Microstructural Evolution and Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites with Different Carbon Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization and Test
3. Results and Discussion
3.1. Microstructure of Porous Cf/Cm Composites
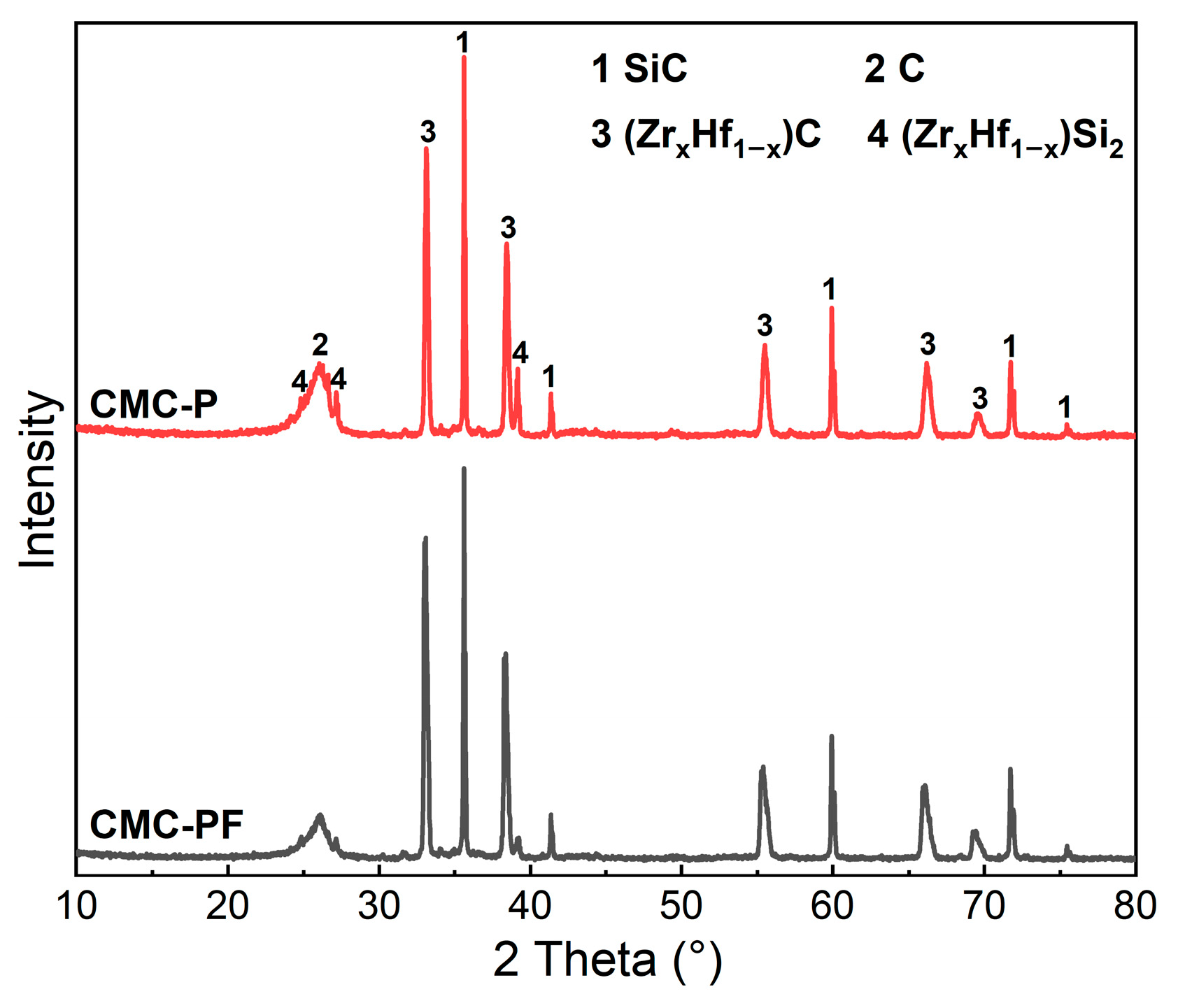
3.2. Phase Composition and Microstructure of Cf/Cm-SiC-(ZrxHf1−x)C Composites
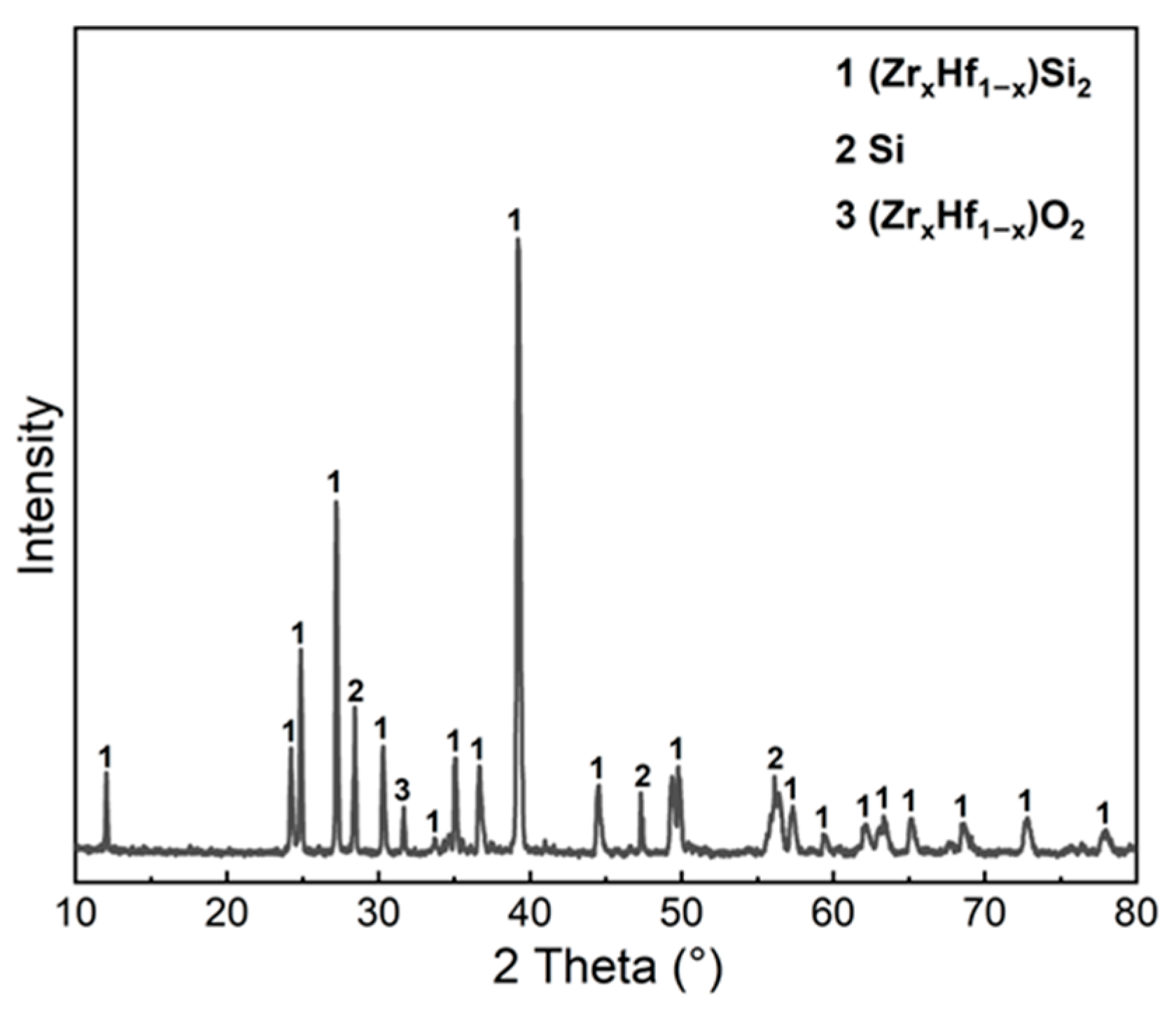
3.3. Microstructural Evolution of Cf/Cm-SiC-(ZrxHf1−x)C Composites
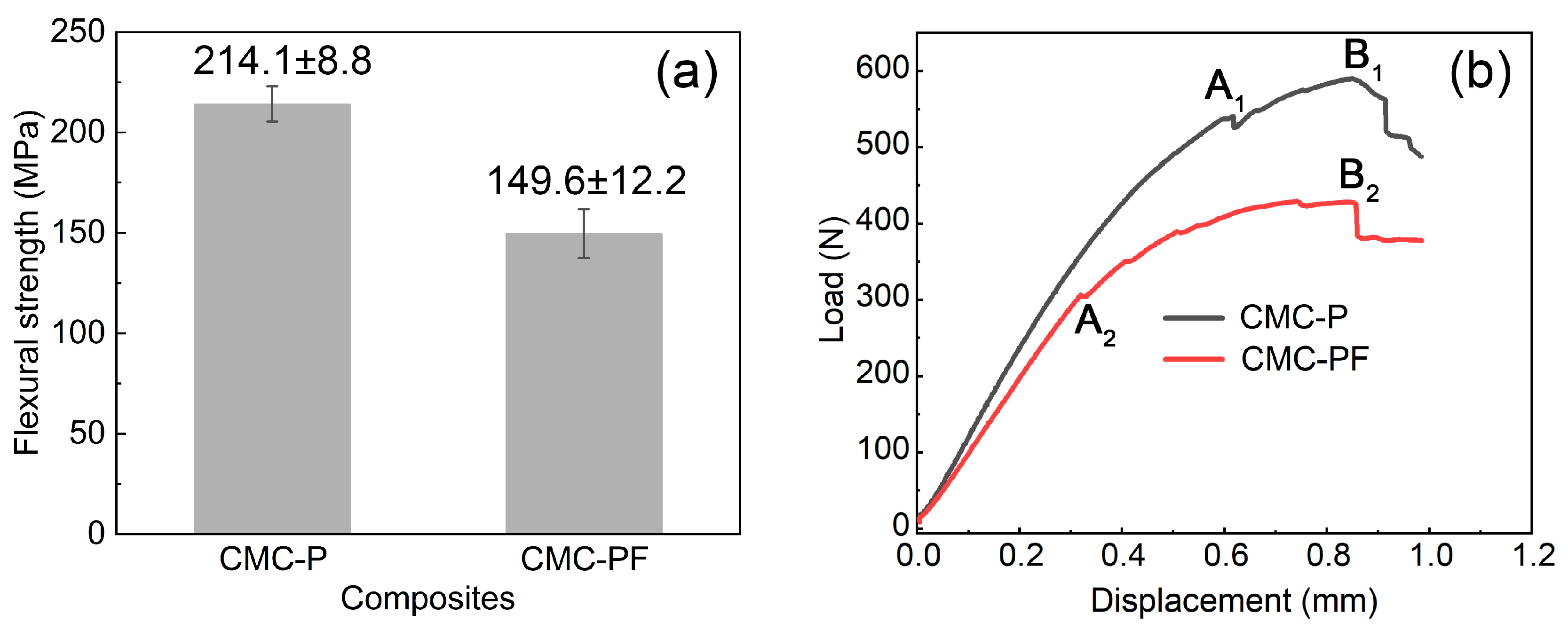
3.4. Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mikinka, E.; Siwak, M. Recent advances in electromagnetic interference shielding properties of carbon-fibre-reinforced polymer composites—A topical review. J. Mater. Sci. Mater. Electron. 2021, 32, 24585–24643. [Google Scholar] [CrossRef]
- Dai, H.; Dai, W.; Hu, Z.; Zhang, W.; Zhang, G.; Gu, R. Advanced composites inspired by biological structures and functions in nature: Architecture design, strengthening mechanisms, and mechanical-functional response. Adv. Sci. 2023, 10, 2207192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, Q.G.; Wang, B.B.; Liu, T.Y.; Sun, J. The ablation behavior and mechanical property of C/C-SiC-ZrB2 composites fabricated by reactive melt infiltration. Ceram. Int. 2017, 43, 6138–6147. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, S.J.; Hao, Y.W.; Yang, Y.N.; Xu, C.H. Mechanical properties and fracture behavior of ultrahigh temperature ceramics at ultrahigh temperatures. Ceram. Int. 2023, 49, 28532–28537. [Google Scholar] [CrossRef]
- Tong, Y.G.; Zhang, H.; Hu, Y.L.; Zhang, P.; Hua, M.Y.; Liang, X.B.; Chen, Y.X.; Zhang, Z.B. RMI-C/C-SiC-ZrSi2 composite serving in inert atmosphere up to 2100 °C: Thermal shock resistance, microstructure and damage mechanism. Ceram. Int. 2021, 47, 20371–20378. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Li, K.Z.; Li, W.; Liu, Q.; Kou, G.; Zhang, Y.L. Preparation, ablation behavior and mechanism of C/C-ZrC-SiC and C/C-SiC composites. Ceram. Int. 2018, 44, 7481–7490. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, M.Y.; He, H.W.; Wang, X.D.; Tong, K.; Wang, Y.F.; Xu, P.; Zheng, L.; Zeng, G.; Su, Z.A.; et al. The effect of carbon source addition order during sol-gel process on the properties of C/C-ZrC-SiC composites. Ceram. Int. 2017, 47, 35366–35377. [Google Scholar] [CrossRef]
- He, Z.J.; Xu, H.N.; Sun, L.B.; Li, C.; Si, X.Q.; Cao, J. Microstructure and properties of C/C and C/SiC composites brazed by reactive infiltration of Si-Zr filler alloy. Mater. Charact. 2024, 213, 114038. [Google Scholar] [CrossRef]
- Tang, S.; Deng, J.; Wang, S.; Liu, W.; Yang, K. Ablation behaviors of ultra-high temperature ceramic composites. Mater. Sci. Eng. A 2007, 465, 1–7. [Google Scholar] [CrossRef]
- Kou, S.; Ma, J.; Ma, Y.; Liu, Y.; Luan, C.; Yang, S.; Fan, S.; Deng, J.; Wang, P. Microstructure and flexural strength of C/HfC-ZrC-SiC composites prepared by reactive melt infiltration method. J. Eur. Ceram. Soc. 2023, 43, 1864–1873. [Google Scholar] [CrossRef]
- Wang, Z.W.; Fang, G.D.; Wang, B.; Dong, S.; Meng, S.H. In-plane compressive properties and failure mechanisms of gradient Cf/ZrB2–SiC composites in high-temperature environment. Compos. Commun. 2024, 48, 101944. [Google Scholar] [CrossRef]
- Zoli, L.; Servadei, F.; Bassi, G.; Rossi, A.; Montesi, M.; Vinci, A.; Sciti, D.; Panseri, S. From outer space to inside the body: Ultra-high temperature ceramic matrix composites for biomedical applications. J. Eur. Ceram. Soc. 2024, 44, 729–737. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, P.; Zhang, B.G.; Li, Y.; Lu, Y.H. Preparation and tribological properties of C/C–SiC brake composites modified by in situ grown carbon nanofibers. Ceram. Int. 2015, 41, 11733–11740. [Google Scholar] [CrossRef]
- Park, T.H.; Yeo, J.S.; Jang, S.M.; Jin, M.; Isao, M.; Yoon, S.H. Synthesis of silicon monoxide–pyrolytic carbon–carbon nanofiber composites and their hybridization with natural graphite as a means of improving the anodic performance of lithium-ion batteries. Nanotechnology 2012, 23, 355601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.K.; Xiong, X. Microstructure, mechanical properties and oxidation behavior of carbon fiber reinforced PyC/C-TaC/PyC layered-structure ceramic matrix composites prepared by chemical vapor infiltration. Mater. Chem. Phys. 2013, 141, 613–619. [Google Scholar] [CrossRef]
- Wang, Y.L.; Xiong, X.; Zhao, X.J.; Li, G.D.; Chen, Z.K.; Sun, W. Structural evolution and ablation mechanism of a hafnium carbide coating on a C/C composite in an oxyacetylene torch environment. Corros. Sci. 2012, 61, 156–161. [Google Scholar] [CrossRef]
- Chen, B.W.; Ni, D.W.; Wang, J.X.; Jiang, Y.L.; Ding, Q.; Gao, L.; Zhang, X.Y.; Ding, Y.S.; Dong, S.M. Ablation behavior of Cf/ZrC-SiC-based composites fabricated by an improved reactive melt infiltration. J. Eur. Ceram. Soc. 2019, 39, 4617–4624. [Google Scholar] [CrossRef]
- Liu, Z.D.; Wang, Y.L.; Xiong, X.; Ye, Z.Y.; Long, Q.Y.; Wang, J.M.; Li, T.Q.; Liu, C.C. Microstructure and ablation behavior of C/C-SiC-(ZrxHf1−x)C composites prepared by reactive melt infiltration method. Materials 2023, 16, 2120. [Google Scholar] [CrossRef]
- Liu, Y.C.; Xia, Z.X.; Ma, L.K.; Yang, P.N.; Chen, B.B.; Feng, Y.C.; Zeng, Y.; Li, C.L.; Zhang, J.R.; Duan, Y.F. Microstructure and ablation mechanism of C/C-ZrC-SiC composite in the solid scramjet plumes environment. Mater. Charact. 2023, 198, 112754. [Google Scholar] [CrossRef]
- Long, Y.; Javed, A.; Zhao, Y.; Chen, Z.K.; Xiong, X.; Xiao, P. Fiber/matrix interfacial shear strength of C/C composites with PyC-TaC-PyC and PyC-SiC-TaC-PyC multi-interlayers. Ceram. Int. 2013, 39, 6489–6496. [Google Scholar] [CrossRef]
- Tan, W.L.; Li, K.Z.; Li, H.J.; Zhang, J.P.; Ni, C.; Cao, A.Z.; Ma, C.H. Ablation behavior and mechanism of C/C-HfC-SiC composites. Vacuum 2015, 116, 124–129. [Google Scholar] [CrossRef]
- Li, K.Z.; Xie, J.; Fu, Q.G.; Li, H.J.; Guo, L.J. Effects of porous C/C density on the densification behavior and ablation property of C/C-ZrC-SiC composites. Carbon 2013, 57, 161–168. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Sciti, D. Influence of SiC content on the oxidation of carbon fibre reinforced ZrB2/SiC composites at 1500 and 1650 °C in air. J. Eur. Ceram. Soc. 2018, 38, 3767–3776. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Sciti, D.; Watts, J.; Hilmas, G.E.; Fahrenholtz, W.G. Mechanical behaviour of carbon fibre reinforced TaC/SiC and ZrC/SiC composites up to 2100 °C. J. Eur. Ceram. Soc. 2019, 39, 780–787. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Li, K.Z.; Li, W.; Liu, Q.; Kou, G.; Zhang, Y.L. Ablation behavior of C/C-ZrC-SiC composites prepared by reactive melt infiltration under oxyacetylene torch at two heat fluxes. Ceram. Int. 2018, 44, 17345–17358. [Google Scholar] [CrossRef]
- Wang, R.N.; Zhang, J.P.; Fei, J.; Liu, C.D.; Fu, Q.G. Effect of ZrB2 on the ablation behavior of Cf/ZrB2-ZrC-SiC sharp leading edge composites by PIP and RMI. Mater. Charact. 2024, 211, 113898. [Google Scholar] [CrossRef]
- Chen, X.W.; Ni, D.W.; Kan, Y.M.; Jiang, Y.L.; Zhou, H.J.; Wang, Z.; Dong, S.M. Reaction mechanism and microstructure development of ZrSi2 melt-infiltrated Cf/SiC-ZrC-ZrB2 composites: The influence of preform pore structures. J. Materiomics 2018, 4, 266–275. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.L.; Chen, H.M.; Li, W.; Chen, Z.H. Effects of carbon matrix on microstructure and properties of 3-D C/ZrC composites prepared by reactive melt infiltration. Ceram. Int. 2014, 40, 7307–7314. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Xiong, X.; Chen, Z.K.; Xiao, P.; Huang, B.Y. Influence factors of C/C–SiC dual matrix composites prepared by reactive melt infiltration. Mater. Des. 2009, 30, 3738–3742. [Google Scholar] [CrossRef]
- Liu, Z.D.; Wang, Y.L.; Xiong, X.; Zhang, H.B.; Ye, Z.Y.; Long, Q.Y.; Wen, Q.B.; Wang, J.M.; Li, T.Q.; Liu, C.C. Structural optimization and air-plasma ablation behaviors of C/C-SiC-(ZrxHf1−x)C composites prepared by reactive melt infiltration method. Corros. Sci. 2023, 222, 111408. [Google Scholar] [CrossRef]
- Lopatin, S.I.; Stolyarova, V.L.; Sevastyanov, V.G.; Nosatenko, P.Y.; Gorskii, V.V.; Sevast’yanov, D.V.; Kuznetsov, N.T. Determination of the saturation vapor pressure of silicon by Knudsen cell mass spectrometry. Russ. J. Inorg. Chem. 2012, 57, 219–225. [Google Scholar] [CrossRef]
- Drevet, B.; Eustathopoulos, N. Wetting of ceramics by molten silicon and silicon alloys: A review. J. Mater. Sci. 2012, 47, 8247–8260. [Google Scholar] [CrossRef]
- Roger, J.; Chollon, G. Mechanisms and kinetics during reactive infiltration of molten silicon in porous graphite. Ceram. Int. 2019, 45, 8690–8699. [Google Scholar] [CrossRef]
- Giuranno, D.; Polkowska, A.; Polkowski, W.; Novakovic, R. Wetting behavior and reactivity of liquid Si-10Zr alloy in contact with glassy carbon. J. Alloys Compd. 2020, 822, 153643. [Google Scholar] [CrossRef]
- Makurunje, P.; Middleburgh, S.C.; Lee, W.E. Addressing high processing temperatures in reactive melt infiltration for multiphase ceramic composites. J. Eur. Ceram. Soc. 2023, 43, 183–197. [Google Scholar] [CrossRef]
- Sangsuwan, P.; Tewari, S.N.; Gatica, J.E.; Singh, M.; Dickerson, R. Reactive infiltration of silicon melt through microporous amorphous carbon preforms. Metall. Mater. Trans. B 1999, 30, 933–944. [Google Scholar] [CrossRef]
- Chen, X.W.; Dong, S.M.; Kan, Y.M.; Jin, X.H.; Zhou, H.J.; Ni, D.W.; Wang, D.K. Microstructure and mechanical properties of three dimensional Cf/SiC-ZrC-ZrB2 composites prepared by reactive melt infiltration method. J. Eur. Ceram. Soc. 2016, 36, 3969–3976. [Google Scholar] [CrossRef]
- Jiang, J.M.; Wang, S.; Li, W.; Chen, Z.H. Fabrication of Cf/ZrC–SiC composites using Zr–8.8Si alloy by melt infiltration. Ceram. Int. 2015, 41, 8488–8493. [Google Scholar] [CrossRef]
- Gibson, R.F. Principles of Composite Material Mechanics, 4th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 243–246. [Google Scholar]
- Khatri, S.C.; Koczak, M.J. Thick-section AS4-graphite/E-glass/PPS hybrid composites: Part I. tensile behavior. Compos. Sci. Technol. 1996, 56, 181–192. [Google Scholar] [CrossRef]
- Albiez, A.; Schwaiger, R. Size effect on the strength and deformation behavior of glassy carbon nanopillars. MRS Adv. 2019, 4, 133–138. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Y.; Xiong, X.; Zhang, H.; Ye, Z.; Long, Q.; Wang, J.; Li, T.; Liu, C. Microstructural Evolution and Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites with Different Carbon Matrices. J. Compos. Sci. 2024, 8, 303. https://doi.org/10.3390/jcs8080303
Liu Z, Wang Y, Xiong X, Zhang H, Ye Z, Long Q, Wang J, Li T, Liu C. Microstructural Evolution and Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites with Different Carbon Matrices. Journal of Composites Science. 2024; 8(8):303. https://doi.org/10.3390/jcs8080303
Chicago/Turabian StyleLiu, Zaidong, Yalei Wang, Xiang Xiong, Hongbo Zhang, Zhiyong Ye, Quanyuan Long, Jinming Wang, Tongqi Li, and Congcong Liu. 2024. "Microstructural Evolution and Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites with Different Carbon Matrices" Journal of Composites Science 8, no. 8: 303. https://doi.org/10.3390/jcs8080303
APA StyleLiu, Z., Wang, Y., Xiong, X., Zhang, H., Ye, Z., Long, Q., Wang, J., Li, T., & Liu, C. (2024). Microstructural Evolution and Mechanical Behaviors of Cf/Cm-SiC-(ZrxHf1−x)C Composites with Different Carbon Matrices. Journal of Composites Science, 8(8), 303. https://doi.org/10.3390/jcs8080303






