Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Synthesis Method
2.2.1. Co-Fe LDH Synthesis
2.2.2. Ni-Fe LDH Synthesis
2.2.3. Zn-Ti LDH Synthesis
2.3. Characterization Methods
2.4. Study of U(VI) Sorption on Obtained Materials
2.4.1. Sorption of U(VI) from Distilled Water
2.4.2. Sorption of U(VI) from Seawater
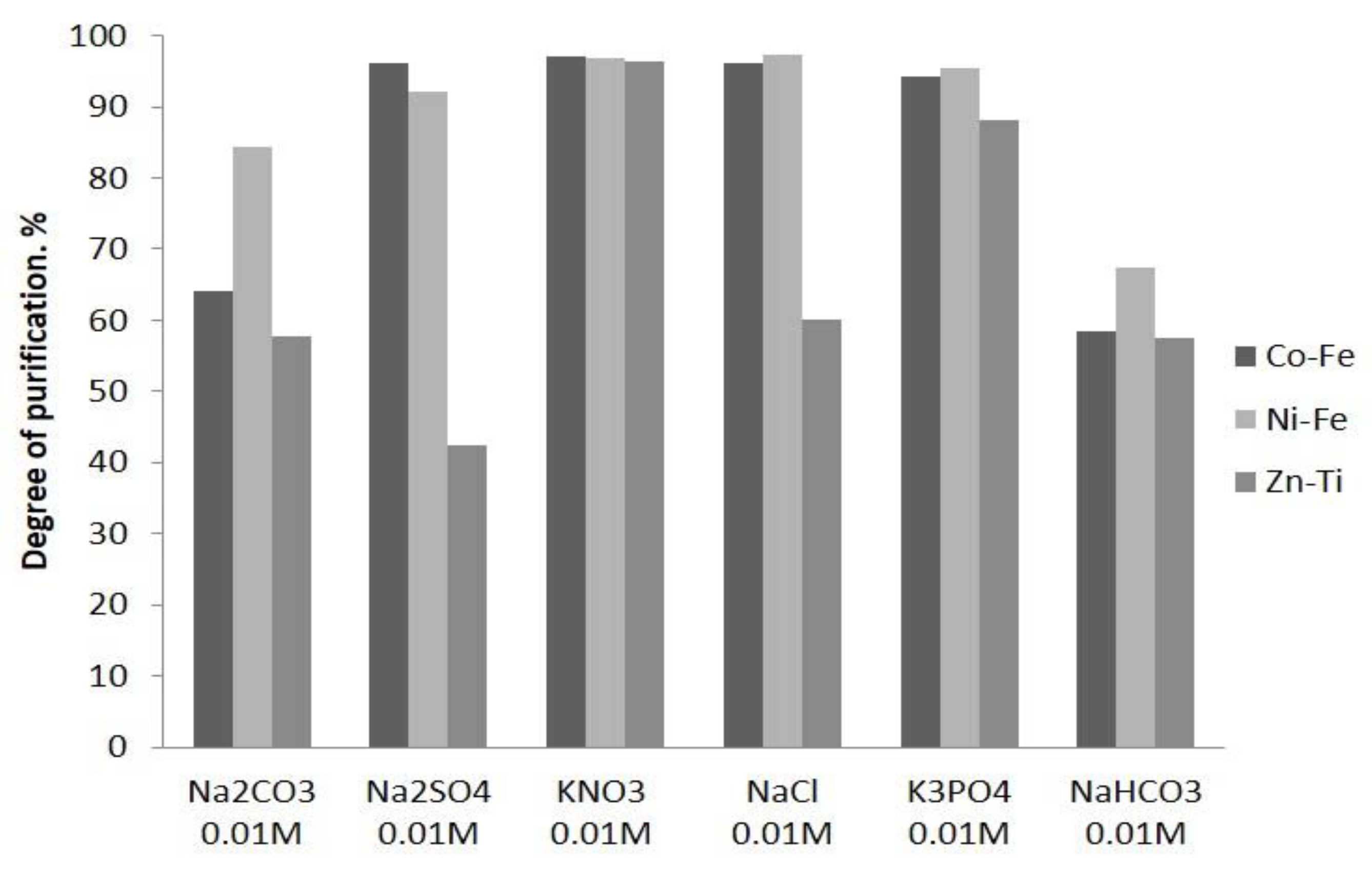
2.4.3. Sorption of U(VI) in Presence of Competing Ions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.J.; Zhang, X.W.; Xie, C.; Wu, X.-Y.; Luo, C.-W.; Li, M.; Peng, Y. Effective capture of aqueous uranium using a novel magnetic goethite: Properties and mechanism. J. Solid State Chem. 2021, 300, 122236. [Google Scholar] [CrossRef]
- Jana, A.; Unni, A.; Ravuru, S.S.; Das, A.; Das, D.; Biswas, S.; Sheshadri, H.; De, S. In-situ polymerization into the basal spacing of LDH for selective and enhanced uranium adsorption: A case study with real life uranium alkaline leach liquor. Chem. Eng. J. 2022, 428, 131180. [Google Scholar] [CrossRef]
- Guo, X.; Ruan, Y.; Diao, Z.; Shih, K.; Su, M.; Song, G.; Chen, D.; Wang, S.; Kong, L. Environmental-friendly preparation of Ni–Co layered double hydroxide (LDH) hierarchical nanoarrays for efficient removing uranium (VI). J. Clean. Prod. 2021, 308, 127384. [Google Scholar] [CrossRef]
- Yuan, X.; Jing, X.Y.; Xu, H.; Zhang, X.; Xu, J. Ni–Al layered double hydroxides modified sponge skeleton with polydopamine coating for enhanced U(VI) extraction from aqueous solution. Chemosphere 2022, 287, 131919. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, S.; Li, L.; Jiang, L.; Ahmed, Z.; Dang, Z.; Wu, P. Memory effect induced the enhancement of uranium (VI) immobilization on low-cost MgAl-double oxide: Mechanism insight and resources recovery. J. Hazard. Mater. 2021, 401, 123447. [Google Scholar] [CrossRef] [PubMed]
- Nestroinia, O.V.; Ryl’tsova, I.G.; Yapryntsev, M.N.; Lebedeva, O.E. Effect of the Synthesis Method on the Phase Composition and Magnetism of Layered Double Hydroxides. Inorg. Mater. 2020, 56, 747–753. [Google Scholar] [CrossRef]
- Ebitani, K.; Motokura, K.; Mori, K.; Mizugaki, T.; Kaneda, K. Reconstructed hydrotalcite as a highly active heterogeneous base catalyst for carbon-carbon bond formations in the presence of water. J. Org. Chem. 2006, 71, 5440–5447. [Google Scholar] [CrossRef] [PubMed]
- Pavel, O.D.; Bîrjega, R.; Che, M.; Costentin, G.; Angelescu, E.; Şerban, S. The activity of Mg/Al reconstructed hydrotalcites by “memory effect” in the cyanoethylation reaction. Catal. Commun. 2008, 9, 1974–1978. [Google Scholar] [CrossRef]
- Li, Q.; Xing, L.; Lu, X.; Li, N.; Xu, M. Magnetic properties of Mg/Co(II)-Al/Fe(III) layered double hydroxides. Inorg. Chem. Commun. 2015, 52, 46–49. [Google Scholar] [CrossRef]
- Bunney, K.G.; Cumberland, S.; Douglas, G. Mechanisms of Uranyl Sequestration by Hydrotalcite. ACS Omega 2017, 2, 7112–7119. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Wang, X.; Sheng, G.; Wang, S.; Ai, Y.; Ji, Y.; Liu, Y.; Hayat, T.; Wang, X. Glycerol-Modified Binary Layered Double Hydroxide Nanocomposites for Uranium Immobilization via Extended X-ray Absorption Fine Structure Technique and Density Functional Theory Calculation. ACS Sustain. Chem. Eng. 2017, 5, 3583–3595. [Google Scholar] [CrossRef]
- Linghu, W.; Yang, H.; Sun, Y.; Sheng, G.; Huang, Y. One-Pot Synthesis of LDH/GO Composites as Highly Effective Adsorbents for Decontamination of U(VI). ACS Sustain. Chem. Eng. 2017, 5, 5608–5616. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, B.; Zhang, S.; Yao, H.; Cai, Z.; Han, Y.; Li, C.; Li, Y.; Ma, S. Intercalation of salicylaldoxime into layered double hydroxide: Ultrafast and highly selective uptake of uranium from different water systems via versatile binding modes. J. Colloid Interface Sci. 2023, 642, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ou, H.; Yang, Y.; Oldfield, D.T.; Thomsen, L.; Subhash, B.; Hamilton, J.L.; Wright, J.T.; Bedford, N.M.; Carolan, J.V. Enhanced uranium extraction selectivity from seawater using dopant engineered layered double hydroxides. Energy Adv. 2023, 2, 1134–1147. [Google Scholar] [CrossRef]
- Foster, C.; Shaw, S.; Neill, T.S.; Bryan, N.; Sherriff, N.; Natrajan, L.S.; Wilson, H.; Lopez-Odriozola, L.; Rigby, B.; Haigh, S.J.; et al. Hydrotalcite Colloidal Stability and Interactions with Uranium(VI) at Neutral to Alkaline pH. Langmuir 2022, 38, 2576–2589. [Google Scholar] [CrossRef] [PubMed]
- Pshinko, G.N. Layered double hydroxides as effective adsorbents for U(VI) and toxic heavy metals removal from aqueous media. J. Chem. 2013, 2013, 347178. [Google Scholar] [CrossRef]
- Pshinko, G.N.; Puzyrnaya, L.N.; Shunkov, V.S.; Kosorukov, A.A.; Demchenko, V.Y. Removal of Radiocesium from Aqueous Media with Zinc–Aluminum Layered Double Hydroxide Intercalated with Copper(II) Hexacyanoferrate. Radiochemistry 2018, 60, 395–399. [Google Scholar] [CrossRef]
- Keimirov, M.A. Sorbents with the structure of layered double hydroxides for aquatic media treatment from U(VI). J. Water Chem. Technol. 2016, 38, 128–133. [Google Scholar] [CrossRef]
- Wang, X.; Yu, S.; Wu, Y.; Pang, H.; Yu, S.; Chen, Z.; Hou, J.; Alsaedi, A.; Hayat, T.; Wang, S. The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chem. Eng. J. 2018, 342, 321–330. [Google Scholar] [CrossRef]
- Yuan, X.; Yin, C.; Zhang, Y.; Chen, Z.; Xu, Y.; Wang, J. Synthesis of C@Ni-Al LDH HSS for efficient U-entrapment from seawater. Sci. Rep. 2019, 9, 5807. [Google Scholar] [CrossRef]
- Guo, Y.; Gong, Z.; Li, C.; Gao, B.; Li, P.; Wang, X.; Zhang, B.; Li, X. Efficient removal of uranium (VI) by 3D hierarchical Mg/Fe-LDH supported nanoscale hydroxyapatite: A synthetic experimental and mechanism studies. Chem. Eng. J. 2020, 392, 123682. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wei, J.-J.; Zeng, G.-M.; Zhang, H.-Q.; Tan, X.-F.; Ma, C.; Li, X.-C.; Li, Z.-H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Mei, H.; Tan, X.; Tan, L.; Meng, Y.; Chen, C.; Fang, M.; Wang, X. Retention of U(VI) by the Formation of Fe Precipitates from Oxidation of Fe(II). ACS Earth Space Chem. 2018, 2, 968–976. [Google Scholar] [CrossRef]
- Pan, Z.; Li, W.; Fortner, J.D.; Giammar, D.E. Measurement and Surface Complexation Modeling of U(VI) Adsorption to Engineered Iron Oxide Nanoparticles. Environ. Sci. Technol. 2017, 51, 9219–9226. [Google Scholar] [CrossRef]
- Scott, T.B.; Allen, G.C.; Heard, P.J.; Lewis, A.C.; Lee, D.F. The extraction of uranium from groundwaters on iron surfaces. Proc. R. Soc. A Math. Phys. Eng. Sci. 2005, 461, 1247–1259. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; He, J.; Qiao, J.; Yang, Y.; Xiong, Z.; Wang, G. Adsorption of uranium (VI) by a MgAl-DHBDC/LDH composite: Kinetic, mechanistic and thermodynamic studies. J. Radioanal. Nucl. Chem. 2023, 332, 4255–4269. [Google Scholar] [CrossRef]
- Yusheng, W.; Ying, D.; Wenmei, H.; Jinhua, X.; Qinqin, T. Uranium-specific adsorbent L-serine intercalated Mg-Al layered bimetallic (hydrogen) oxides for selectively treating uranium-containing wastewater. J. Radioanal. Nucl. Chem. 2023, 332, 3741–3752. [Google Scholar] [CrossRef]
- Yarusova, S.B.; Shichalin, O.O.; Belov, A.A.; Azon, S.; Buravlev, I.Y.; Golub, A.; Mayorov, V.Y.; Gerasimenko, A.; Papynov, E.; Ivanets, A.; et al. Synthesis of amorphous KAlSi3O8 for cesium radionuclide immobilization into solid matrices using spark plasma sintering technique. Ceram. Int. 2022, 48, 3808–3817. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Nepomnyushchaya, V.A.; Ivanets, A.; Belov, A.; Dran’kov, A.; Yarusova, S.; Buravlev, I.; Tarabanova, A.; Fedorets, A.; et al. Hydrothermal synthesis and spark plasma sintering of NaY zeolite as solid-state matrices for cesium-137 immobilization. J. Eur. Ceram. Soc. 2022, 42, 3004–3014. [Google Scholar] [CrossRef]
- Papynov, E.K.; Dran’kov, A.N.; Tkachenko, I.A.; Buravlev, I.Y.; Mayorov, V.Y.; Merkulov, E.B.; Fedorets, A.N.; Ognev, A.V.; Samardak, A.S.; Drenin, A.S.; et al. Synthesis and Sorption Characteristics of Magnetic Materials Based on Cobalt Oxides and Their Reduced Forms. Russ. J. Inorg. Chem. 2020, 65, 820–828. [Google Scholar] [CrossRef]
- Dran’kov, A.; Shichalin, O.; Papynov, E.; Nomerovskii, A.; Mayorov, V.; Pechnikov, V.; Ivanets, A.; Buravlev, I.; Yarusova, S.; Zavjalov, A.; et al. Hydrothermal synthesis, structure and sorption performance to cesium and strontium ions of nanostructured magnetic zeolite composites. Nucl. Eng. Technol. 2022, 54, 1991–2003. [Google Scholar] [CrossRef]
- María, A.A.; Yolanda, A.; Victoriano, B.; José, M.L.; José, M.M.; José, R.R.; Francisco, J.U. Thermal decomposition of Mg/Al and Mg/Ga layered-double hydroxides: A spectroscopic study. J. Mater. Chem. 1999, 9, 1603–1607. [Google Scholar]
- Yang, W.; Kim, Y.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg-Al-CO4 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Roelofs, J.C.A.A.; van Bokhoven, J.A.; van Dillen, A.J.; Geus, J.W.; Jong, K.P. The thermal decomposition of Mg-Al hydrotalcites: Effects of interlayer anions and characteristics of the final structure. Chem.-A Eur. J. 2002, 8, 5571–5579. [Google Scholar] [CrossRef]
- Wei, G.; Liu, X.; Chen, S.; Shao, D.; Yuan, W.; Lu, X.; Xie, Y.; Shu, X. Direct immobilization of simulated nuclear waste in preformed Gd2Zr2O7 pyrochlore via spark plasma sintering reaction. Mater. Chem. Phys. 2022, 291, 126711. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, P.; Gong, B.; Fang, Y.; Zhu, N. Fabrication and photocatalytic properties of a visible-light responsive nanohybrid based on self-assembly of carboxyl graphene and ZnAl layered double hydroxides. J. Mater. Chem. A 2014, 2, 5534–5540. [Google Scholar] [CrossRef]
- Giles, C.N.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 786, 3973–3993. [Google Scholar] [CrossRef]
- Papynov, E.K.; Tkachenko, I.A.; Maiorov, V.Y.; Pechnikov, V.S.; Fedorets, A.N.; Portnyagin, A.S.; Dran’kov, A.N.; Buravlev, I.Y.; Grishin, A.V.; Tananaev, I.G.; et al. Nanostructured Magnetic Sorbents for Selective Recovery of Uranium(VI) from Aqueous Solutions. Radiochemistry 2019, 61, 28–36. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Ma, C.; Hu, H.; Shen, C.; He, S.; Li, P. Highly efficient and reusable Mg-Fe layered double hydroxides anchored in attapulgite for uranium uptake from wastewater. Chemosphere 2023, 321, 138055. [Google Scholar] [CrossRef]

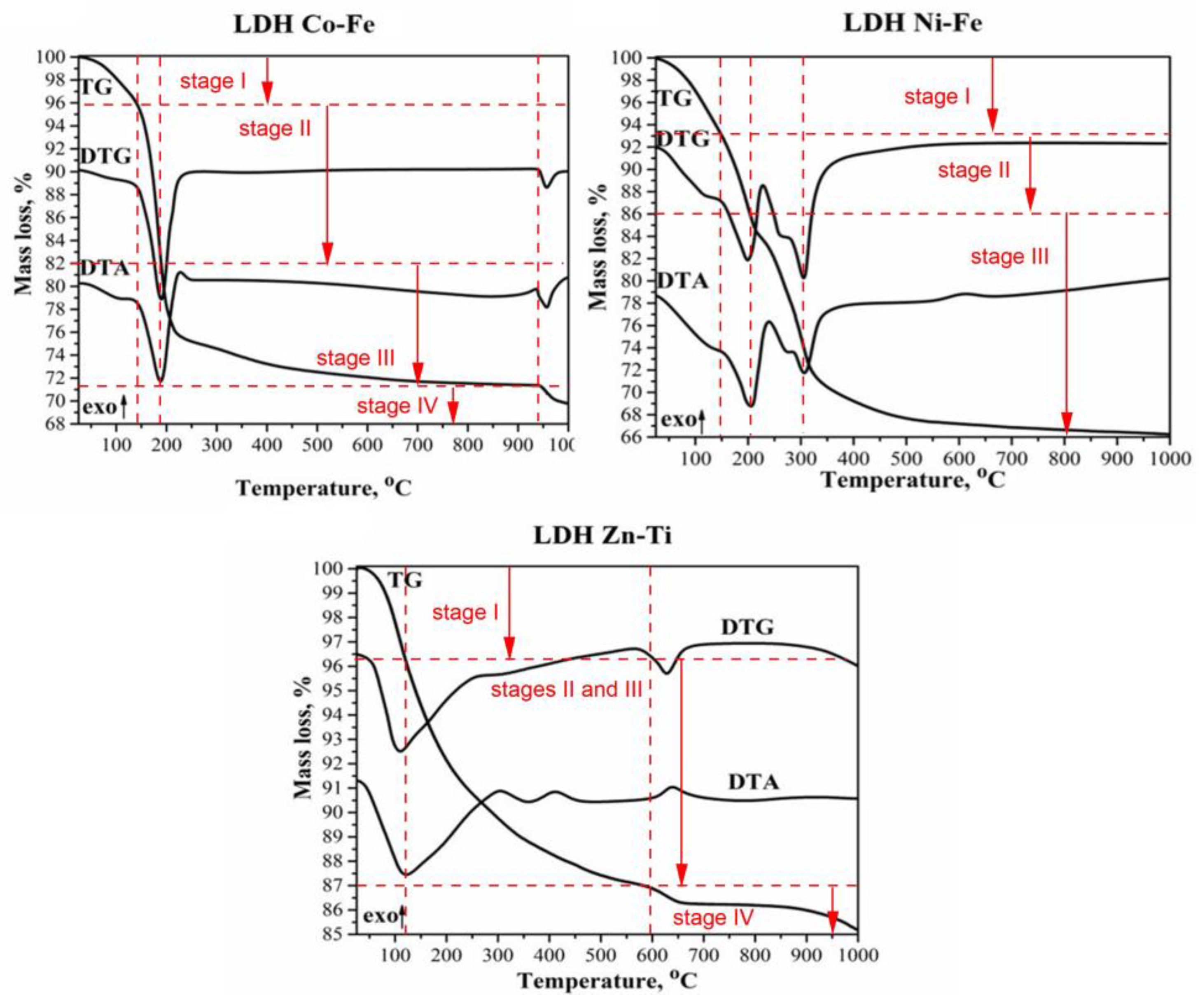





| Sample | Stage (Temperature, °C) | Weight Loss, % | Total Weight Loss, % |
|---|---|---|---|
| Co-Fe-LDH | Stage I (20–140 °C) Stage II (140–190 °C) Stage III (190–940 °C) Stage IV (940–1000 °C) | 4.1 13.9 10.8 1.4 | 30.2 |
| Ni-Fe-LDH | Stage I (20–150 °C) Stage II (150–205 °C) Stage III (205–1000 °C) | 6.8 7.2 19.7 | 33.7 |
| Zn-Ti-LDH | Stage I (20–120 °C) Stages II–III (120–600 °C) Stage IV (600–1000 °C) | 3.7 9.3 1.8 | 14.8 |
| Sample | wt, % | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Cl | Co | Fe | Ni | Zn | Ti | |
| Co-Fe LDH | 7.66 | 36.84 | - | 35.12 | 20.39 | - | - | - |
| Ni-Fe LDH | - | 48.21 | 0.49 | - | 15.3 | 36 | - | - |
| Zn-Ti LDH | - | 45.11 | 6.82 | - | - | - | 18.72 | 9.35 |
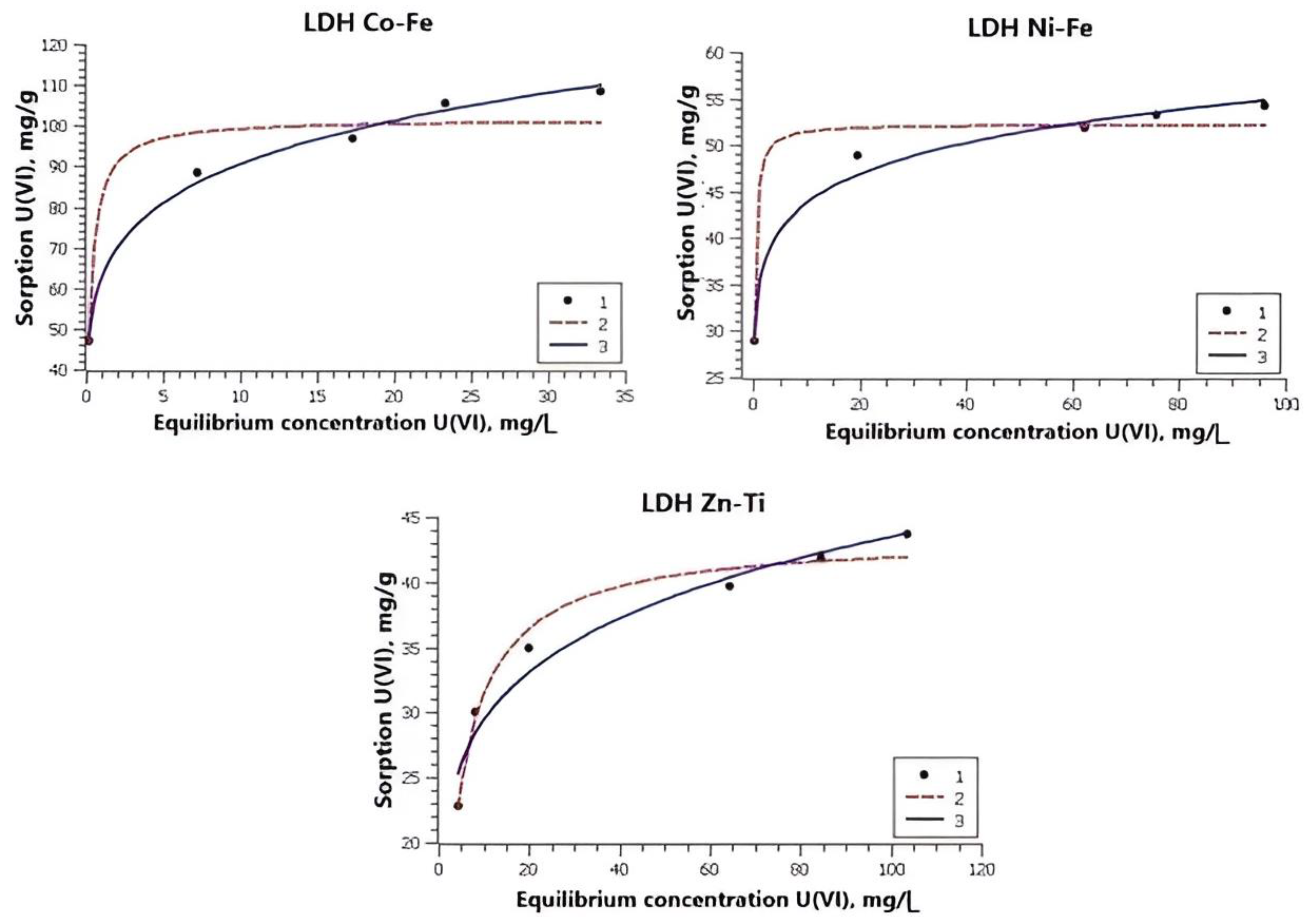
| Equation | Parameters | Co-Fe LDH | Ni-Fe LDH | Zn-Ti LDH | |||
|---|---|---|---|---|---|---|---|
| Seawater | Distilled Water | Seawater | Distilled Water | Seawater | Distilled Water | ||
| Freundlich | Kf | 62.47 ± 1.79 | 68.91 ± 2.95 | 34.78 ± 0.97 | 49.80 ± 4.11 | 19.88 ± 1.53 | 49.96 ± 0.32 |
| n | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.17 ± 0.02 | 0.09 | |
| R2 | 0.99 | 0.98 | 0.98 | 0.94 | 0.956 | 0.99 | |
| Langmuir | qmax | 10.60 ± 4.26 | 114.12 ± 4.34 | 51.85 ± 0.95 | 73.82 ± 4.97 | 43.51 ± 0.93 | 69.46 ± 2.10 |
| KL | 4.16 ± 1.41 | 2.78 ± 0.83 | 6.24 ± 1.06 | 31.27 ± 17.33 | 0.26 ± 0.03 | 35.2 ± 8.80 | |
| R2 | 0.92 | 0.94 | 0.96 | 0.80 | 0.976 | 0.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balybina, V.A.; Dran’kov, A.N.; Shichalin, O.O.; Savel’eva, N.Y.; Kokorina, N.G.; Kuular, Z.C.; Ivanov, N.P.; Krasitskaya, S.G.; Ivanets, A.I.; Papynov, E.K. Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials. J. Compos. Sci. 2023, 7, 458. https://doi.org/10.3390/jcs7110458
Balybina VA, Dran’kov AN, Shichalin OO, Savel’eva NY, Kokorina NG, Kuular ZC, Ivanov NP, Krasitskaya SG, Ivanets AI, Papynov EK. Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials. Journal of Composites Science. 2023; 7(11):458. https://doi.org/10.3390/jcs7110458
Chicago/Turabian StyleBalybina, Valeria A., Artur N. Dran’kov, Oleg O. Shichalin, Natalia Yu. Savel’eva, Nadezhda G. Kokorina, Zhanna C. Kuular, Nikita P. Ivanov, Svetlana G. Krasitskaya, Andrei I. Ivanets, and Evgeniy K. Papynov. 2023. "Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials" Journal of Composites Science 7, no. 11: 458. https://doi.org/10.3390/jcs7110458
APA StyleBalybina, V. A., Dran’kov, A. N., Shichalin, O. O., Savel’eva, N. Y., Kokorina, N. G., Kuular, Z. C., Ivanov, N. P., Krasitskaya, S. G., Ivanets, A. I., & Papynov, E. K. (2023). Mesoporous Layered Double Hydroxides: Synthesis for High Effective Uranium Ions Sorption from Seawater and Salt Solutions on Nanocomposite Functional Materials. Journal of Composites Science, 7(11), 458. https://doi.org/10.3390/jcs7110458









