Geens, T.; Harrad, S.; Law, R.J. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J. Chromatogr. A 2009, 1216, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, K.; Jiang, J.; Li, J.; Liu, Y. Highly dispersed melamine cyanurate flame-retardant epoxy resin composites. Polym. Int. 2017, 66, 85–91. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. The investigation on the flame retardancy mechanism of nitrogen flame retardant melamine cyanurate in polyamide 6. J. Polym. Res. 2009, 16, 583–589. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 2024.
- ASTM C1353/C1353M-20 (2020); Standard Test Method for Abrasion Resistance of Dimension Stone Subjected to Foot Traffic Using a Rotary Platform Abraser. ASTM: West Conshohocken, PA, USA, 2024; Volume 04.07, p. 6.
- ASTM C482-20 (2020); Standard Test Method for Bond Strength of Ceramic Tile to Portland Cement Paste. ASTM: West Conshohocken, PA, USA, 2024; Volume 15.02, p. 5.
- Baigorri Garcia, P. Chemical and abrasion resistance of ceramic glazes for floors. Ceram. Inf. 1986, 21, 145–149. [Google Scholar]
- Thiel, G.A. Relative resistance to abrasion of mineral grains of sand size. J. Sediment. Petrol. 1940, 10, 103–124. [Google Scholar]
- Skuthan, R. Abrasion resistant glazes for floor tiles. Interceram 1977, 26, 52–53. [Google Scholar]
- CC Mosaics; United States Ceramic Tile: Miami, FL, USA, 2020.
- ANSI A137.1:2022; American National Standards Specifications for Ceramic Tile. ANSI: Washington, DC, USA, 2022.


| FR Added | FR wt. % | Sample Number | Combustion Time (s) [a] | Damaged Length (in) [b] | Classification |
|---|---|---|---|---|---|
| None | 0 | 1 | 3 | 1.125 | 94HB |
| 2 | 14 | 1 | |||
| 3 | 25 | 1 | |||
| TCEP | 10 | 1 | 10 | 0.375 | 94HB |
| 2 | 20 | 0.437 | |||
| 3 | 6 | 0.062 | |||
| TCP | 10 | 1 | 20 | 0.375 | 94HB |
| 2 | 19 | 0.812 | |||
| 3 | 16 | 0.125 | |||
| HBCD | 10 | 1 | 8 | 1.500 | 94HB |
| 2 | 5 | 0.687 | |||
| 3 | 12 | 1.125 | |||
| MA | 10 | 1 | 34 | 0.125 | 94HB |
| 2 | 11 | 0.250 | |||
| 3 | 9 | 0 | |||
| TBBPA | 10 | 1 | 3 | 0.250 | 94HB |
| 2 | 11 | 0 | |||
| 3 | 12 | 0.500 | |||
| 15 | 1 | 4 | 1.375 | 94HB | |
| 2 | 9 | 1.375 | |||
| 3 | 5 | 1.375 | |||
| 20 | 1 | 3 | 0.687 | 94HB | |
| 2 | 2 | 0.875 | |||
| 3 | 2 | 1 |
| FR Added | FR wt. % | Set no. | Sample No. | Flaming Combustion (s) [a] | Flaming Combustion (s) [b] | Glowing Combustion (s) [c] | Cotton Ignited (Y/N) [d] | Burned > 5-Inch Mark (Y/N) [e] | Classification |
|---|---|---|---|---|---|---|---|---|---|
| None | 0 | 1 | 1 | 120 | 0 | 0 | Y | Y | NR |
| 2 | 130 | 0 | 0 | Y | Y | ||||
| 3 | 10 | 14 | 2 | Y | N | ||||
| 4 | 150 | 0 | 0 | Y | Y | ||||
| 5 | 38 | 100 | 0 | Y | Y | ||||
| 2 | 1 | 50 | 64 | 0 | Y | N | |||
| 2 | 123 | 0 | 2 | Y | Y | ||||
| 3 | 140 | 0 | 0 | Y | Y | ||||
| 4 | 97 | 0 | 0 | Y | Y | ||||
| 5 | 76 | 35 | 0 | Y | N | ||||
| TCEP | 10 | 1 | 1 | 15 | 17 | 3 | Y | N | NR |
| 2 | 8 | 20 | 8 | Y | N | 94V-2 | |||
| 3 | 52 | 15 | 2 | Y | N | NR | |||
| 4 | 19 | 35 | 3 | Y | N | NR | |||
| 5 | 11 | 45 | 3 | Y | N | NR | |||
| 2 | 1 | 64 | 7 | 2 | Y | N | NR | ||
| 2 | 21 | 8 | 3 | N | N | 94V-1 | |||
| 3 | 20 | 25 | 3 | Y | N | NR | |||
| 4 | 13 | 8 | 5 | Y | N | 94V-2 | |||
| 5 | 15 | 21 | 2 | Y | N | NR | |||
| TCP | 10 | 1 | 1 | 10 | 13 | 4 | Y | N | 94V-2 |
| 2 | 17 | 4 | 2 | N | N | 94V-1 | |||
| 3 | 12 | 27 | 3 | Y | N | NR | |||
| 4 | 32 | 24 | 2 | Y | N | NR | |||
| 5 | 16 | 3 | 2 | N | N | 94V-1 | |||
| 2 | 1 | 38 | 6 | 4 | Y | N | NR | ||
| 2 | 22 | 22 | 2 | Y | N | NR | |||
| 3 | 12 | 8 | 2 | N | N | 94V-1 | |||
| 4 | 23 | 6 | 3 | Y | N | 94V-2 | |||
| 5 | 33 | 7 | 4 | Y | N | NR | |||
| HBCD | 10 | 1 | 1 | 6 | 8 | 2 | N | N | 94V-1 |
| 2 | 4 | 3 | 3 | Y | N | 94V-2 | |||
| 3 | 11 | 21 | 2 | N | N | NR | |||
| 4 | 6 | 2 | 2 | N | N | 94V-1 | |||
| 5 | 10 | 23 | 3 | Y | N | NR | |||
| 2 | 1 | 13 | 8 | 2 | Y | N | 94V-2 | ||
| 2 | 13 | 4 | 2 | Y | N | 94V-2 | |||
| 3 | 6 | 4 | 2 | Y | N | 94V-2 | |||
| 4 | 8 | 5 | 2 | Y | N | 94V-2 | |||
| 5 | 13 | 6 | 3 | Y | N | 94V-2 | |||
| MA | 10 | 1 | 1 | 10 | 5 | 2 | Y | N | 94V-2 |
| 2 | 7 | 8 | 4 | N | N | 94V-1 | |||
| 3 | 5 | 13 | 3 | Y | N | 94V-2 | |||
| 4 | 6 | 16 | 2 | Y | N | 94V-2 | |||
| 5 | 4 | 37 | 3 | N | N | NR | |||
| 2 | 1 | 11 | 5 | 2 | Y | N | 94V-2 | ||
| 2 | 4 | 15 | 3 | Y | N | 94V-2 | |||
| 3 | 6 | 14 | 2 | Y | N | 94V-2 | |||
| 4 | 2 | 6 | 1 | N | N | 94V-1 | |||
| 5 | 4 | 26 | 2 | Y | N | 94V-2 | |||
| TBBPA | 10 | 1 | 1 | 3 | 3 | 3 | N | N | 94V-1 |
| 2 | 9 | 19 | 2 | Y | N | 94V-2 | |||
| 3 | 7 | 6 | 4 | Y | N | 94V-2 | |||
| 4 | 7 | 4 | 2 | N | N | 94V-1 | |||
| 5 | 12 | 12 | 2 | Y | N | 94V-2 | |||
| 2 | 1 | 7 | 6 | 3 | Y | N | 94V-2 | ||
| 2 | 10 | 11 | 2 | N | N | 94V-1 | |||
| 3 | 6 | 7 | 2 | Y | N | 94V-2 | |||
| 4 | 6 | 17 | 3 | Y | N | 94V-2 | |||
| 5 | 5 | 10 | 1 | Y | N | 94V-2 | |||
| TBBPA | 15 | 1 | 1 | 5 | 9 | 2 | N | N | 94V-1 |
| 2 | 11 | 5 | 1 | N | N | 94V-1 | |||
| 3 | 11 | 6 | 2 | N | N | 94V-1 | |||
| 4 | 7 | 7 | 1 | N | N | 94V-1 | |||
| 5 | 5 | 11 | 2 | Y | N | 94V-2 | |||
| 2 | 1 | 6 | 6 | 2 | N | N | 94V-1 | ||
| 2 | 5 | 6 | 2 | N | N | 94V-1 | |||
| 3 | 6 | 6 | 1 | N | N | 94V-1 | |||
| 4 | 7 | 7 | 1 | N | N | 94V-1 | |||
| 5 | 4 | 5 | 2 | N | N | 94V-2 | |||
| 20 | 1 | 1 | 3 | 5 | 0 | N | N | 94V-0 | |
| 2 | 2 | 3 | 1 | N | N | 94V-0 | |||
| 3 | 1 | 2 | 0 | N | N | 94V-0 | |||
| 4 | 1 | 2 | 0 | N | N | 94V-0 | |||
| 5 | 2 | 3 | 0 | N | N | 94V-0 | |||
| 2 | 1 | 4 | 4 | 1 | N | N | 94V-0 | ||
| 2 | 3 | 4 | 0 | N | N | 94V-0 | |||
| 3 | 3 | 6 | 1 | N | N | 94V-0 | |||
| 4 | 3 | 3 | 1 | N | N | 94V-0 | |||
| 5 | 1 | 2 | 0 | Y | N | 94V-2 |
| Material | FR wt. % | /°C | /°C | /°C |
|---|---|---|---|---|
| SunBG90 | 0 | 228 | 118.5 | –36.2 |
| SunBG90/HBCD | 10 | 213 | 114.1 | –35.2 |
| SunBG90/MA | 10 | 214 | 118.8 | NA |
| SunBG90/TBBPA | 10 | 217 | 115.6 | –36.0 |
| SunBG90/TBBPA | 15 | 216 | 114.6 | –36.3 |
| SunBG90/TBBPA | 20 | 216 | 114.1 | –36.7 |
| cyclo-S8 | NA | 229 | 118 | NA |
| Materials | Abrasion Resistance (IW, HA) |
|---|---|
| SunBG90 | 16 |
| SunBG90/TBBPA (20 wt. %) | 52 |
| Limestone | 10 |
| Marble | 10 |
| Granite | 25 |
| Materials | Bond Strength (psi) |
|---|---|
| SunBG90 | 15 |
| SunBG90/TBBPA (20 wt. %) | 26 |
| Mosaic tile | 50 or greater |
| Porcelain tile | 50 or greater |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauceda-Oloño, P.Y.; Guinati, B.G.S.; Smith, A.D.; Smith, R.C. Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite. J. Compos. Sci. 2024, 8, 304. https://doi.org/10.3390/jcs8080304
Sauceda-Oloño PY, Guinati BGS, Smith AD, Smith RC. Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite. Journal of Composites Science. 2024; 8(8):304. https://doi.org/10.3390/jcs8080304
Chicago/Turabian StyleSauceda-Oloño, Perla Y., Bárbara G. S. Guinati, Ashlyn D. Smith, and Rhett C. Smith. 2024. "Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite" Journal of Composites Science 8, no. 8: 304. https://doi.org/10.3390/jcs8080304
APA StyleSauceda-Oloño, P. Y., Guinati, B. G. S., Smith, A. D., & Smith, R. C. (2024). Influence of Additives on Flame-Retardant, Thermal, and Mechanical Properties of a Sulfur–Triglyceride Polymer Composite. Journal of Composites Science, 8(8), 304. https://doi.org/10.3390/jcs8080304








