Micrographite (μG) and Polypropylene (PP) Composites: Preparation and Influence of Filler Content on Property Modifications
Abstract
1. Introduction
2. Materials and Characterization Methods
2.1. Preparation of Polymer Composites
2.2. Characterization
2.2.1. Mechanical Properties
2.2.2. Thermal Properties
2.2.3. XRD Analysis
2.2.4. Microstructural and FTIR Analysis
2.2.5. Rheology of Polymer Composites
3. Results and Discussion
3.1. Tensile Strength
3.2. Flexural and Impact Strength
3.3. Crystallography, Polarized Light Microscopy, and FTIR
3.4. Thermal Analysis
3.5. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahu, D.; Sutar, H.; Senapati, P.; Murmu, R.; Roy, D. Graphene, Graphene-Derivatives and Composites: Fundamentals, Synthesis Approaches to Applications. J. Compos. Sci. 2021, 5, 181. [Google Scholar] [CrossRef]
- Fang, C.; Xiao, H.; Zheng, T.; Bai, H.; Liu, G. Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. J. Compos. Sci. 2021, 5, 188. [Google Scholar] [CrossRef]
- Tu, F.; Xie, J.; Cao, G.; Zhao, X. Self-Assembly of Bi2Te3-Nanoplate/Graphene-Nanosheet Hybrid by One-Pot Route and Its Improved Li-Storage Properties. Materials 2012, 5, 1275–1284. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, D.; Wu, M.; Ruan, S.; Castro, J.M.; Lee, L.J.; Chen, F. Impacts of Carbonaceous Particulates on Extrudate Semicrystalline Polyethylene Terephthalate Foams: Nonisothermal Crystallization, Rheology, and Infrared Attenuation Studies. Ind. Eng. Chem. Res. 2020, 59, 15586–15597. [Google Scholar] [CrossRef]
- Lakatos, Á.; Csík, A. Multiscale Thermal Investigations of Graphite Doped Polystyrene Thermal Insulation. Polymers 2022, 14, 1606. [Google Scholar] [CrossRef]
- Naumova, L.; Minakova, T.; Gorlenko, N.; Kurzina, I.; Vasenina, I. Oxidative Destruction of Organic Pollutants on the Polypropylene Fiber Modified by Nanodispersed Iron. Environments 2018, 5, 82. [Google Scholar] [CrossRef]
- Kostic, S.; Kocovic, V.; Petrovic Savic, S.; Miljanic, D.; Miljojkovic, J.; Djordjevic, M.; Vukelic, D. The Influence of Friction and Twisting Angle on the Tensile Strength of Polypropylene Baling Twine. Appl. Sci. 2024, 14, 3046. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Appl. Therm. Eng. 2007, 27, 1271–1277. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energ. 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Krupa, I.; Novák, I.; Chodák, I. Electrically and thermally conductive polyethylene/graphite composites and their mechanical properties. Synth. Met. 2004, 145, 245–252. [Google Scholar] [CrossRef]
- Zhang, W.; Dehghani-Sanij, A.A.; Blackburn, R.S. Carbon based conductive polymer composites. J. Mater. Sci. 2007, 42, 3408–3418. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Alshammari, B.A.; Wilkinson, A.N.; AlOtaibi, B.M.; Alotibi, M.F. Influence of Carbon Micro- and Nano-Fillers on the Viscoelastic Properties of Polyethylene Terephthalate. Polymers 2022, 14, 2440. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H. Graphene Nanocomposites as Innovative Materials for Energy Storage and Conversion—Design and Headways. Int. J. Mol. Sci. 2023, 24, 11593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lei, Y.; Zou, H. High thermally conducting composites obtained via in situ exfoliation process of expandable graphite filled polyamide 6. Polym. Compos. 2013, 34, 1816–1823. [Google Scholar] [CrossRef]
- Wu, J.W.; Ji, X.Y.; Yang, J.D. Preparation and properties of polypropylene/graphite composites with high thermal conductivity. Eng. Plast. Appl. 2016, 44, 36–40. [Google Scholar]
- Myalski, J.; Godzierz, M.; Olesik, P. Effect of Carbon Fillers on the Wear Resistance of PA6 Thermoplastic Composites. Polymers 2020, 12, 2264. [Google Scholar] [CrossRef]
- Huang, Y.; Kormakov, S.; He, X.; Gao, X.; Zheng, X.; Liu, Y.; Sun, J.; Wu, D. Conductive Polymer Composites from Renewable Resources: An Overview of Preparation, Properties, and Applications. Polymers 2019, 11, 187. [Google Scholar] [CrossRef]
- Zambrzycki, M.; Sokolowski, K.; Gubernat, M.; Fraczek-Szczypta, A. Effect of Secondary Carbon Nanofillers on the Electrical, Thermal, and Mechanical Properties of Conductive Hybrid Composites Based on Epoxy Resin and Graphite. Materials 2021, 14, 4169. [Google Scholar] [CrossRef]
- Pączkowski, P.; Sigareva, N.V.; Gorelov, B.M.; Terets, M.I.; Sementsov, Y.I.; Kartel, M.T.; Gawdzik, B. The Influence of Carbon Nanotubes on the Physical and Chemical Properties of Nanocomposites Based on Unsaturated Polyester Resin. Nanomaterials 2023, 13, 2981. [Google Scholar] [CrossRef] [PubMed]
- Sutar, H.; Mishra, B.; Senapati, P.; Murmu, R.; Sahu, D. Mechanical, Thermal, and Morphological Properties of Graphene Nanoplatelet-Reinforced Polypropylene Nanocomposites: Effects of Nanofiller Thickness. J. Compos. Sci. 2021, 5, 24. [Google Scholar] [CrossRef]
- Patra, S.C.; Swain, S.; Senapati, P.; Sahu, H.; Murmu, R.; Sutar, H. Polypropylene and Graphene Nanocomposites: Effects of Selected 2D-Nanofiller’s Plate Sizes on Fundamental Physicochemical Properties. Inventions 2023, 8, 8. [Google Scholar] [CrossRef]
- Gautneb, H.; Rønning, J.S.; Engvik, A.K.; Henderson, I.H.C.; Larsen, B.E.; Solberg, J.K.; Ofstad, F.; Gellein, J.; Elvebakk, H.; Davidsen, B. The Graphite Occurrences of Northern Norway, a Review of Geology, Geophysics, and Resources. Minerals 2020, 10, 626. [Google Scholar] [CrossRef]
- ASTM D638-02a; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D790; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D256-A; Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. ASTM International: West Conshohocken, PA, USA, 2023.
- Kinloch, A.J.; Young, R.J. Fracture Behaviour of Polymers; Applied Science Publishers: Essex, UK, 1983. [Google Scholar]
- Oswald, T.A.; Menges, G. Materials Science of Polymers for Engineers, 2nd ed.; Hanser: Munich, Germany, 2003; p. 622. [Google Scholar]
- Arencón, D.; Velasco, J.I. Fracture Toughness of Polypropylene-Based Particulate Composites. Materials 2009, 2, 2046–2094. [Google Scholar] [CrossRef]
- Friedrich, K. Crazes and shear bands in semy-crystalline thermoplastics. In Crazing in Polymers; Hausch, H.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 225–274. [Google Scholar]
- Mózczó, J.; Pukánszky, B. Polymer micro and nanocomposites: Structure, interactions, properties. J. Ind. Eng. Chem. 2008, 14, 535–563. [Google Scholar] [CrossRef]
- Zhuk, A.V.; Knunyants, N.N.; Oshmyan, V.G.; Topolkaraev, V.A.; Berlin, A.A. Debonding microprocesses and interfacial strength in particle-filled polymer materials. J. Mater. Sci. 1993, 28, 4995–5606. [Google Scholar] [CrossRef]
- Hutar, P.; Majer, Z.; Nahlik, L.; Shestakova, L.; Knesl, Z. Influence of particle size on the fracture toughness of a PP-based particulate composite. Mech. Compos. Mater. 2009, 45, 281–286. [Google Scholar] [CrossRef]
- Wang, K.; Wu, J.; Ye, L.; Zeng, H. Mechanical properties and toughening mechanisms of polypropylene/barium sulfate composites. Compos. Part A 2003, 34, 1192–1205. [Google Scholar] [CrossRef]
- Tsui, C.P.; Tang, C.Y.; Lee, T.C. Finite element analysis of polymer composites filled by interphase coated particles. J. Mater. Process. Technol. 2001, 117, 105–110. [Google Scholar] [CrossRef]
- Liang, J.Z.; Li, R.K.Y.; Tjong, S.C. Effects of filler content and size on drop-weight dart impact fracture behaviour of glass bead-filled polypropylene composites. J. Thermoplast. Compos. Mater. 2000, 13, 241–245. [Google Scholar] [CrossRef]
- Cui, L.; Wang, P.; Zhang, Y.; Zhang, L.; Chen, Y.; Wang, L.; Guo, X. Combined effect of α-nucleating agents and glass fiber reinforcement on a polypropylene composite: A balanced approach. RSC Adv. 2017, 7, 42783–42791. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R.S. Synthesis of SiO2 Nanoparticles by Sol-Gel Method and Their Optical and Structural Properties. Rom. J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Sutar, H.; Sahoo, P.C.; Sahu, P.S.; Sahoo, S.; Murmu, R.; Swain, S.; Mishra, S.C. Mechanical, Thermal and Crystallization Properties of Polypropylene (PP) Reinforced Composites with High Density Polyethylene (HDPE) as Matrix. Mater. Sci. Appl. 2018, 9, 502–515. [Google Scholar] [CrossRef]
- Xiao, W.; Wu, P.; Feng, J. Effect of β-nucleating agents on crystallization and melting behavior of isotactic polypropylene. J. Appl. Polym. Sci. 2008, 108, 3370–3379. [Google Scholar] [CrossRef]
- Um, J.G.; Jun, Y.-S.; Alhumade, H.; Krithivasan, H.; Lui, G.; Yu, A. Investigation of the size effect of graphene nano-platelets (GnPs) on the anti-corrosion performance of polyurethane/GnPcomposites. RSC Adv. 2018, 8, 17091–17100. [Google Scholar] [CrossRef]
- Saleem, A.; Zhang, Y.; Gong, H.; Majeed, M.K.; Jing, J.; Lin, X.; Ashfaq, M.Z. Enhanced thermal conductivity and mechanical properties of a GNP reinforced Si3N4 composite. RSC Adv. 2019, 9, 39986–39992. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Gómez, M.A.; Martínez, G. Polymeric modification of graphene through esterification of graphite oxide and poly (vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Verdejo, R.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Antonio de Saja, J.; Lopez-Manchado, M.A. Functionalized graphene sheet filled silicone foam nanocomposites. J. Mater. Chem. 2008, 18, 2221. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, J.; Choi, K.Y.; Rhee, J.M. Glass transition behavior of polypropylene/polystyrene/styrene-ethylene-propylene block copolymer blends. Polym. Bull. 1998, 40, 765–771. [Google Scholar] [CrossRef]
- Rahman, N.A.; Hassan, A.; Yahya, R.; Lafia-Araga, R.; Hornsby, P. Polypropylene/glass fiber/nanoclay hybrid composites: Morphological, thermal, dynamic mechanical and impact behaviors. J. Reinf. Plast. Compos. 2012, 31, 1247–1257. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Jiménez-Suárez, A.; Moriche, R.; Ureña, A. Graphene nanoplatelets thickness and lateral size influence on the morphology and behavior of epoxy composites. Eur. Polym. J. 2014, 53, 292–301. [Google Scholar] [CrossRef]
- Mayoral, B.; Harkin-Jones, E.; Khanam, P.N.; AlMaadeed, M.A.; Ouederni, M.; Hamilton, A.R.; Sun, D. Melt processing and characterisation of polyamide 6/graphene nanoplatelet composites. RSC Adv. 2015, 5, 52395–52409. [Google Scholar] [CrossRef]











| Material | Polypropylene | Graphite | ||
|---|---|---|---|---|
| Properties | Melt Flow Index (ASTM Standard) | 11 g/10 min | Molecular Weight | 12.01 |
| Density | 0.900 g/cm3 | Particle Size | >50 µm | |
| Pellet Size | 3–5 mm | Bulk Density | 20–30 g/100 mL | |
| Grade | M110 Homopolymer | BET Surface Area | 28.35 m2/g | |
| Graphite Content, wt.% | Sample Code |
|---|---|
| 0 | PP |
| 3 | PP-3G |
| 6 | PP-6G |
| 9 | PP-9G |
| 12 | PP-12G |
| 15 | PP-15G |
| Properties | Type of Sample and Results | |||||
|---|---|---|---|---|---|---|
| PP | PP-3G | PP-6G | PP-9G | PP-12G | PP-15G | |
| Tensile stress at maximum load, MPa (SD = 1) | 28.68 | 35.07 | 35.49 | 34.18 | 34.53 | 33.65 |
| Maximum load, N (SD = 52) | 1221.63 | 1395.19 | 1421.14 | 1369.53 | 1391.82 | 1356.11 |
| Tensile stress at break, MPa (SD = 1) | 16.46 | 29.91 | 29.94 | 30.28 | 33.14 | 33.50 |
| Load at break, N (SD = 59) | 1109.38 | 1189.82 | 1198.83 | 1213.16 | 1335.72 | 1350.40 |
| Tensile strain at maximum load, % (SD = 1) | 10.68 | 5.14 | 5.72 | 4.52 | 4.78 | 3.93 |
| Tensile strain at break, % (SD = 3) | 120.32 | 11.12 | 10.97 | 9.13 | 6.41 | 4.09 |
| Modulus, MPa (SD = 66) | 1412.13 | 1538.89 | 1460.58 | 1602.43 | 1558.00 | 1640.19 |
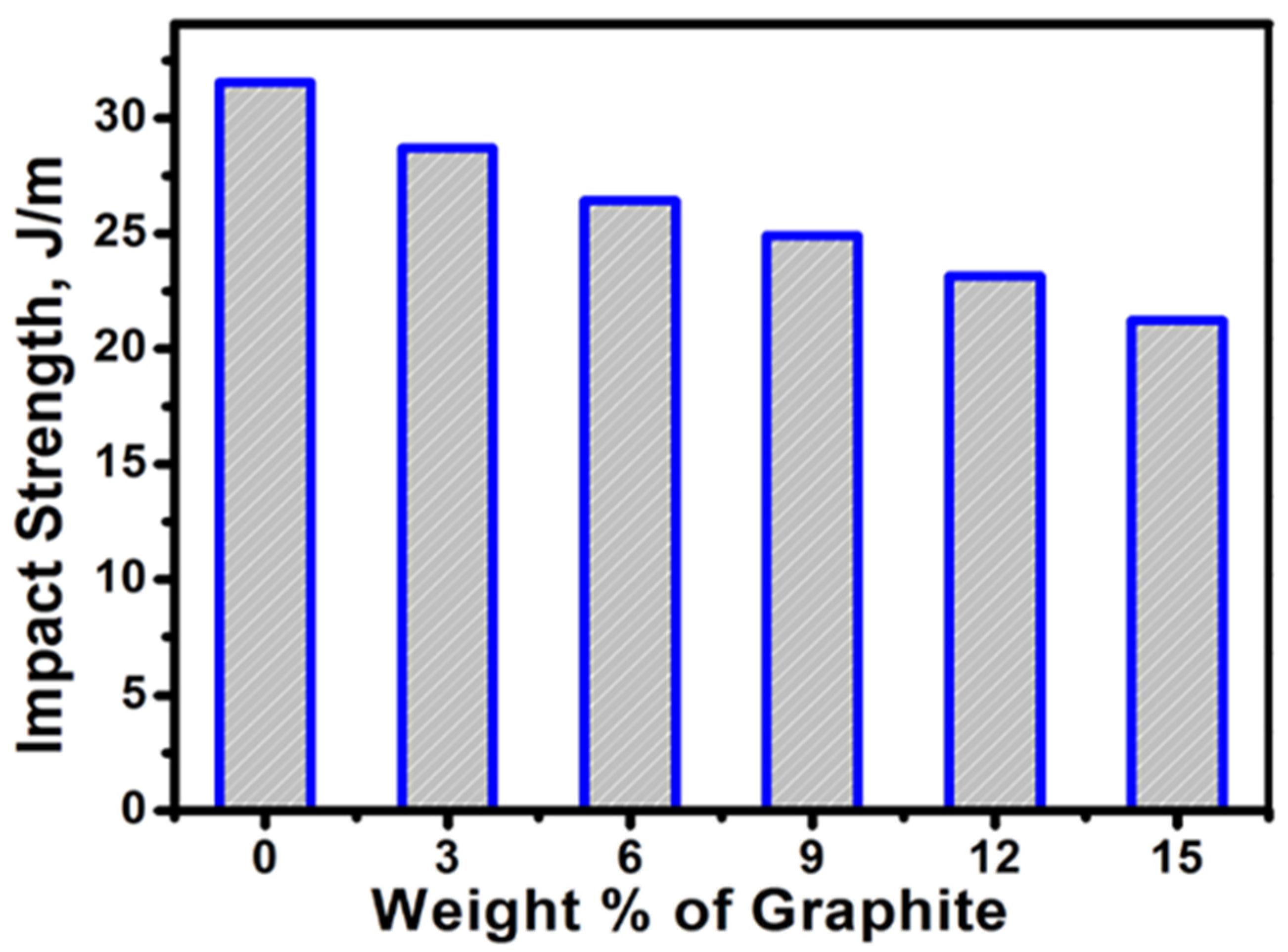
| Type of Sample | Flexural Properties | Impact Strength, J/m (SD = 3) | ||
|---|---|---|---|---|
| Maximum Load, N (SD = 2) | Maximum Stress, MPa (SD = 1) | Modulus, MPa (SD = 123) | ||
| PP | 55.82 | 33.43 | 1182.13 | 31.56 |
| PP-3G | 61.58 | 37.24 | 1230.60 | 28.70 |
| PP-6G | 62.63 | 37.87 | 1348.50 | 26.43 |
| PP-9G | 64.05 | 38.73 | 1438.78 | 24.91 |
| PP-12G | 65.92 | 39.96 | 1534.45 | 23.16 |
| PP-15G | 67.04 | 40.67 | 1601.59 | 21.24 |
| Test Type | Properties | Sample Type | |||||
|---|---|---|---|---|---|---|---|
| PP | PP-3G | PP-6G | PP-9G | PP-12G | PP-15G | ||
| DSC | Tm, °C | 162.40 | 164.76 | 165.11 | 165.66 | 166.74 | 167.18 |
| ΔHm, J/g | 34.72 | 35.82 | 36.94 | 37.89 | 40.12 | 42.68 | |
| Tc, °C | 111.45 | 125.36 | 124.26 | 124.42 | 124.89 | 128.16 | |
| ΔHc, J/g | 51.14 | 44.19 | 37.04 | 36.56 | 33.65 | 29.23 | |
| TGA | Total weight loss, % | 99.26 | 98.42 | 97.03 | 93.18 | 92.63 | 89.01 |
| TR, °C | 474.12 | 465.76 | 464.22 | 479.35 | 479.85 | 482.44 | |
| Tonset, °C | 385.12 | 345.43 | 411.12 | 424.26 | 335.81 | 433.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharai, R.; Sutar, H.; Murmu, R.; Roy, D. Micrographite (μG) and Polypropylene (PP) Composites: Preparation and Influence of Filler Content on Property Modifications. J. Compos. Sci. 2024, 8, 298. https://doi.org/10.3390/jcs8080298
Dharai R, Sutar H, Murmu R, Roy D. Micrographite (μG) and Polypropylene (PP) Composites: Preparation and Influence of Filler Content on Property Modifications. Journal of Composites Science. 2024; 8(8):298. https://doi.org/10.3390/jcs8080298
Chicago/Turabian StyleDharai, Rabindra, Harekrushna Sutar, Rabiranjan Murmu, and Debashis Roy. 2024. "Micrographite (μG) and Polypropylene (PP) Composites: Preparation and Influence of Filler Content on Property Modifications" Journal of Composites Science 8, no. 8: 298. https://doi.org/10.3390/jcs8080298
APA StyleDharai, R., Sutar, H., Murmu, R., & Roy, D. (2024). Micrographite (μG) and Polypropylene (PP) Composites: Preparation and Influence of Filler Content on Property Modifications. Journal of Composites Science, 8(8), 298. https://doi.org/10.3390/jcs8080298







