Preparation and Properties of Flexible CuI/Polyvinylpyrrolidone Nanocomposite Thermoelectric Film
Abstract
:1. Introduction
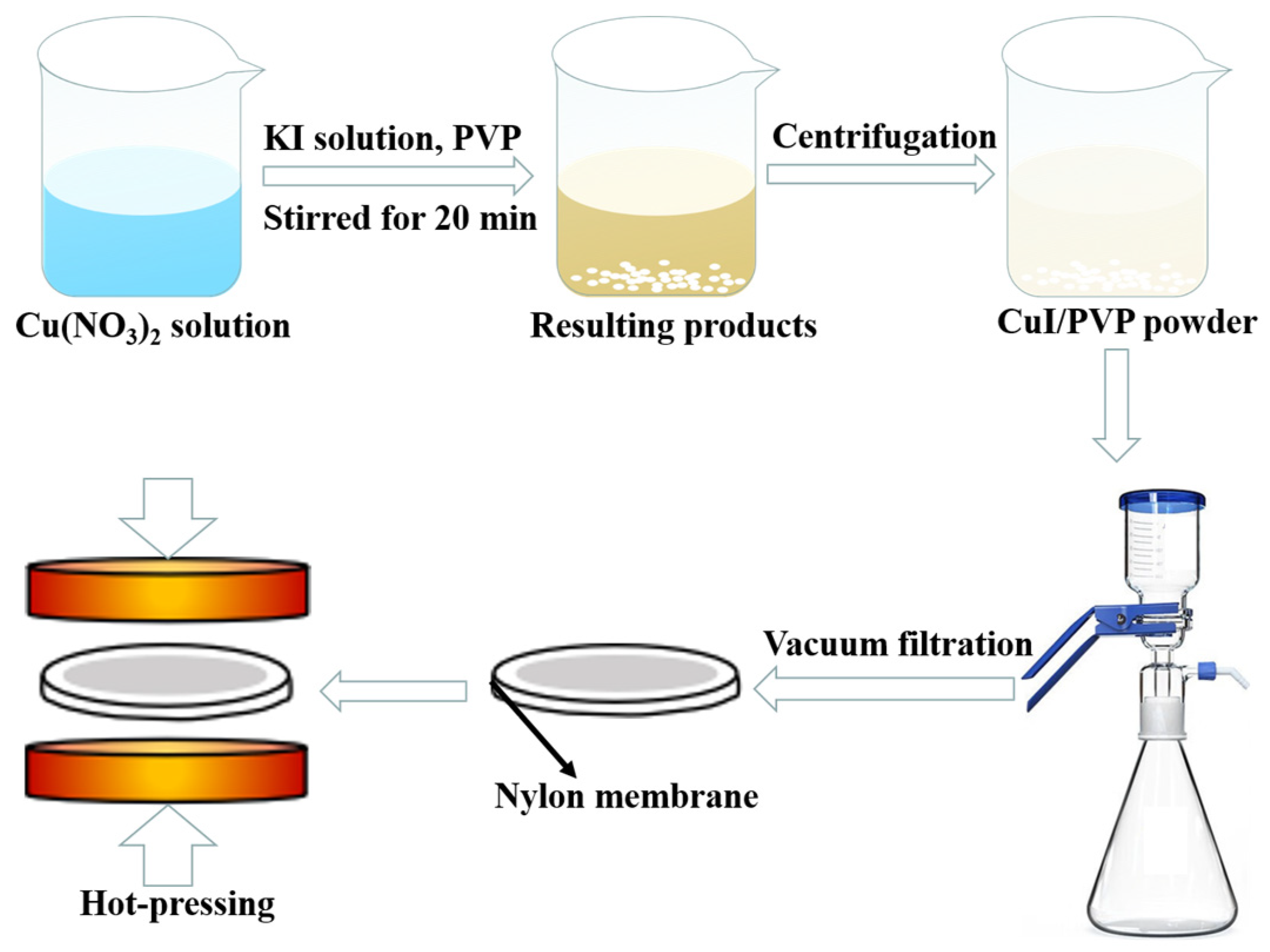
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Leonov, V.; Vullers, R.J.M. Wearable electronics self-powered by using human body heat: The state of the art and the perspective. J. Renew. Sustain. Energy 2009, 1, 062701. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Liu, Y.; Wang, B.; Fang, L.; Qiu, J.; Zhang, K.; Wang, S. Exceptional thermoelectric properties of flexible organic-inorganic hybrids with monodispersed and periodic nanophase. Nat. Commun. 2018, 9, 3817. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, e2106052. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shi, H.; Wang, S.; Wang, D.; Qin, B.; Wang, Y.; Chang, C.; Zhao, L. Enhancing Carrier Mobility and Seebeck Coefficient by Modifying Scattering Factor. Adv. Energy Mater. 2023, 13, 2300312. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Trinh, T.L.; Chang, C.; Zhao, L.-D.; Nguyen, T.H.; Duong, V.T.; Duong, A.T.; Park, J.H.; Park, S.; Kim, J.; et al. Unidentified major p-type source in SnSe: Multivacancies. NPG Asia Mater. 2022, 14, 42. [Google Scholar] [CrossRef]
- Jin, Z.; Mao, T.; Qiu, P.; Yue, Z.; Wang, L.; Zhao, K.; Ren, D.; Shi, X.; Chen, L. Thermoelectric properties and service stability of Ag-containing Cu2Se. Mater. Today Phys. 2021, 21, 100550. [Google Scholar] [CrossRef]
- Li, M.; Cortie, D.L.; Liu, J.; Yu, D.; Islam, S.M.K.N.; Zhao, L.; Mitchell, D.R.; Mole, R.A.; Cortie, M.B.; Dou, S.; et al. Ultra-high thermoelectric performance in graphene incorporated Cu2Se: Role of mismatching phonon modes. Nano Energy 2018, 53, 993–1002. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Zeng, Z.; Embs, J.; Pei, Y.; Ma, J.; Chen, Y. Soft-mode dynamics in the ferroelectric phase transition of GeTe. npj Comput. Mater. 2021, 7, 118. [Google Scholar] [CrossRef]
- Abdellaoui, L.; Chen, Z.; Yu, Y.; Luo, T.; Hanus, R.; Schwarz, T.; Villoro, R.B.; Cojocaru-Mirédin, O.; Snyder, G.J.; Raabe, D.; et al. Parallel Dislocation Networks and Cottrell Atmospheres Reduce Thermal Conductivity of PbTe Thermoelectrics. Adv. Funct. Mater. 2021, 31, 2101214. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer–inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820–841. [Google Scholar] [CrossRef]
- Hewitt, C.A.; Kaiser, A.B.; Roth, S.; Craps, M.; Czerw, R.; Carroll, D.L. Multilayered carbon nanotube/polymer composite based thermoelectric fabrics. Nano Lett. 2012, 12, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Si, Y.; Zhao, C.; Yu, D.; Wang, W.; Sun, G. Flexible and Washable Poly(Ionic Liquid) Nanofibrous Membrane with Moisture Proof Pressure Sensing for Real-Life Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 27200–27209. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, M.; Gao, G. Preparation and application of graphene-based wearable sensors. Nano Res. 2022, 15, 9850–9865. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Xiao, Z.-C.; Yang, Y.; Deng, B.-W.; Yin, B.; Ke, K.; Yang, M.-B. Flexible TPU strain sensors with tunable sensitivity and stretchability by coupling AgNWs with rGO. J. Mater. Chem. C 2020, 8, 4040–4048. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.-J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Fan, Z.; Razavi, H.; Do, J.-W.; Moriwaki, A.; Ergen, O.; Chueh, Y.-L.; Leu, P.W.; Ho, J.C.; Takahashi, T.; Reichertz, L.A.; et al. Three-dimensional nanopillar-array photovoltaics on low-cost and flexible substrates. Nat. Mater. 2009, 8, 648–653. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Sun, Z.; Zhang, Q.; Wei, P.; Mu, X.; Zhou, H.; Li, C.; Ma, S.; He, D.; et al. Superparamagnetic enhancement of thermoelectric performance. Nature 2017, 549, 247–251. [Google Scholar] [CrossRef]
- Qu, S.; Yao, Q.; Wang, L.; Chen, Z.; Xu, K.; Zeng, H.; Shi, W.; Zhang, T.; Uher, C.; Chen, L. Highly anisotropic P3HT films with enhanced thermoelectric performance via organic small molecule epitaxy. NPG Asia Mater. 2016, 8, e292. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.; Shen, S. A facile chemical reduction approach for effectively tuning thermoelectric properties of PEDOT films. Org. Electron. 2015, 17, 151–158. [Google Scholar] [CrossRef]
- Wang, J.; Cai, K.; Song, H.; Shen, S. Simultaneously enhanced electrical conductivity and Seebeck coefficient in Poly (3,4-ethylenedioxythiophene) films treated with hydroiodic acid. Synth. Met. 2016, 220, 585–590. [Google Scholar] [CrossRef]
- Ni, D.; Song, H.; Chen, Y.; Cai, K. Free-standing highly conducting PEDOT films for flexible thermoelectric generator. Energy 2019, 170, 53–61. [Google Scholar] [CrossRef]
- Alamer, F.A.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y.; et al. Review on PEDOT:PSS-Based Conductive Fabric. ACS Omega 2022, 7, 35371–35386. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, Y.; Wang, W.; Hu, M.; Huang, X.; Mao, D.; Huang, S.; Xie, L.; Lin, P.; Jiang, B.; et al. Staggered-layer-boosted flexible Bi2Te3 films with high thermoelectric performance. Nat. Nanotechnol. 2023, 1–8. [Google Scholar] [CrossRef]
- Kim, W.S.; Anoop, G.; Jeong, I.-S.; Lee, H.J.; Bin Kim, H.; Kim, S.H.; Goo, G.W.; Lee, H.; Lee, H.J.; Kim, C.; et al. Feasible tuning of barrier energy in PEDOT:PSS/Bi2Te3 nanowires-based thermoelectric nanocomposite thin films through polar solvent vapor annealing. Nano Energy 2020, 67, 104207. [Google Scholar] [CrossRef]
- Huang, X.-L.; Ao, D.-W.; Chen, T.-B.; Chen, Y.-X.; Li, F.; Chen, S.; Liang, G.-X.; Zhang, X.-H.; Zheng, Z.-H.; Fan, P. High-performance copper selenide thermoelectric thin films for flexible thermoelectric application. Mater. Today Energy 2021, 21, 100743. [Google Scholar] [CrossRef]
- Xie, J.; Han, M.; Zeng, X.; Mao, D.; Li, H.; Zeng, X.; Liu, R.; Ren, L.; Sun, R.; Xu, J. Flexible pCu2Se-nAg2Se thermoelectric devices via in situ conversion from printed Cu patterns. Chem. Eng. J. 2022, 435, 135172. [Google Scholar] [CrossRef]
- Varghese, T.; Hollar, C.; Richardson, J.; Kempf, N.; Han, C.; Gamarachchi, P.; Estrada, D.; Mehta, R.J.; Zhang, Y. High-performance and flexible thermoelectric films by screen printing solution-processed nanoplate crystals. Sci. Rep. 2016, 6, 33135. [Google Scholar] [CrossRef]
- Norimasa, O.; Chiba, T.; Hase, M.; Komori, T.; Takashiri, M. Improvement of thermoelectric properties of flexible Bi2Te3 thin films in bent states during sputtering deposition and post-thermal annealing. J. Alloys Compd. 2022, 898, 162889. [Google Scholar] [CrossRef]
- Goo, G.; Anoop, G.; Unithrattil, S.; Kim, W.S.; Lee, H.J.; Kim, H.B.; Jung, M.-H.; Park, J.; Ko, H.C.; Jo, J.Y. Proton-Irradiation Effects on the Thermoelectric Properties of Flexible Bi2Te3/PEDOT:PSS Composite Films. Adv. Electron. Mater. 2019, 5, 1800786. [Google Scholar] [CrossRef]
- Jin, Q.; Shi, W.; Zhao, Y.; Qiao, J.; Qiu, J.; Sun, C.; Lei, H.; Tai, K.; Jiang, X. Cellulose Fiber-Based Hierarchical Porous Bismuth Telluride for High-Performance Flexible and Tailorable Thermoelectrics. ACS Appl. Mater. Interfaces 2018, 10, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Sanyal, B. First-principles study of thermoelectric properties of CuI. Mater. Res. Express 2014, 1, 15708. [Google Scholar] [CrossRef]
- Thomas, S.; Hildreth, O.; Zaeem, M.A. Unveiling the effect of vacancy defects on structural, mechanical, electronic and diffusion properties of copper (I) iodide. Scr. Mater. 2022, 213, 114634. [Google Scholar] [CrossRef]
- Xu, Z.L.; Yang, C.; Wu, Y.N. Temperature-dependent electronic structure of gamma-phase CuI: First-principles insights. J. Phys. Condens. Matter 2022, 34, 134002. [Google Scholar]
- Yuan, P.; Chen, R.; Zhang, X.; Chen, F.; Yan, J.; Sun, C.; Ou, D.; Peng, J.; Lin, S.; Zheng, N.; et al. Ether-Soluble Cu53 Nanoclusters as an Effective Precursor of High-Quality CuI Films for Optoelectronic Applications. Angew. Chem. Int. Ed. Engl. 2019, 58, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Kawashima, M.; Rödl, C.; Botti, S. Layered CuI: A path to 2D p-type transparent conducting materials. J. Mater. Chem. C 2021, 9, 11284–11291. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, J.; Gao, G.; Xu, J.; Zhao, L.; Zhou, W.; Wang, Q.; Yuan, S.; Sun, J. Transparent p-type CuI film based self-powered ultraviolet photodetectors with ultrahigh speed, responsivity and detectivity. J. Mater. Chem. C 2022, 10, 13040–13046. [Google Scholar] [CrossRef]
- Cha, J.-H.; Jung, D.-Y. Air-Stable Transparent Silver Iodide-Copper Iodide Heterojunction Diode. ACS Appl. Mater. Interfaces 2017, 9, 43807–43813. [Google Scholar] [CrossRef]
- Kneiß, M.; Yang, C.; Barzola-Quiquia, J.; Benndorf, G.; von Wenckstern, H.; Esquinazi, P.; Lorenz, M.; Grundmann, M. Suppression of Grain Boundary Scattering in Multifunctional p-Type Transparent γ-CuI Thin Films due to Interface Tunneling Currents. Adv. Mater. Interfaces 2018, 5, 1701411. [Google Scholar] [CrossRef]
- Mulla, R.; Rabinal, M.K. Defect-Controlled Copper Iodide: A Promising and Ecofriendly Thermoelectric Material. Energy Technol. 2018, 6, 1178–1185. [Google Scholar] [CrossRef]
- Murmu, P.P.; Karthik, V.; Liu, Z.; Jovic, V.; Mori, T.; Yang, W.L.; Smith, K.E.; Kennedy, J.V. Influence of Carrier Density and Energy Barrier Scattering on a High Seebeck Coefficient and Power Factor in Transparent Thermoelectric Copper Iodide. ACS Appl. Energy Mater. 2020, 3, 10037–10044. [Google Scholar] [CrossRef]
- Yang, C.; Souchay, D.; Kneiß, M.; Bogner, M.; Wei, H.M.; Lorenz, M.; Oeckler, O.; Benstetter, G.; Fu, Y.Q.; Grundmann, M. Transparent flexible thermoelectric material based on non-toxic earth-abundant p-type copper iodide thin film. Nat. Commun. 2017, 8, 16076. [Google Scholar] [CrossRef] [PubMed]
- Klochko, N.; Barbash, V.; Klepikova, K.; Kopach, V.; Tyukhov, I.; Yashchenko, O.; Zhadan, D.; Petrushenko, S.; Dukarov, S.; Sukhov, V.; et al. Efficient biodegradable flexible hydrophobic thermoelectric material based on biomass-derived nanocellulose film and copper iodide thin nanostructured layer. Sol. Energy 2020, 212, 231–240. [Google Scholar] [CrossRef]
- Coroa, J.; Faustino, B.M.M.; Marques, A.; Bianchi, C.; Koskinen, T.; Juntunen, T.; Tittonen, I.; Ferreira, I. Highly transparent copper iodide thin film thermoelectric generator on a flexible substrate. RSC Adv. 2019, 9, 35384–35391. [Google Scholar] [CrossRef]
- Klochko, N.; Zhadan, D.; Klepikova, K.; Petrushenko, S.; Kopach, V.; Khrypunov, G.; Lyubov, V.; Dukarov, S.; Khrypunova, A. Semi-transparent copper iodide thin films on flexible substrates as p-type thermolegs for a wearable thermoelectric generator. Thin Solid Films 2019, 683, 34–41. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Jiang, C.; Wei, P.; Ding, Y.; Cai, K.; Tong, L.; Gao, Q.; Lu, Y.; Zhao, W.; Chen, S. Ultrahigh performance polyvinylpyrrolidone/Ag2Se composite thermoelectric film for flexible energy harvesting. Nano Energy 2021, 80, 105488. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Q.; Yang, J.; Cai, K.; Liu, Y.; Lu, Y.; Qiu, Y.; Lu, Y.; Wang, Z.; Wu, M.; et al. Exceptionally High Power Factor Ag2Se/Se/Polypyrrole Composite Films for Flexible Thermoelectric Generators. Adv. Funct. Mater. 2021, 32, 2106902. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Cai, K.; Gao, M.; Li, Y.; Wang, Z.; Wu, M.; Wei, P.; Zhao, W.; Du, Y.; et al. Exceptional power factor of flexible Ag/Ag2Se thermoelectric composite films. Chem. Eng. J. 2022, 434, 134739. [Google Scholar] [CrossRef]
- Wu, M.; Cai, K.; Li, X.; Li, Y.; Liu, Y.; Lu, Y.; Wang, Z.; Zhao, W.; Wei, P. Ultraflexible and High-Thermoelectric-Performance Sulfur-Doped Ag2Se Film on Nylon for Power Generators. ACS Appl. Mater. Interfaces 2022, 14, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Wang, Z.; Li, J.; Wei, P.; Zhao, W.; Chen, L.; Cai, K. High performance Ag2Se films by a one-pot method for a flexible thermoelectric generator. J. Mater. Chem. A 2022, 10, 25644–25651. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, Y.; Cai, K.; Tong, L.; Lu, Y.; Zhao, W.; Wei, P. Ultrahigh Performance of n-Type Ag2Se Films for Flexible Thermoelectric Power Generators. ACS Appl. Mater. Interfaces 2020, 12, 9646–9655. [Google Scholar] [CrossRef]
- Khan, W.S.; Hamadneh, N.N.; Khan, W.A. Prediction of thermal conductivity of polyvinylpyrrolidone (PVP) electrospun nanocomposite fibers using artificial neural network and prey-predator algorithm. PLoS ONE 2017, 12, e0183920. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, Y.; Liu, Y.; Wu, M.; Li, Y.; Wang, Z.; Cai, K. CuI/Nylon Membrane Hybrid Film with Large Seebeck Effect. Chin. Phys. Lett. 2021, 38, 126701. [Google Scholar] [CrossRef]
- Grundmann, M.; Schein, F.L.; Lorenz, M.; Böntgen, T.; Lenzner, J.; von Wenckstern, H. Cuprous iodide—A p-type transparent semiconductor: History and novel applications. Phys. Status Solidi A 2013, 210, 1671–1703. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, B.; Lu, W.; Xie, D.; Ou, H.; Han, X.; Dai, J.; Lu, X.; Han, G.; Wang, G.; et al. Twin Engineering in Solution-Synthesized Nonstoichiometric Cu5FeS4 Icosahedral Nanoparticles for Enhanced Thermoelectric Performance. Adv. Funct. Mater. 2018, 28, 1705117. [Google Scholar] [CrossRef]
- Dun, C.; Hewitt, C.A.; Huang, H.; Xu, J.; Zhou, C.; Huang, W.; Cui, Y.; Zhou, W.; Jiang, Q.; Carroll, D.L. Flexible n-type thermoelectric films based on Cu-doped Bi2Se3 nanoplate and Polyvinylidene Fluoride composite with decoupled Seebeck coefficient and electrical conductivity. Nano Energy 2015, 18, 306–314. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Wong, I.H.K.; Lin, M.; Young, D.J.; Hor, T.S.A. A thermoelectric copper-iodide composite from the pyrolysis of a well-defined coordination polymer. Dalton Trans. 2018, 47, 5564–5569. [Google Scholar] [CrossRef]
- Li, H.; Zong, Y.; Li, X.; Ding, Q.; Jiang, Y.; Han, W. Biodegradable CuI/BCNF composite thermoelectric film for wearable energy harvesting. Cellulose 2021, 28, 10707–10714. [Google Scholar] [CrossRef]
- Zhou, C.; Dun, C.; Wang, Q.; Wang, K.; Shi, Z.; Carroll, D.L.; Liu, G.; Qiao, G. Nanowires as Building Blocks to Fabricate Flexible Thermoelectric Fabric: The Case of Copper Telluride Nanowires. ACS Appl. Mater. Interfaces 2015, 7, 21015–21020. [Google Scholar] [CrossRef] [PubMed]




| Sample | Mass Ratio (PVP:CuI) | S (μVK−1) | σ (Scm−1) | PF (μWm−1K−2) |
|---|---|---|---|---|
| P0-film | 0:1 | 522 | 0.18 | 4.94 |
| P1-film | 0.0095:1 | 605 | 0.22 | 8.05 |
| P2-film | 0.0286:1 | 374 | 0.17 | 2.38 |
| P3-film | 0.0477:1 | 332 | 0.14 | 1.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Zuo, X.; Liu, Y.; Wang, Z.; Cai, K. Preparation and Properties of Flexible CuI/Polyvinylpyrrolidone Nanocomposite Thermoelectric Film. J. Compos. Sci. 2023, 7, 461. https://doi.org/10.3390/jcs7110461
Han X, Zuo X, Liu Y, Wang Z, Cai K. Preparation and Properties of Flexible CuI/Polyvinylpyrrolidone Nanocomposite Thermoelectric Film. Journal of Composites Science. 2023; 7(11):461. https://doi.org/10.3390/jcs7110461
Chicago/Turabian StyleHan, Xiaowen, Xinru Zuo, Ying Liu, Zixing Wang, and Kefeng Cai. 2023. "Preparation and Properties of Flexible CuI/Polyvinylpyrrolidone Nanocomposite Thermoelectric Film" Journal of Composites Science 7, no. 11: 461. https://doi.org/10.3390/jcs7110461
APA StyleHan, X., Zuo, X., Liu, Y., Wang, Z., & Cai, K. (2023). Preparation and Properties of Flexible CuI/Polyvinylpyrrolidone Nanocomposite Thermoelectric Film. Journal of Composites Science, 7(11), 461. https://doi.org/10.3390/jcs7110461






