TEM Study of a Layered Composite Structure Produced by Ion-Plasma Treatment of Aluminum Coating on the Ti-6Al-4V Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
- -
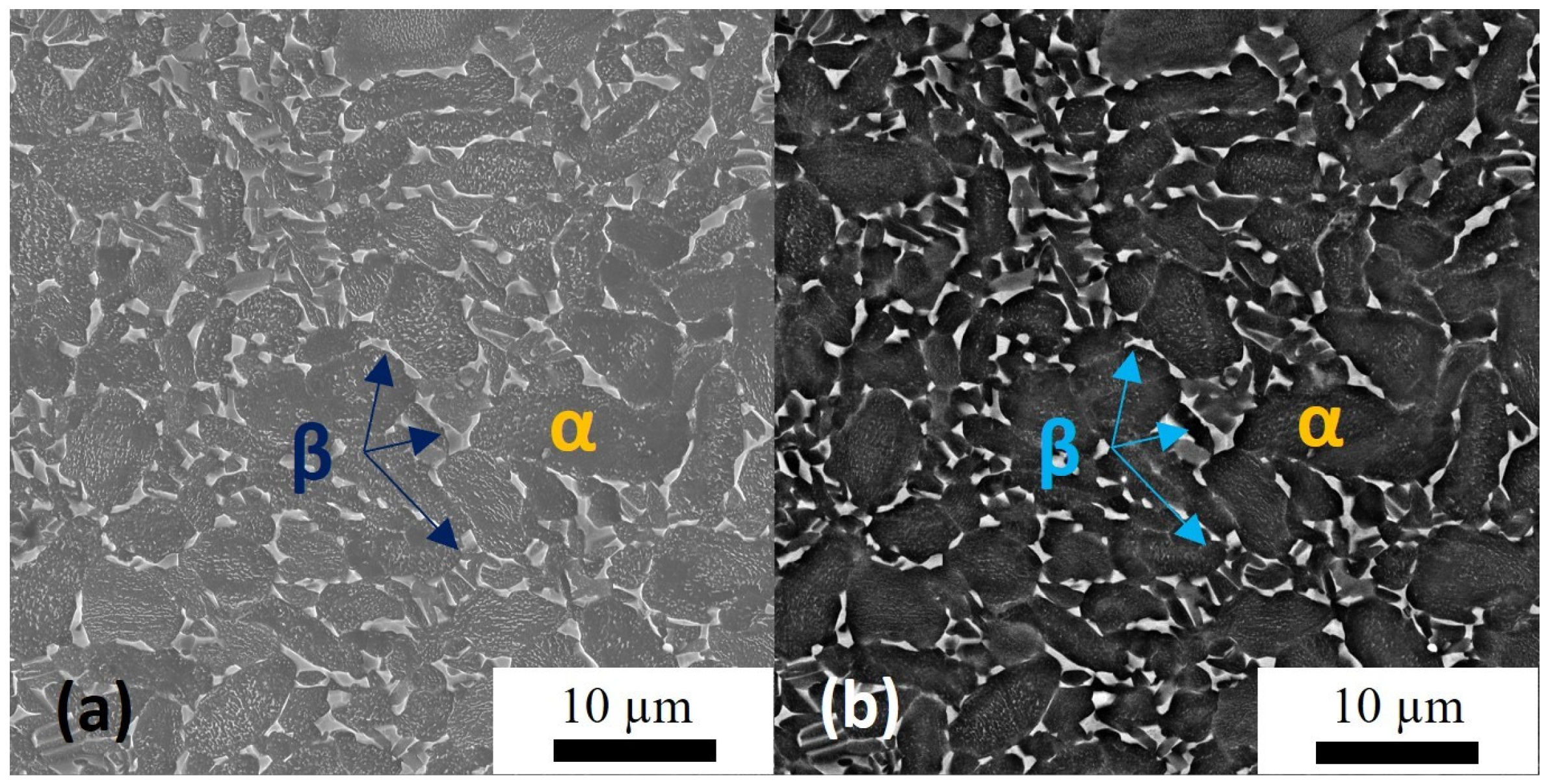
- The CIPT processing allows formation of the intermetallic coatings and surface-modified layers in the titanium alloy. The main phase is the TiAl3 intermetallide, which correlates with previous research [31,45,46,47,48]. In Ti alloy, a desirable phase TiAl and titanium-enriched Ti3Al phase are also present. Therefore, the variation of the treatment regime is needed to reduce the content of the TiAl3 phase and to increase the fraction of the TiAl one.
- -
- Despite the fact that TiAl3 is the main intermetallic phase both in the coating and in the base material, it possesses different morphologies in either. The sequential variation of the microstructure (grain size and distribution) was observed when one moves from the surface of the specimen to its depth (IM—intermetallics, Al—Al-based solid solution, Ti—Ti-based solid solution):Fine grains (IM) → fine grains (IM) + nanocrystallites (nc-(Al(Ti) + α-Ti)) → nanocrystallites (nc-(Al(Ti) + α-Ti)) → interface “coating/substrate” → ultrafine grains (IM) → fine grains (Ti)During the phase transformation under the CIPT, the temperature-assisted (500 °C) grain growth of the intermetallic phase TiAl3 occurs in Al-based and Ti-based parts with principally different melting and recrystallization temperatures [2]. The grain size in the Al-based coating is obviously higher than that of the Ti-based one.
- -
- Phase transformation and recrystallization are not realized in nanocrystalline regions of the aluminum coating near the “titanium alloy/coating” interface. Elemental EDS analysis and TEM diffraction analysis were completed, and confirmed the preservation of the nanoscale-sized fragments and high concentrations of Al in this region. A high fraction of the grain boundaries in nanocrystalline regions can favor the diffusion of the elements in both directions and, therefore, stimulates phase transformation in coarser grains situated under and over the nanocrystalline layer. These diffusion flows can suppress grain boundary migration and support the stability of the nanocrystalline structure.
4. Conclusions
- The deposition of Al on the Ti-6Al-4V alloy is accompanied by the formation of a layered aluminum coating with a gradient microstructure—nanocrystalline near the “coating/substrate” interface and fine-grained in the outer part of the coating. The α-stabilized region of ≈5 µm thickness is formed in the surface layer of the base titanium alloy due to the diffusion of the aluminum during the deposition of the coating.
- After the CIPT, the coating and the surface of the base titanium alloy have a layered morphology, and each of the layers possesses different grain structure and composition. Moving from the surface of the former Al coating to the depth of the specimen, the following evolution of the microstructure and phase composition is observed.Phase composition:TiAl3 → TiAl3 + nc-(Al(Ti) + α-Ti) → nc-(Al(Ti) + α-Ti) → TiAl3 → TiAl3 + TiAl → TiAl → Ti3Al → α-Ti alloy → (α + β)-Ti alloyMicrostructure:Fine grains (IM) → fine grains (IM) + nanocrystallites (nc-(Al(Ti) + α-Ti)) → nanocrystallites (nc-(Al(Ti) + α-Ti)) → interface “coating/substrate” → ultrafine grains (IM) → fine grains (Ti),IM—intermetallides, Al—Al-based solid solution, Ti—Ti-based solid solution.
- The nanocrystalline aluminum layer, which is formed during the deposition of the aluminum coating near the “titanium alloy/coating” interface, does not undergo recrystallization under the CIPT. The layer can favor the diffusion of the elements in both directions and, therefore, stimulates phase transformation in coarser grains situated under and over the nanocrystalline layer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti–6Al–4V alloy. J. Alloys Compd. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2003; ISBN 9783527305346. [Google Scholar]
- Liu, Z.; Zhang, X.; Xuan, F.; Wang, Z.; Tu, S. In situ synthesis of TiN/Ti3Al intermetallic matrix composite coatings on Ti6Al4V alloy. Mater. Des. 2012, 37, 268–273. [Google Scholar] [CrossRef]
- Kustas, F.M.; Misra, M.S. Friction and wear of titanium alloys. In ASM Handbook, Friction, Lubrication, and Wear Technology; Blau, P.J., Ed.; ASM International: Geauga County, OH, USA, 1992; Volume 18, pp. 778–784. [Google Scholar]
- Budinski, K.G. Tribological properties of titanium alloys. Wear 1991, 151, 203–217. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Xing, Y.Z.; Wang, G.; Zhang, Y.; Chen, Y.N.; Dargusch, M. Development in plasma surface diffusion techniques of Ti-6Al-4V alloy: A review. Int. J. Adv. Manuf. Technol. 2017, 92, 1901–1912. [Google Scholar] [CrossRef]
- Avelar-Batista, J.C.; Spain, E.; Housden, J.; Matthews, A.; Fuentes, G.G. Plasma nitriding of Ti6Al4V alloy and AISI M2 steel substrates using DC glow discharge under a triode configuration. Surf. Coat. Technol. 2005, 200, 1954–1961. [Google Scholar] [CrossRef]
- Li, X.; Tang, B.; Ye, J. Fabrication of Zr and Zr-N surface alloying layers and hardness improvement of Ti-6Al-4V alloy by plasma surface alloying technique. Appl. Surf. Sci. 2012, 258, 1981–1984. [Google Scholar] [CrossRef]
- Cassar, G.; Matthews, A.; Leyland, A. Triode plasma diffusion treatment of titanium alloys. Surf. Coat. Technol. 2012, 212, 20–31. [Google Scholar] [CrossRef]
- Cassar, G.; Banfield, S.; Wilson, J.A.B.; Housden, J.; Matthews, A.; Leyland, A. Tribological properties of duplex plasma oxidised, nitrided and PVD coated Ti-6Al-4V. Surf. Coat. Technol. 2011, 206, 395–404. [Google Scholar] [CrossRef]
- Casadei, F.; Tului, M. Combining thermal spraying and PVD technologies: A new approach of duplex surface engineering for Ti alloys. Surf. Coat. Technol. 2013, 237, 415–420. [Google Scholar] [CrossRef]
- Attard, B.; Matthews, A.; Leyland, A.; Cassar, G. Enhanced surface performance of Ti-6Al-4V alloy using a novel duplex process combining PVD-Al coating and triode plasma oxidation. Surf. Coat. Technol. 2014, 257, 154–164. [Google Scholar] [CrossRef]
- Ryabchikov, A.I.; Sivin, D.O.; Bozhko, I.A.; Stepanov, I.B.; Shevelev, A.E. Microstructure of titanium alloy modified by high-intensity implantation of low-and high-energy aluminium ions. Surf. Coat. Technol. 2020, 391, 125722. [Google Scholar] [CrossRef]
- Liang, W.P.; Miao, Q.; Ben, N.J.; Ren, B.L. Tribological behaviors of Ti-6Al-4V alloy with surface plasma molybdenized layer. Surf. Coat. Technol. 2013, 228, S249–S253. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Kukareko, V.A.; Zinchenko, V.F.; Eroshenko, A.Y.; Kuchina, A.S. Increasing the cyclic life of submicrocrystalline and large-grain titanium in high-intensity ionic implantation. Steel Transl. 2010, 40, 729–732. [Google Scholar] [CrossRef]
- Romankov, S.; Kaloshkin, S.D.; Hayasaka, Y.; Hayasaka, N.; Kasai, E.; Komarov, S.V. Effect of process parameters on the formation of Ti-Al coatings fabricated by mechanical milling. J. Alloys Compd. 2009, 484, 665–673. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M.; Kaysser, W.A. Intermetallic Ti-Al Coatings for Protection of Titanium Alloys: Oxidation and Mechanical Behavior. Surf. Coat. Technol. 1997, 94, 34–40. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Y.; Lin, X.; Zhou, B.; Qian, T. Microstructure and high temperature oxidation resistance of Ti-Ni gradient coating on TA2 titanium alloy fabricated by laser cladding. Opt. Laser Technol. 2016, 83, 140–147. [Google Scholar] [CrossRef]
- Vojtěch, D.; Novák, P.; Macháč, P.; Morťaniková, M.; Jurek, K. Surface protection of titanium by Ti5Si3 silicide layer prepared by combination of vapour phase siliconizing and heat treatment. J. Alloys Compd. 2008, 464, 179–184. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.; Weatherly, G.; Wilkinson, D.; Kovalev, A.; Wainstein, D. The role of chromium in protective alumina scale formation during the oxidation of ternary TiAlCr alloys in air. Intermetallics 2004, 12, 165–180. [Google Scholar] [CrossRef]
- Lazurenko, D.V.; Bataev, I.A.; Laptev, I.S.; Ruktuev, A.A.; Maliutina, I.N.; Golkovsky, M.G.; Bataev, A.A. Formation of Ti-Al intermetallics on a surface of titanium by non-vacuum electron beam treatment. Mater. Charact. 2017, 134, 202–212. [Google Scholar] [CrossRef]
- Wendler, B.G.; Kaczmarek, Ł. Oxidation resistance of nanocrystalline microalloyed γ-TiAl coatings under isothermal conditions and thermal fatigue. J. Mater. Process. Technol. 2005, 164–165, 947–953. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Wendler, B.; Siniarski, D.; Rylski, A.; Bieliński, D.; Dobrowolski, O.; Lipiński, D. Oxidation Resistance of Refractory γ-TiAlW Coatings. Surf. Coat. Technol. 2007, 201, 6167–6170. [Google Scholar] [CrossRef]
- Cizek, J.; Man, O.; Roupcova, P.; Loke, K.; Dlouhy, I. Oxidation performance of cold spray Ti-Al barrier coated γ-TiAl intermetallic substrates. Surf. Coat. Technol. 2015, 268, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Teer, D.G.; Salem, F.B. The formation of low friction wear-resistant surfaces on titanium by ion plating. Thin Solid Film. 1977, 45, 583–589. [Google Scholar] [CrossRef]
- Hampshire, J.; Kelly, P.J.; Teer, D.G. The tribological properties of co-deposited aluminium–titanium alloy coatings. Thin Solid Film. 2004, 447, 392–398. [Google Scholar] [CrossRef]
- Flom, D.G.; Komanduri, R. Some indentation and sliding experiments on single crystal and polycrystalline materials. Wear 2002, 252, 401–429. [Google Scholar] [CrossRef]
- Appel, F.; Paul, J.D.H.; Oehring, M. Gamma Titanium Aluminide Alloys: Science and Technology; Wiley-VCH: Weinheim, Germany, 2011; pp. 5–248. [Google Scholar]
- Zhang, Y.; Li, H.; Zhang, K. Investigation of the Laser Melting Deposited TiAl Intermetallic Alloy on Titanium Alloy. Adv. Mater. Res. 2011, 146, 1638–1641. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Y.Y.; Hao, Y.L.; Yang, R. Growth of intermetallic layer in multi-laminated Ti/Al diffusion couples. Mater. Sci. Eng. Struct. 2006, 435, 638–647. [Google Scholar] [CrossRef]
- Ramos, A.; Vieira, M.; Morgiel, J.; Grzonka, J.; Simões, S.; Vieira, M. Production of intermetallic compounds from Ti/Al and Ni/Al multilayer thin films—A comparative study. J. Alloys Compd. 2009, 484, 335–340. [Google Scholar] [CrossRef]
- Lyu, S.; Sun, Y.; Li, G.; Xiao, W.; Ma, C. Effect of layer sequence on the mechanical properties of Ti/TiAl laminates. Mater. Des. 2018, 143, 160–168. [Google Scholar] [CrossRef]
- Gachon, J.-C.; Rogachev, A.; Grigoryan, H.; Illarionova, E.; Kuntz, J.-J.; Kovalev, D.; Nosyrev, A.; Sachkova, N.; Tsygankov, P. On the mechanism of heterogeneous reaction and phase formation in Ti/Al multilayer nanofilms. Acta Mater. 2005, 53, 1225–1231. [Google Scholar] [CrossRef]
- Romankov, S.E.; Mamaeva, A.; Vdovichenko, E.; Ermakov, E. Effect of ion irradiation on the interdiffusion growth of aluminide phases in Ti–Al diffusion couple. Nucl. Instrum. Methods Phys. Res. Sect. Beam Interact. Mater. At. 2005, 237, 575–584. [Google Scholar] [CrossRef]
- Luo, J.G.; Acoff, V.L. Using cold roll bonding and annealing to process Ti/Al multi-Layered composites from elemental foils. Mater. Sci. Eng. 2004, 379, 164–172. [Google Scholar] [CrossRef]
- Thuillard, M.; Tran, L.T.; Nicolet, M.A. Al3Ti formation by diffusion of aluminum through titanium. Thin Solid Film. 1988, 166, 21–28. [Google Scholar] [CrossRef]
- Zhao, X.-A.; So, F.; Nicolet, N.-A. TiAl3 formation by furnace annealing of Ti/Al bilayers and the effects of impurities. J. Appl. Phys. 1988, 63, 2600–2607. [Google Scholar] [CrossRef] [Green Version]
- Van Loo, F.J.J.; Rieck, G.D. Diffusion in the titanium-aluminium system—I. Interdiffusion between solid Al and Ti or Ti-Al alloys. Acta Metall. 1973, 21, 61–71. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Wang, D.N.; Wang, B.Y.; Yu, R.S.; Wei, L. Annealing studies of Ti/Al multilayer film by slow positron beam. Appl. Surf. Sci. 2007, 253, 7309–7312. [Google Scholar] [CrossRef]
- van Loo, F.J.J.; Rieck, G.D. Diffusion in the titanium-aluminium system—II. Interdiffusion in the composition range between 25 and 100 at.% Ti. Acta Metall. 1973, 21, 73–84. [Google Scholar] [CrossRef]
- Mackowiak, J.; Shreir, L.L. The nature and growth of interaction layers formed during the reaction between solid titanium and liquid aluminium. J. Less Common Met. 1959, 1, 456–466. [Google Scholar] [CrossRef]
- Hibino, A. Analysis of combustion synthesis process of TiAl intermetallic compound by dipping experiment of Ti wire into Al melt. J. Jpn. Inst. Met. 1997, 61, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Sujata, M.; Bhargava, S.; Sangal, S. On the formation of TiAl3 during reaction between solid Ti and liquid Al. J. Mater. Sci. Lett. 1997, 16, 1175–1178. [Google Scholar] [CrossRef]
- Raman, A.; Schubert, K. Über den Aufbau einiger zu TiAl3 verwandter Legierungsreihen. II. Untersuchungen in einigen T-Al-Si- und T4…6-In-Systemen. Z. Metallkde 1965, 56, 44–52. [Google Scholar]
- Zhao, H.; Yu, M.; Jiang, Z.; Zhou, L.; Song, X. Interfacial microstructure and mechanical properties of Al/Ti dissimilar joints fabricated via friction stir welding. J. Alloys Compd. 2019, 789, 139–149. [Google Scholar] [CrossRef]
- Choi, J.-W.; Liu, H.; Fujii, H. Dissimilar friction stir welding of pure Ti and pure Al. Mater. Sci. Eng. 2018, 730, 168–176. [Google Scholar] [CrossRef]
- Assari, A.H.; Eghbali, B. Solid State Diffusion Bonding Characteristics at the Interfaces of Ti and Al Layers. J. Alloys Compd. 2019, 773, 50–58. [Google Scholar] [CrossRef]
- Rogachev, A.S. Exothermic reaction waves in multilayer nanofilms. Russ. Chem. Rev. 2008, 77, 21–37. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Gachon, J.C.; Grigoryan, H.E.; Illeková, E.; Kochetov, N.F.; Nosyrev, F.N.; Sachkova, N.V.; Schuster, J.C.; Sharafutdinov, M.R.; Shkodich, N.F.; et al. Diffraction of synchrotron radiation for in situ study of the heterogeneous reaction mechanisms in lamellar composites obtained by mechanical activation and magnetron sputtering. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2007, 575, 126–129. [Google Scholar] [CrossRef]
- Ramos, A.S.; Calinas, R.; Vieira, M.T. The formation of γ-TiAl from Ti/Al multilayers with different periods. Surf. Coat. Technol. 2006, 200, 6196–6200. [Google Scholar] [CrossRef]
- Sun, Y.; Haly, J.; Kulkarni, K.; Aindow, M.; Lavernia, E.J. Influence of electric current on microstructure evolution in Ti/Al and Ti/TiAl3 during spark plasma sintering. J. Alloys Compd. 2015, 648, 1097–1103. [Google Scholar] [CrossRef]
- Conrad, H. Effects of electric current on solid state phase transformations in metals. Mater. Sci. Eng. 2000, 287, 227–237. [Google Scholar] [CrossRef]
- Garbacz, H.; Pouquet, J.M.; García-Lecina, E.; Díaz-Fuentes, M.; Wieciński, P.; Martin, R.H.; Wierzchoń, T.; Kurzydlowski, K.J. Microstructure, fatigue and corrosion properties of the Ti–Al intermetallic layers. Surf. Coat. Technol. 2011, 205, 4433–4440. [Google Scholar] [CrossRef]
- Garbacz, H.; Wieciński, P.; Ossowski, M.; Ortore, G.; Wierzchoń, T.; Kurzydłowski, K.J. Surface engineering techniques used for improving the mechanical and tribological properties of the Ti6A14V alloy. Surf. Coat. Technol. 2008, 202, 2453–2457. [Google Scholar] [CrossRef]
- Wiecinski, P.; Garbacz, H.; Ossowski, M.; Wierzchoń, T.; Kurzydlowski, K.J. Ti–Al Intermetallic Layers Produced on Titanium Alloy by Duplex Method. Key Eng. Mater. 2007, 333, 285–288. [Google Scholar] [CrossRef]
- Romankov, S.; Mamaeva, A.; Kaloshkin, S.D.; Komarov, S.V. Pulsed plasma treatment of Ti–Al coatings produced by mechanical alloying method. Mater. Lett. 2007, 61, 5288–5291. [Google Scholar] [CrossRef]
- Sahu, B.P.; Wu, W.; Wang, J.; Misra, A. Deformation behavior of crystalline/amorphous Al-Si nanocomposites with nanolaminate or nanofibrous microstructures. Phys. Rev. Mater. 2022, 6, 094002. [Google Scholar] [CrossRef]
- Williams, D.B.; Carter, C.B. Transmission Electron Microscopy. In Textbook for Materials Science; Springer: Greer, SC, USA, 2009. [Google Scholar]
- Perevalova, O.B.; Panin, A.V.; Kazachenok, M.S.; Sinyakova, E.A. Effect of Ultrasonic Impact Treatment on Structural Phase Transformations in Ti-6Al-4V Titanium Alloy. Phys. Mesomech. 2022, 25, 248–258. [Google Scholar] [CrossRef]
- Shekhtman, S.R.; Migranov, M.S. Influence of ion bombardment of a substrate on the quality of vacuum-plasma Ti-TiN coatings. IOP Conf. Ser. J. Phys. Conf. Ser. 2017, 872, 012023. [Google Scholar] [CrossRef] [Green Version]
- Bay, B.; Hansen, N. Recrystallization in commercially pure aluminum. Metall. Trans. 1984, 15, 287–297. [Google Scholar] [CrossRef]
- Hamphreys, F.J.; Hatherly, M. Recrystallization and Related Phemonena; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Okeke, U.; Yilmazer, H.; Sato, S.; Boehler, C.J. Strength enhancement of an aluminum alloy through high pressure torsion. Mater. Sci. Eng. 2019, 760, 195–205. [Google Scholar] [CrossRef]
- Ye, W.; Kumar, P.; Mishra, M.; Mushongera, L.T. Local damage in grain boundary stabilized nanocrystalline aluminum. Mater. Lett. 2021, 300, 130153. [Google Scholar] [CrossRef]
- Nazarov, A.A.; Romanov, A.E.; Valiev, R.Z. On the structure, stress fields and energy of nonequilibrium grain boundaries. Acta Metall. Mater. 1993, 41, 1033–1040. [Google Scholar] [CrossRef]
- Yu, S.; Zhipeng, W.; Lianxi, H.; Binghua, W.; Taiqing, D. Characterization of solid phase diffusion reaction behavior and diffusion reaction kinetics of Ti/Al. Rare Metal Mater. Eng. 2017, 46, 2080–2086. [Google Scholar] [CrossRef]
- Divinski, S.V.; Reglitz, G.; Rösner, H.; Estrin, Y.; Wilde, G. Ultra-fast diffusion channels in pure Ni severely deformed by equial-channel angular pressing. Acta Mater. 2011, 59, 1974–1985. [Google Scholar] [CrossRef]








| Experimental Values | Calculated Values | |||||
|---|---|---|---|---|---|---|
| α-Ti | Al | Al3Ti | ||||
| dhkl, Å | hkl | d, Å | hkl | d, Å | hkl | d, Å |
| 2.556 | 100 | 2.558 | 111 | 2.338 | 002 | 4.291 |
| 101 | 3.515 | |||||
| 002 | 2.341 | 110 | 2.724 | |||
| 2.23 | 101 | 2.244 | 002 | 2.025 | 112 | 2.300 |
| 103 | 2.297 | |||||
| 004 | 2.146 | |||||
| 1.73 | 102 | 1.729 | - | - | 200 | 1.927 |
| 202 | 1.758 | |||||
| 211 | 1.690 | |||||
| 114 | 1.686 | |||||
| 1.48 | 110 | 1.475 | 022 | 1.432 | 105 | 1.568 |
| 213 | 1.476 | |||||
| 204 | 1.434 | |||||
| 006 | 1.431 | |||||
| 1.34 | 103 | 1.326 | - | - | 220 | 1.363 |
| 1.26 | 200 | 1.275 | 113 | 1.221 | 222 | 1.299 |
| 112 | 1.244 | 301 | 1.270 | |||
| 116 | 1.267 | |||||
| Spectrum | Al | Ti | V | Predicted Phase |
|---|---|---|---|---|
| Spectrum 1 | 77.59 | 21.47 | 0.94 | TiAl3 |
| Spectrum 2 | 76.44 | 22.89 | 0.67 | |
| Spectrum 3 | 97.85 | 2.15 | 0 | Al(Ti,V) + TiAl3 |
| Spectrum 4 | 98.16 | 1.65 | 0.19 | |
| Spectrum 5 | 97.24 | 2.76 | 0 | |
| Spectrum 6 | 80.69 | 18.93 | 0.38 | TiAl3 |
| Spectrum 7 | 78.10 | 21.06 | 0.84 | |
| Spectrum 8 | 77.78 | 21.04 | 1.18 | |
| Spectrum 9 | 79.29 | 20.09 | 0.62 | |
| Spectrum 10 | 70.98 | 28.23 | 0.79 | |
| Spectrum 11 | 77.62 | 21.17 | 1.21 | |
| Spectrum 12 | 52.70 | 46.41 | 0.89 | TiAl |
| Spectrum 13 | 57.68 | 42.18 | 0.14 | |
| Spectrum 14 | 53.95 | 46.05 | 0 | |
| Spectrum 15 | 12.73 | 85.95 | 1.32 | Ti(Al,V) |
| Spectrum 16 | 12.76 | 84.81 | 2.43 | |
| Spectrum 17 | 14.51 | 83.95 | 1.54 | |
| Spectrum 18 | 12.70 | 85.91 | 1.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, A.; Ramazanov, K.; Nazarov, A.; Mukhamadeev, V.; Zagibalova, E.; Astafurova, E. TEM Study of a Layered Composite Structure Produced by Ion-Plasma Treatment of Aluminum Coating on the Ti-6Al-4V Alloy. J. Compos. Sci. 2023, 7, 271. https://doi.org/10.3390/jcs7070271
Nikolaev A, Ramazanov K, Nazarov A, Mukhamadeev V, Zagibalova E, Astafurova E. TEM Study of a Layered Composite Structure Produced by Ion-Plasma Treatment of Aluminum Coating on the Ti-6Al-4V Alloy. Journal of Composites Science. 2023; 7(7):271. https://doi.org/10.3390/jcs7070271
Chicago/Turabian StyleNikolaev, Aleksey, Kamil’ Ramazanov, Almaz Nazarov, Vener Mukhamadeev, Elena Zagibalova, and Elena Astafurova. 2023. "TEM Study of a Layered Composite Structure Produced by Ion-Plasma Treatment of Aluminum Coating on the Ti-6Al-4V Alloy" Journal of Composites Science 7, no. 7: 271. https://doi.org/10.3390/jcs7070271
APA StyleNikolaev, A., Ramazanov, K., Nazarov, A., Mukhamadeev, V., Zagibalova, E., & Astafurova, E. (2023). TEM Study of a Layered Composite Structure Produced by Ion-Plasma Treatment of Aluminum Coating on the Ti-6Al-4V Alloy. Journal of Composites Science, 7(7), 271. https://doi.org/10.3390/jcs7070271






