Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Preparation
2.3. Proximate Analysis
- Ash: the total ash was determined by the difference in sample mass before and after heating in an electric oven at 730 °C for 8 h. The ash content is given by Equation (3).
- Volatile matter: the sample was deposited in a crucible closed by a lid and placed in an oven at 950 °C for 7 min. The percentage of volatile matter is calculated according to Equation (4).
- Fixed carbon content: this refers to the non-volatile solid fraction resulting from the volatile matter and ash test, as defined by the ASTM and calculated by Equation (5).
2.4. Iodine Index
2.5. Characterization
2.5.1. FTIR
2.5.2. pHpzc
2.5.3. SEM/EDS
2.5.4. BET
2.6. Adsorption Study
2.6.1. Equilibrium Study
2.6.2. Kinetics Study
2.6.3. Isotherm Study
2.6.4. Statistical Study
2.7. Thermodynamic Study
2.8. Desorption and Regeneration
3. Results
3.1. Proximate Analysis, Iodine Index and Characterization
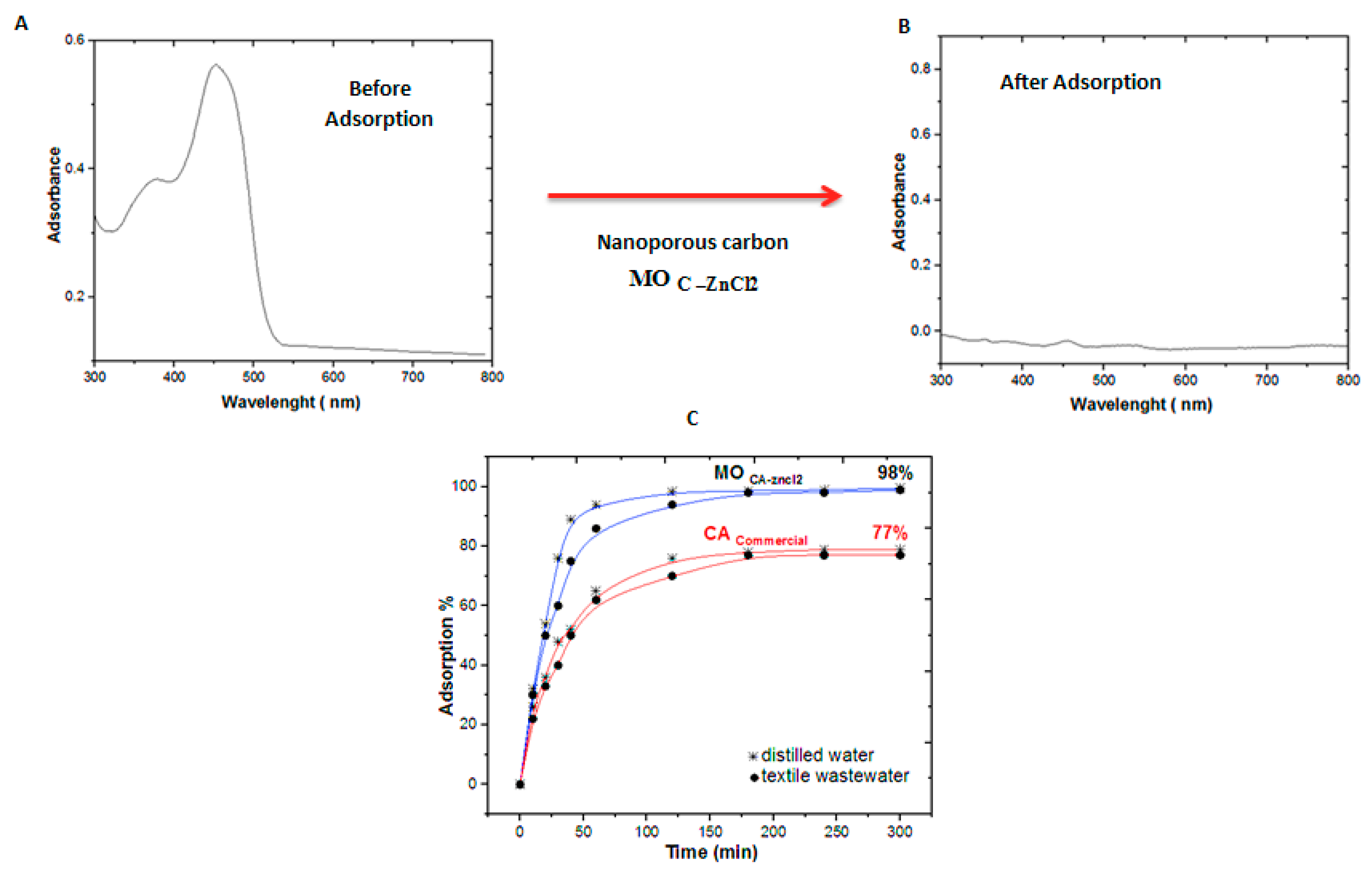
3.2. Adsorption Study
3.3. Thermodynamics Study
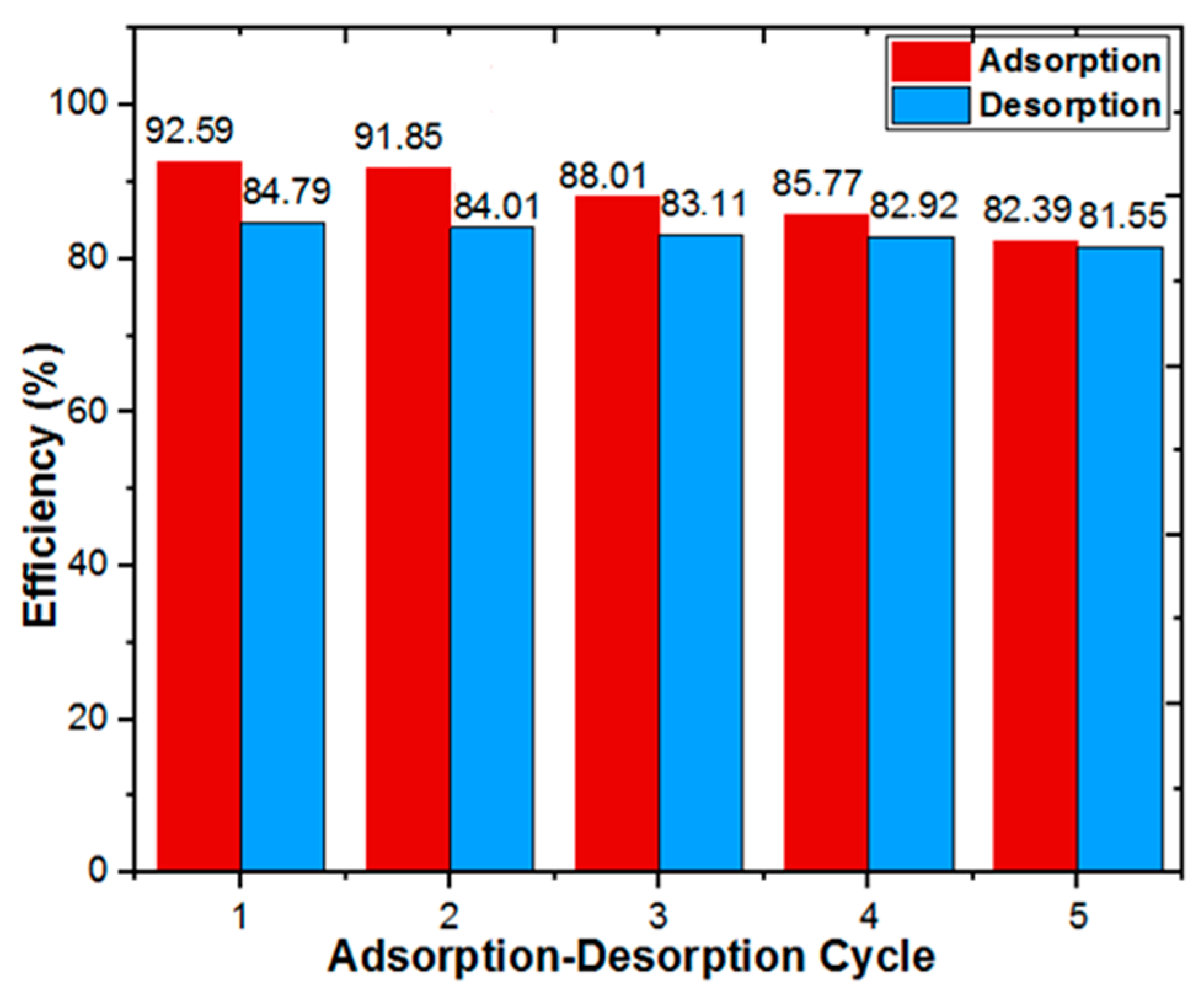
3.4. Adsorption–Desorption
3.5. Treatment of the Real Textile Effluent
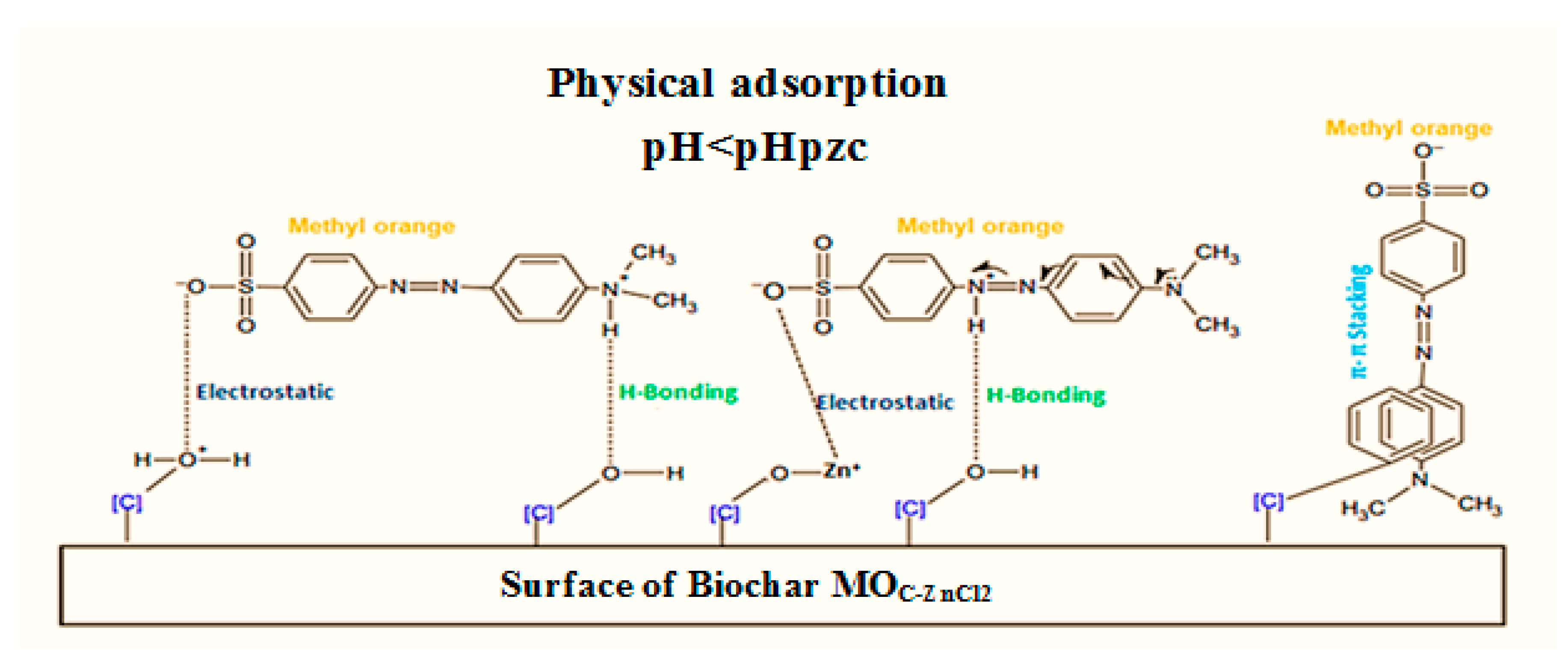
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Radia, J.; Romana, S. Biodegradation of Synthetic Dyes of Textile Effluent by Microorganisms: An Environmentally and Economically Sustainable Approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar]
- Gayathiri, E.; Prakash, P.; Selvam, K.; Awasthi, M.K.; Gobinath, R.; Karri, R.R.; Ragunathan, M.G.; Jayanthi, J.; Mani, V.; Poudineh, M.A.; et al. Plant Microbe Based Remediation Approaches in Dye Removal: A Review. Bioengineered 2022, 13, 7798–7828. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and Applications of Activated Carbons as Adsorbents from Olive Stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Kalsoom; Khan, S.; Ullah, R.; Adil, M.; Waheed, A.; Khan, K.A.; Ghramh, H.A.; Alharby, H.F.; Alzahrani, Y.M.; Alghamdi, S.A.; et al. Adsorption of Pesticides Using Wood-Derived Biochar and Granular Activated Carbon in a Fixed-Bed Column System. Water 2022, 14, 2937. [Google Scholar] [CrossRef]
- Boulika, H.; El Hajam, M.; Hajji Nabih, M.; Riffi Karim, I.; Idrissi Kandri, N.; Zerouale, A. Definitive Screening Design Applied to Cationic & Anionic Adsorption Dyes on Almond Shells Activated Carbon: Isotherm, Kinetic and Thermodynamic Studies. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Dungani, R.; Munawar, S.S.; Karliati, T.; Malik, J.; Aditiawati, P. Sulistyono Study of Characterization of Activated Carbon from Coconut Shells on Various Particle Scales as Filler Agent in Composite Materials. J. Korean Wood Sci. Technol. 2022, 50, 256–271. [Google Scholar] [CrossRef]
- Mechnou, I.; Meskini, S.; El Ayar, D.; Lebrun, L.; Hlaibi, M. Olive Mill Wastewater from a Liquid Biological Waste to a Carbon/Oxocalcium Composite for Selective and Efficient Removal of Methylene Blue and Paracetamol from Aqueous Solution. Bioresour. Technol. 2022, 365, 128162. [Google Scholar] [CrossRef]
- Gopalan, J.; Buthiyappan, A.; Abdul Raman, A.A. Insight into Metal-Impregnated Biomass Based Activated Carbon for Enhanced Carbon Dioxide Adsorption: A Review. J. Ind. Eng. Chem. 2022, 113, 72–95. [Google Scholar] [CrossRef]
- Gomes, H.d.O.; Freire, P.d.T.C.; do Nascimento, R.F.; Pereira Teixeira, R.N. Removal of Contaminants from Water Using Moringa oleifera Lam. as Biosorbent: An Overview of the Last Decade. J. Water Process Eng. 2022, 46, 102576. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.-K.; Kim, S.-J.; Kim, B.-H.; Yoon, H.-S.; Jung, S.-C. Rapid Degradation of Methyl Orange Using Hybrid Advanced Oxidation Process and Its Synergistic Effect. J. Ind. Eng. Chem. 2016, 35, 205–210. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of Photocatalytic Degradation of Methyl Orange Using Immobilized Scoria-Ni/TiO2 Nanoparticles. J. Nanostructure Chem. 2020, 10, 143–159. [Google Scholar] [CrossRef]
- Oyarce, E.; Butter, B.; Santander, P.; Sánchez, J. Polyelectrolytes Applied to Remove Methylene Blue and Methyl Orange Dyes from Water via Polymer-Enhanced Ultrafiltration. J. Environ. Chem. Eng. 2021, 9, 106297. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Ye, J.; Wang, G. Electrochemical Degradation of Azo Dye Methyl Orange by Anodic Oxidation on Ti4O7 Electrodes. J. Mater. Sci. Mater. Electron. 2018, 29, 14065–14072. [Google Scholar] [CrossRef]
- Mechnou, I.; Mourtah, I.; Raji, Y.; Chérif, A.; Lebrun, L.; Hlaibi, M. Effective Treatment and the Valorization of Solid and Liquid Toxic Discharges from Olive Oil Industries, for Sustainable and Clean Production of Bio-Coal. J. Clean. Prod. 2021, 288, 125649. [Google Scholar] [CrossRef]
- Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Preparation and Surface Characterization of Activated Carbons from Euphorbia Rigida by Chemical Activation with ZnCl2, K2CO3, NaOH and H3PO4. Appl. Surf. Sci. 2012, 261, 247–254. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Lagergren, B.K.S. Svenska Zur Theorie Der Sogenannten Adsorption Geloster Stofe, Kungliga Svenska Vetenskapsakademiens. Handlingar 1989, 24, 1–39. [Google Scholar]
- Amrhar, O.; Nassali, H.; Elyoubi, M. Application of Nonlinear Regression Analysis to Select the Optimum Absorption Isotherm for Methylene Blue Adsorption onto Natural Illitic Clay. Bull. De La Société R. Des Sci. De Liège 2015, 84, 116–130. [Google Scholar]
- Shinogi, Y.; Kanri, Y. Pyrolysis of Plant, Animal and Human Waste: Physical and Chemical Characterization of the Pyrolytic Products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent Advances and Challenges in Biomass-Derived Porous Carbon Nanomaterials for Supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Raji, Y.; Mechnou, I.; Yassine, W.; Kadri, Z.; Oumghar, K.; Cherkaoui, O.; Zyade, S. Extraction of the Natural Indigo Carmine Pigment from the Isatis Plant, Characterization and Dyeing of Wool. IOP Conf. Ser. Mater. Sci. Eng. 2020, 948, 012017. [Google Scholar] [CrossRef]
- Vunain, E.; Kenneth, D.; Biswick, T. Synthesis and Characterization of Low-Cost Activated Carbon Prepared from Malawian Baobab Fruit Shells by H3PO4 Activation for Removal of Cu(II) Ions: Equilibrium and Kinetics Studies. Appl. Water Sci. 2017, 7, 4301–4319. [Google Scholar] [CrossRef]
- Wexler, A.S. Integrated Intensities of Absorption Bands in Infrared Spectroscopy. Appl. Spectrosc. Rev. 1967, 1, 29–98. [Google Scholar] [CrossRef]
- Bates, J.B. Fourier Transform Infrared Spectroscopy. Science 1976, 191, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, R.N.; Patra, D. Modification of Nanostructured ZnO Surfaces with Curcumin: Fluorescence-Based Sensing for Arsenic and Improving Arsenic Removal by ZnO. RSC Adv. 2016, 6, 17256–17268. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hosni, K.; Hamdi, N.; Srasra, E. Kinetics and Equilibrium Studies on Removal of Methylene Blue and Methyl Orange by Adsorption onto Activated Carbon Prepared from Date Pits-A Comparative Study. Korean J. Chem. Eng. 2015, 32, 274–283. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Salamanca, M.; Diaz-Corrales, J.D.; Flórez, E.; Silva-Agredo, J.; Torres-Palma, R.A. Understanding the Removal of an Anionic Dye in Textile Wastewaters by Adsorption on ZnCl2activated Carbons from Rice and Coffee Husk Wastes: A Combined Experimental and Theoretical Study. J. Environ. Chem. Eng. 2021, 9, 105685. [Google Scholar] [CrossRef]
- Argun, M.E.; Güclü, D.; Karatas, M. Adsorption of Reactive Blue 114 Dye by Using a New Adsorbent: Pomelo Peel. J. Ind. Eng. Chem. 2014, 20, 1079–1084. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and Kinetic Studies of Methyl Orange and Methyl Violet Adsorption on Activated Carbon Derived from Phragmites Australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Gong, R.; Ye, J.; Dai, W.; Yan, X.; Hu, J.; Hu, X.; Li, S.; Huang, H. Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with Finger-Citron-Residue-Based Activated Carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, Y.; Li, H.; Li, X.; Zhang, C.; Guo, F.; Liu, X.; Wang, K.; Zhao, X.S. Waste-Cellulose-Derived Porous Carbon Adsorbents for Methyl Orange Removal. Chem. Eng. J. 2019, 371, 55–63. [Google Scholar] [CrossRef]
- Spessato, L.; Cazetta, A.L.; Melo, S.; Pezoti, O.; Tami, J.; Ronix, A.; Fonseca, J.M.; Martins, A.F.; Silva, T.L.; Almeida, V.C. Synthesis of Superparamagnetic Activated Carbon for Paracetamol Removal from Aqueous Solution. J. Mol. Liq. 2020, 300, 112282. [Google Scholar] [CrossRef]
- Bello, O.S.; Lasisi, B.M.; Adigun, O.J.; Ephraim, V. Scavenging Rhodamine B Dye Using Moringa Oleifera Seed Pod. Chem. Speciat. Bioavailab. 2017, 29, 120–134. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- León, G.; García, F.; Miguel, B.; Bayo, J. Equilibrium, Kinetic and Thermodynamic Studies of Methyl Orange Removal by Adsorption onto Granular Activated Carbon. Desalin. Water Treat. 2015, 57, 17104–17117. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.-S. Adsorption of Methyl Orange from Aqueous Solution by Aminated Pumpkin Seed Powder: Kinetics, Isotherms, and Thermodynamic Studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef]
- Santos, T.M.; de Jesus, F.A.; da Silva, G.F.; Pontes, L.A.M. Synthesis of Activated Carbon from Oleifera Moringa for Removal of Oils and Greases from the Produced Water. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100357. [Google Scholar] [CrossRef]
- Myek, B.; Adesina, O.B.; Ochigbo, V.; Batari, M.L. Preliminary Study on the Production of Activated Carbon from Moringa Oleifera Seed Shells by Thermal Activation Method. J. Mod. Chem. Chem. Technol. 2021, 6, 13–16. [Google Scholar]
- Hock, P.E.; Zaini, M.A.A. Activated Carbons by Zinc Chloride Activation for Dye Removal—A Commentary. Acta Chim. Slovaca 2018, 11, 99–106. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and Characterization of Magnetic ZnCl2-Activated Carbon Produced from Coconut Shell for the Adsorption of Methylene Blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Fang, C.; Chen, J.; Chen, H.; Li, H.; Yao, Y. Facile Synthesis of Porous Carbon/Fe3O4 Composites Derived from Waste Cellulose Acetate by One-Step Carbothermal Method as a Recyclable Adsorbent for Dyes. J. Mater. Res. Technol. 2020, 9, 3384–3393. [Google Scholar] [CrossRef]
- Mittal, A.; Malviya, A.; Kaur, D.; Mittal, J.; Kurup, L. Studies on the Adsorption Kinetics and Isotherms for the Removal and Recovery of Methyl Orange from Wastewaters Using Waste Materials. J. Hazard. Mater. 2007, 148, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liang, Y.; He, Y.; Wang, Q. Activated Carbon/NiFe2O4 Magnetic Composite: A Magnetic Adsorbent for the Adsorption of Methyl Orange. J. Environ. Chem. Eng. 2015, 3, 1740–1751. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Hamdi, N.; Kriaa, A.; Srasra, E. Adsorption of Methyl Orange Using Activated Carbon Prepared from Lignin by ZnCl2 Treatment. Russ. J. Phys. Chem. A 2012, 86, 1294–1300. [Google Scholar] [CrossRef]
- EL Khattabi, E.H.; Rachdi, Y.; Bassam, R.; Mourid, E.H.; Naimi, Y.; EL Alouani, M.; Belaaouad, S. Enhanced Elimination of Methyl Orange and Recycling of an Eco-Friendly Adsorbent Activated Carbon from Aqueous Solution. Russ. J. Phys. Chem. B 2021, 15, S149–S159. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic Methyl Orange Removal from Aqueous Solutions by Activated Carbon Reinforced Conducting Polyaniline as Adsorbent: Synthesis, Characterization, Adsorption Behavior, Regeneration and Kinetics Study. J. Polym. Environ. 2022, 30, 886–895. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated Carbon Derived from Waste Orange and Lemon Peels for the Adsorption of Methyl Orange and Methylene Blue Dyes from Wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, Á.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Synthesis, Characterization and Dye Removal Capacities of N-Doped Mesoporous Carbons. J. Colloid Interface Sci. 2015, 450, 91–100. [Google Scholar] [CrossRef]




| A | |||||||
| Adsorbent | Yield % | Volatile % | Ash Content % | Carbon Fixed % | Surface BET (m2/g) | Iodine Index mg/g | |
| MOR | ---- | 60.983 | 5.702 | 33.315 | 92.155 | 77.961 | |
| MOC | |||||||
| T °C | Pyrolysis time | ||||||
| 700 °C | 5 min | 47.196 | 30.691 | 2.126 | 67.183 | 216.525 | 260.786 |
| 700 °C | 10 min | 45.110 | 29.255 | 2.080 | 68.665 | ||
| 800 °C | 5 min | 43.114 | 20.962 | 2.054 | 76.984 | 369.029 | 369.656 |
| 800 °C | 10 min | 42.931 | 20.159 | 2.036 | 77.805 | ||
| 900 °C | 5 min | 41.360 | 12.895 | 2.019 | 85.086 | 422.634 | 420.370 |
| 900 °C | 10 min | 40.169 | 12.113 | 2.012 | 85.875 | ||
| B | |||||||
| MOC-ZnCl2 | |||||||
| Impregnation Rate MOC/ZnCl2 | Yield % | Surface BET (m2/g) | Iodine Index mg/g | ||||
| 1:1 | 40.964 | 625.222 | 589.854 | ||||
| 1:2 | 40.120 | 699.696 | 611.291 | ||||
| 1:3 | 40.033 | 602.005 | 589.855 | ||||
| C0 (mg·L−1) | Qeexp (mg g−1) | Model PFO | Model PSO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KF (min−1) | Qe th (mg g−1) | R2 | R2ajusted | χ2Réduced | KS. 104 (g mg−1 min−1) | Qe th (mg g−1) | R2 | R2ajusted | χ2 Reduced | ||
| 500 | 326.215 | 0.053 | 309.521 | 0.921 | 0.910 | 422.910 | 2.850 | 329.821 | 0.983 | 0.981 | 9.061 |
| First Step | Second Step | Third Step | ||||||
|---|---|---|---|---|---|---|---|---|
| KI1 (mg/g min1/2) | C1 (mg/g) | R2 | KI2 (mg/g min1/2) | C2 (mg/g) | R2 | KI3 (mg/g min1/2) | C3 (mg/g) | R2 |
| 37.487 | 5.339 | 0.982 | 28.139 | 59.575 | 0.991 | 2.538 | 277.134 | 0.927 |
| Isotherms | |||
|---|---|---|---|
| Langmuir | Freundlich | ||
| R2 | 0.994 | R2 | 0.958 |
| R2adjusted | 0.993 | R2adjusted | 0.949 |
| χ2Reduced | 1.161 | χ2Reduced | 9.431 |
| KL (L·mg−1) | 0.053 | KF (mg (1 − 1/n) L (1/n)/g) | 222.269 |
| Qmax (mg·g−1) | 367.835 | n | 8.494 |
| RL | 0.018 | ||
| Temkin | Dubinin–Radushkevich | ||
| R2 | 0.963 | R2 | 0.963 |
| R2adjusted | 0.956 | R2adjusted | 0.956 |
| χ2Reduced | 8.261 | χ2Reduced | 8.262 |
| bT (kJ·mol−1) | 0.009 | qD (mg·g−1) | 353.210 |
| KT (L·g−1) | 18.980 | KD (kmol2·J−2) | 256.493 |
| T (K) | 1/T (K−1) | Ce (mg/L) | Qe (mg/g) | KD | Ln KD | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1 K−1) |
|---|---|---|---|---|---|---|---|---|
| 298 | 0.00335 | 150.131 | 330.211 | 2.199 | 0.788 | −1.903 | 13.593 | 0.052 |
| 303 | 0.00330 | 141.665 | 339.245 | 2.394 | 0.872 | −2.163 | ||
| 313 | 0.00319 | 126.564 | 365.614 | 2.888 | 1.060 | −2.683 | ||
| 323 | 0.00309 | 113.325 | 378.986 | 3.344 | 1.207 | −3.203 |
| Characteristic | Before Adsorption | After Adsorption | Removal | Norms of Limits According to the World Health Organization (WHO) for Wastewater |
|---|---|---|---|---|
| Turbidity (NTU) | 24.71 | 2.19 | 91.13% | <5 |
| COD (mg·L−1) | 519.53 | 11.80 | 97.72% | <90 |
| BOD5 (mg·L−1) | 11.29 | 7.06 | 37.46% | <30 |
| COD/DBO5 | 46.01 (Not biodegradable) | 1.67 (Easily biodegradable) | ---- | <3 (Biodegradable) |
| TSS (mg·L−1) | 1.36 | 0.50 | 63.23% | <20 |
| pH | 7.52 | 6.96 | --- | 6.5–8.5 |
| Temperature (°C) | 25 | 25 | ---- | <30 |
| Color | Light orange | Incolore | Total discoloration | Clair/incolore |
| Odor | Inodore | Inodore | ---- | Inodore |
| Adsorbents | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|
| Activated carbon/NiFe2O4 magnetic composite | 182.82 mg | [46] |
| Activated carbon from lignin | 300 | [47] |
| Activated carbon from date pits | 434.0 | [29] |
| Commercial activated carbon | 113.63 | [48] |
| Activated carbon Reinforced Conducting Polyaniline | 192.52 | [49] |
| Activated carbon from Lemon peels | 33 | [50] |
| Pumpkin seed powder | 200.3 | [39] |
| N-doped mesoporous activated carbon (N-OMC) | 135.8 | [51] |
| Activated carbon prepared from date pits date pits | 434 | [29] |
| Nanoporous carbon from Husks of Moringa oleifera | 367.83 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raji, Y.; Nadi, A.; Rouway, M.; Jamoudi Sbai, S.; Yassine, W.; Elmahbouby, A.; Cherkaoui, O.; Zyade, S. Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism. J. Compos. Sci. 2022, 6, 385. https://doi.org/10.3390/jcs6120385
Raji Y, Nadi A, Rouway M, Jamoudi Sbai S, Yassine W, Elmahbouby A, Cherkaoui O, Zyade S. Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism. Journal of Composites Science. 2022; 6(12):385. https://doi.org/10.3390/jcs6120385
Chicago/Turabian StyleRaji, Yosra, Ayoub Nadi, Marwane Rouway, Sara Jamoudi Sbai, Wafaa Yassine, Abdelfattah Elmahbouby, Omar Cherkaoui, and Souad Zyade. 2022. "Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism" Journal of Composites Science 6, no. 12: 385. https://doi.org/10.3390/jcs6120385
APA StyleRaji, Y., Nadi, A., Rouway, M., Jamoudi Sbai, S., Yassine, W., Elmahbouby, A., Cherkaoui, O., & Zyade, S. (2022). Efficient Adsorption of Methyl Orange on Nanoporous Carbon from Agricultural Wastes: Characterization, Kinetics, Thermodynamics, Regeneration and Adsorption Mechanism. Journal of Composites Science, 6(12), 385. https://doi.org/10.3390/jcs6120385







