Synthesis of Vectorized Nanoparticles Based on a Copolymer of N-Vinyl-2-Pyrrolidone with Allyl Glycidyl Ether and a Carbohydrate Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radical Copolymerization of N-vinyl-2-pyrrolidone and Allyl Glycidyl Ether
2.2. Conjugation of Trisaccharide A with NVPAGE Copolymer
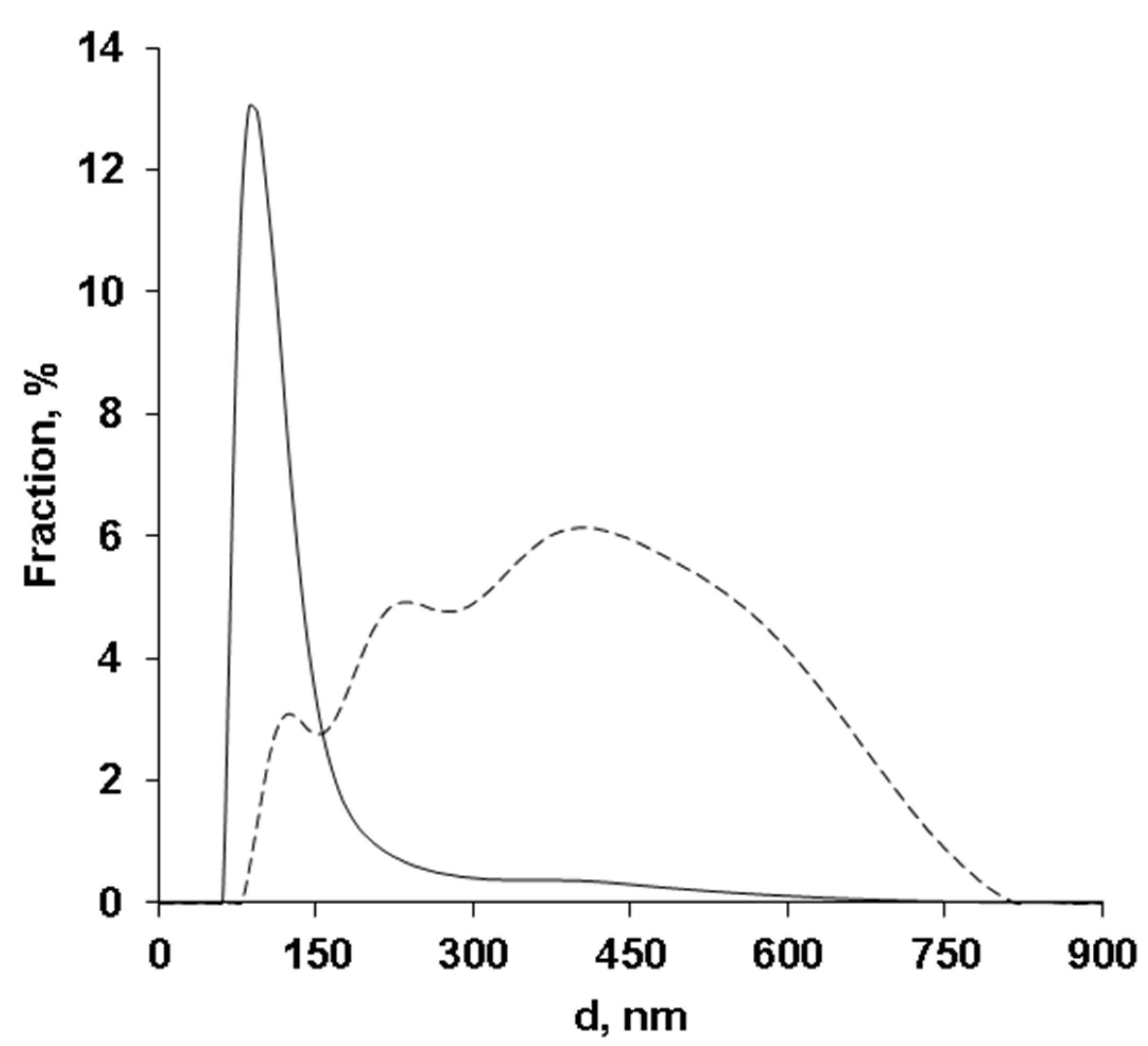
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchige, S.; Artemov, D. Theranostic Pretargeting Drug Delivery and Imaging Platforms in Cancer Precision Medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- MacRitchie, N.; Di Francesco, V.; Ferreira, M.; Guzik, T.J.; Decuzzi, P.; Maffia, P. Nanoparticle theranostics in cardiovascular inflammation. Semin. Immunol. 2021, 56, 101536. [Google Scholar] [CrossRef] [PubMed]
- Manners, N.; Priya, V.; Mehata, A.K.; Rawat, M.; Mohan, S.; Makeen, H.A.; Albratty, M.; Albarrati, A.; Meraya, A.M.; Muthu, M.S. Theranostic Nanomedicines for the Treatment of Cardiovascular and Related Diseases: Current Strategies and Future Perspectives. Pharmaceuticals 2022, 15, 441. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jain, S.; Kumar, D.; Soni, S.; Sharma, M. A Review on Theranostics: An Approach to Targeted Diagnosis and Therapy. Asian J. Pharm. Res. Dev. 2019, 7, 63–69. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; Abucafy, M.P.; Manaia, E.B.; da Silva, B.L.; Rissi, N.C.; Oshiro-Junior, J.A.; Chiavacci, L.A. Drug delivery using theranostics: An Overview of its use, advantages and safety assessment. Curr. Nanosci. 2020, 16, 3–14. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Samanta, S.; Le Joncour, V.; Wegrzyniak, O.; Rangasami, V.K.; Ali-Löytty, H.; Hong, T.; Selvaraju, R.K.; Aberg, O.; Hilborn, J.; Laakkonen, P.; et al. Heparin-Derived Theranostic Nanoprobes Overcome the Blood–Brain Barrier and Target Glioma in Murine Model. Adv. Ther. 2022, 5, 2200001. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based Theranostic Agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Ventola, C.L. The nanomedicine revolution: Part 1—Emerging concepts. Pharm. Ther. 2012, 37, 512–525. [Google Scholar]
- Sevastre, A.-S.; Horescu, C.; Carina Baloi, S.; Cioc, C.E.; Vatu, B.I.; Tuta, C.; Artene, S.A.; Danciulescu, M.M.; Tudorache, S.; Dricu, A. Benefits of Nanomedicine for Therapeutic Intervention in Malignant Diseases. Coatings 2019, 9, 628. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aibani, N.; Callan, J.F.; Callan, B. Recent advances in amphiphilic polymers for simultaneous delivery of hydrophobic and hydrophilic drugs. Ther. Deliv. 2016, 7, 15–31. [Google Scholar] [CrossRef]
- Nutan, B.; Singh Chandel, A.K.; Jewrajka, S.K. Synthesis and Multi-Responsive Self-Assembly of Cationic Poly(caprolactone)–Poly(ethylene glycol) Multiblock Copolymers. Chem. Eur. J. 2017, 23, 8166–8170. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Atanase, L.I.; Winninger, J.; Delaite, C.; Riess, G. Reversible addition–fragmentation chain transfer synthesis and micellar characteristics of biocompatible amphiphilic poly(vinyl acetate)-graft-poly(N-vinyl-2-pyrrolidone) copolymers. Eur. Polym. J. 2014, 53, 109–117. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral Drug-Loaded Biocompatible Polymeric Nanoparticles Obtained by Non-Aqueous Emulsion Polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Bloise, N.; Okkeh, M.; Restivo, E.; Della Pina, C.; Visai, L. Targeting the “Sweet Side” of Tumor with Glycan-Binding Molecules Conjugated-Nanoparticles: Implications in Cancer Therapy and Diagnosis. Nanomaterials 2021, 11, 289. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Nicotra, F.; Russo, L. Glycans in nanomedicine, impact and perspectives. Future Med. Chem. 2019, 11, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Shchegravina, E.S.; Sachkova, A.A.; Usova, S.D.; Nyuchev, A.V.; Gracheva, Y.A.; Fedorov, A.Y. Carbohydrate Systems in Targeted Drug Delivery: Expectation and Reality. Russ. J. Bioorg. Chem. 2021, 47, 71–98. [Google Scholar] [CrossRef]
- Stenzel, M.H. Glycopolymers for Drug Delivery: Opportunities and Challenges. Macromolecules 2022, 55, 4867–4890. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Zhang, J.-G.; Yang, X.; Du, W.-L.; Yu, Z.-L.; Lv, Z.-Y.; Mou, X.-Z. Carbohydrates based stimulus responsive nanocarriers for cancer-targeted chemotherapy: A review of current practices. Expert Opin. Drug Deliv. 2022, 19, 623–640. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef]
- Kazakova, E.D.; Yashunsky, D.V.; Nifantiev, N.E. The Synthesis of Blood Group Antigenic A Trisaccharide and Its Biotinylated Derivative. Molecules 2021, 26, 5887. [Google Scholar] [CrossRef]
- Fomitskaya, P.A.; Argunov, D.A.; Tsvetkov, Y.E.; Lalov, A.V.; Ustyuzhanina, H.E.; Nifantiev, N.E. Further investigation of the 2-azido-phenylselenylation of glycals. Eur. J. Org. Chem. 2021, 2021, 5897–5904. [Google Scholar] [CrossRef]
- Iurisci, I.; Cumashi, A.; Sherman, A.A.; Tsvetkov, Y.E.; Tinari, N.; Piccolo, E.; D’Egidio, M.; Adamo, V.; Natoli, C.; Rabinovich, G.A.; et al. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009, 29, 403–410. [Google Scholar]
- Casset, F.; Peters, T.; Etzler, M.; Korchagina, E.; Nifant’ev, N.; Perez, S.; Imberty, A. Conformational analysis of blood group A trisaccharide in solution and in the binding site of Dolichos biflorus lectin using transient ans transferred NOE and ROE experiments. Eur. J. Biochem. 1996, 239, 710–719. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; You, S.; Mao, R.; Cai, E.; Pan, W.; Zhang, C.; Hu, R.; Shen, J. Mild Hyperthermia-Assisted ROS Scavenging Hydrogels Achieve Diabetic Wound Healing. ACS Macro Lett. 2022, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Xiang, Y.; Qi, X.; Mao, R.; Cai, E.; Lan, Y.; Lu, H.; Shen, J.; Deng, H. Harnessing a biopolymer hydrogel reinforced by copper/tannic acid nanosheets for treating bacteria-infected diabetic wounds. Mater. Today Adv. 2022, 15, 100271. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together is better: Poly(tannic acid) nanorods functionalized polysaccharide hydrogels for diabetic wound healing. Ind. Crops Prod. 2022, 186, 115273. [Google Scholar] [CrossRef]
- Nechaeva, A.; Artyukhov, A.; Luss, A.; Shtilman, M.; Gritskova, I.; Shulgin, A.; Motyakin, M.; Levina, I.; Krivoborodov, E.; Toropygin, I.; et al. Synthesis of Amphiphilic Copolymers of N-Vinyl-2-pyrrolidone and Allyl Glycidyl Ether for Co-Delivery of Doxorubicin and Paclitaxel. Polymers 2022, 14, 1727. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 215–228. [Google Scholar]
- Wong, S.S.; Jameson, D.M. Chemistry of Protein and Nucleic Acid Cross-Linking and Conjugation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 82–92. [Google Scholar]
- Tsatsakis, A.; Stratidakis, A.; Goryachaya, A.; Tzatzarakis, M.; Stivaktakis, P.; Docea, A.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-N-vinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yingyongnarongkul, B.; Radchatawedchakoon, W.; Krajarng, A.; Watanapokasin, R.; Suksamrarn, A. High transfection efficiency and low toxicity cationic lipids with aminoglycerol–diamine conjugate. Bioorg. Med. Chem. 2009, 17, 176–188. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Varankin, A.V.; Luss, A.L.; Dyatlov, V.A.; Tsatsakis, A.M.; Shtilman, M.I.; Korshak, Y.V. Immobilization of dopamine on the copolymer of N-vinyl-2-pyrrolidone and allyl glycidyl ether and synthesis of new hydrogels. Polym. Int. 2020, 69, 1275–1282. [Google Scholar] [CrossRef]
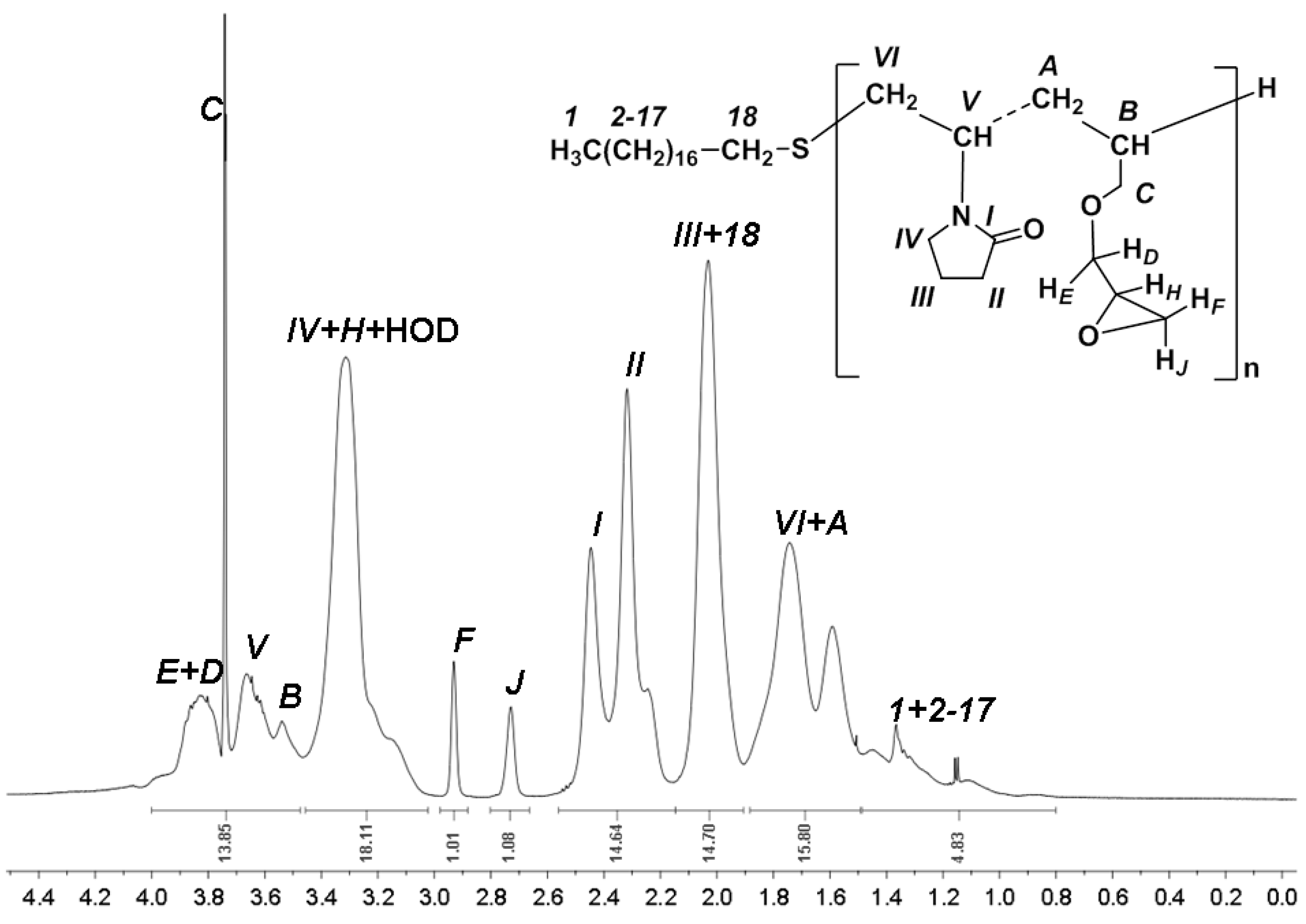


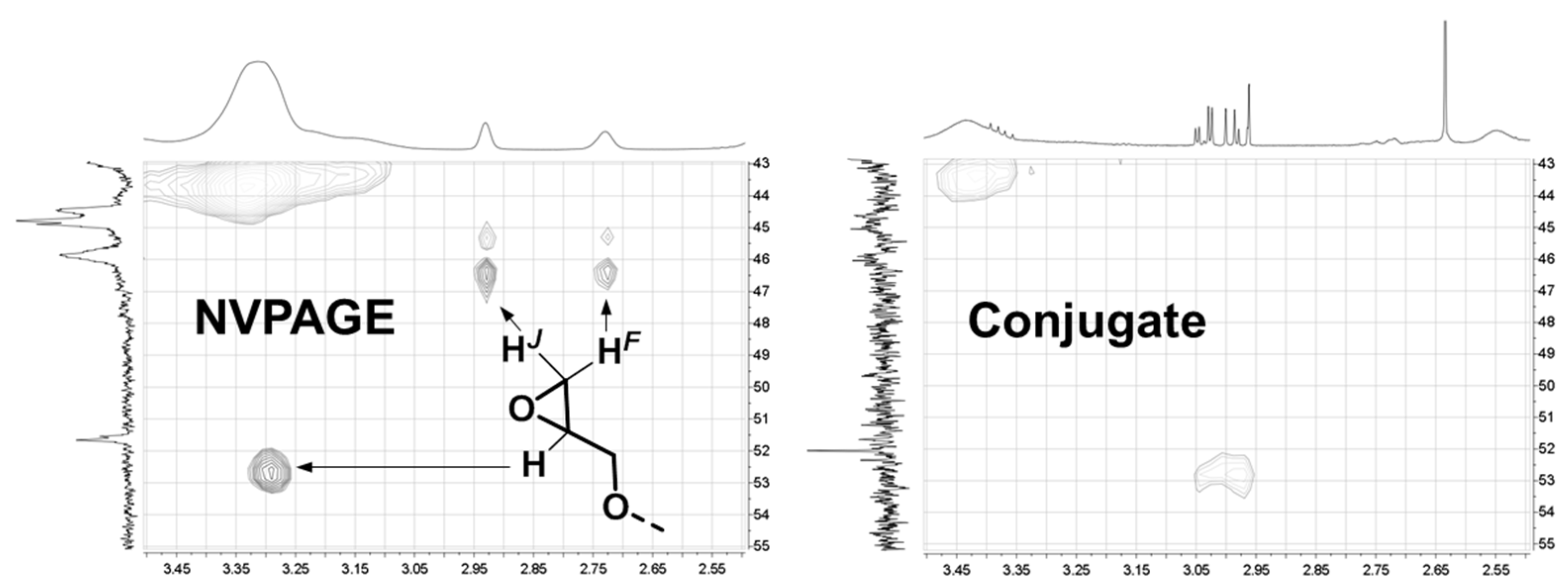


| 1-Gal | 1-GalNAc | 1-Fuc | Me MeGlu | 1a MeGlu | 1b MeGlu | 2 MeGlu | 3 MeGlu | 4 MeGlu | 5 MeGlu | 6a MeGlu | 6b MeGlu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H | 4.52 (t) | 5.16 (d) | 5.28 (d) | 2.53 (s) | 2.88 (m) | 2.92 (m) | 3.98 (m) | 3.76 (m) | 3.64 (m) | 3.76 (m) | 3.64 (m) | 3.80 (dd) |
| 13C | 101.59 | 91.38 | 98.75 | 34.81 | 52.79 | 70.52 | 71.92 | 71.90 | 71.92 | 63.74 | ||
| Me | 1a | 1b | 2 | 3 | 4 | 5 | 6a | 6b | |
|---|---|---|---|---|---|---|---|---|---|
| Meglumine | |||||||||
| 1H | 2.34 | 2.62 | 2.68 | 3.88 | 3.75 | 3.63 | 3.75 | 3.64 | 3.81 |
| 13C | 35.83 | 53.58 | 72.23 | 72.20 | 72.10 | 72.20 | 64.09 | ||
| Conjugate | |||||||||
| 1H | 2.53 | 2.88 | 2.92 | 3.98 | 3.76 | 3.64 | 3.76 | 3.64 | 3.80 |
| 13C | 34.81 | 52.79 | 70.52 | 71.92 | 71.90 | 71.92 | 63.74 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinnitskiy, D.Z.; Luss, A.L.; Krylov, V.B.; Ustyuzhanina, N.E.; Goryachaya, A.V.; Nechaeva, A.M.; Shtilman, M.I.; Nifantiev, N.E.; Mezhuev, Y.O. Synthesis of Vectorized Nanoparticles Based on a Copolymer of N-Vinyl-2-Pyrrolidone with Allyl Glycidyl Ether and a Carbohydrate Vector. J. Compos. Sci. 2022, 6, 247. https://doi.org/10.3390/jcs6090247
Vinnitskiy DZ, Luss AL, Krylov VB, Ustyuzhanina NE, Goryachaya AV, Nechaeva AM, Shtilman MI, Nifantiev NE, Mezhuev YO. Synthesis of Vectorized Nanoparticles Based on a Copolymer of N-Vinyl-2-Pyrrolidone with Allyl Glycidyl Ether and a Carbohydrate Vector. Journal of Composites Science. 2022; 6(9):247. https://doi.org/10.3390/jcs6090247
Chicago/Turabian StyleVinnitskiy, Dmitry Z., Anna L. Luss, Vadim B. Krylov, Nadezhda E. Ustyuzhanina, Anastasiya V. Goryachaya, Anna M. Nechaeva, Mikhail I. Shtilman, Nikolay E. Nifantiev, and Yaroslav O. Mezhuev. 2022. "Synthesis of Vectorized Nanoparticles Based on a Copolymer of N-Vinyl-2-Pyrrolidone with Allyl Glycidyl Ether and a Carbohydrate Vector" Journal of Composites Science 6, no. 9: 247. https://doi.org/10.3390/jcs6090247
APA StyleVinnitskiy, D. Z., Luss, A. L., Krylov, V. B., Ustyuzhanina, N. E., Goryachaya, A. V., Nechaeva, A. M., Shtilman, M. I., Nifantiev, N. E., & Mezhuev, Y. O. (2022). Synthesis of Vectorized Nanoparticles Based on a Copolymer of N-Vinyl-2-Pyrrolidone with Allyl Glycidyl Ether and a Carbohydrate Vector. Journal of Composites Science, 6(9), 247. https://doi.org/10.3390/jcs6090247







