Preparation of Nanochitin from Crickets and Comparison with That from Crab Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
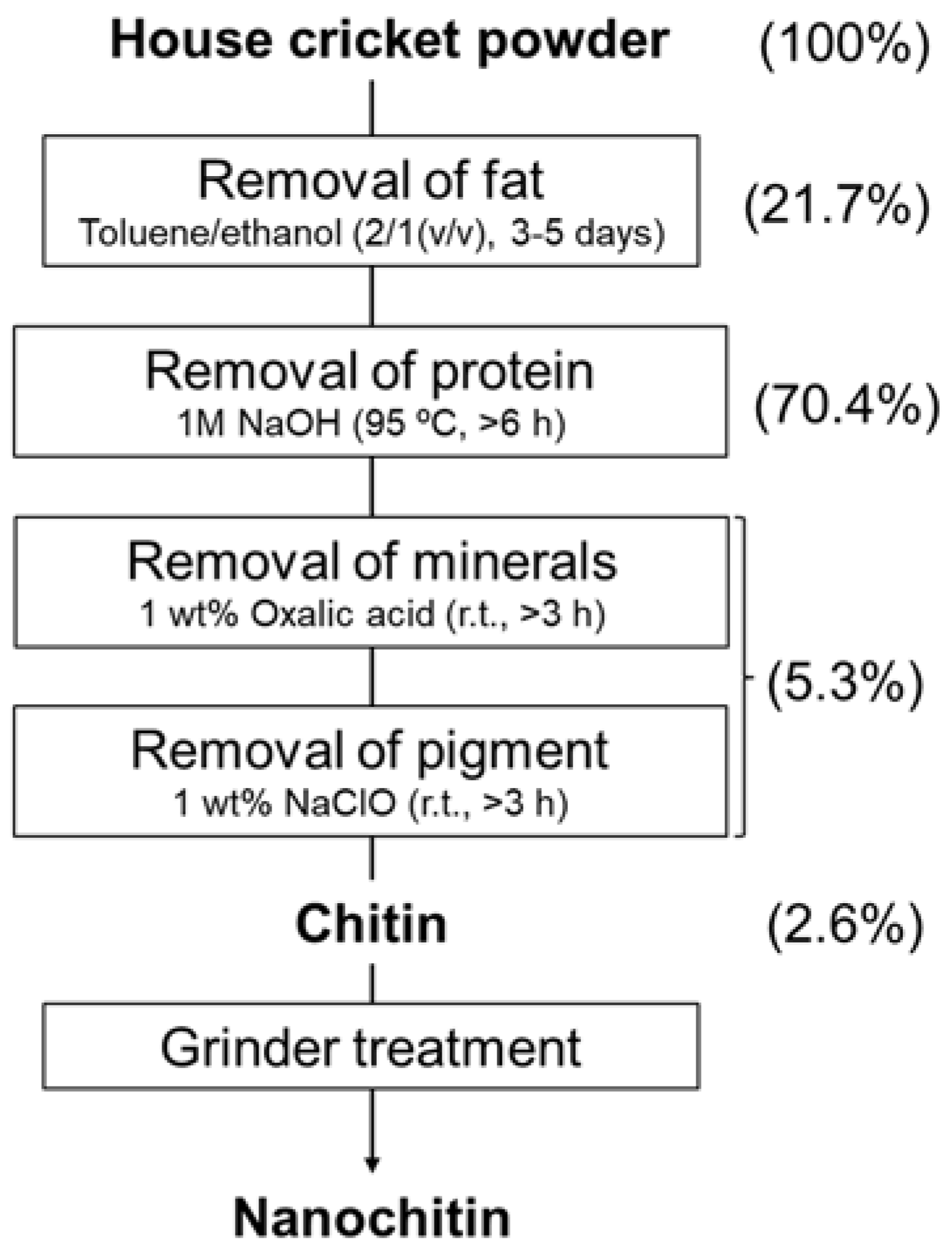
2.2. Preparation of Chitin from Cricket Powder
2.3. Preparation of Nanochitin from Cricket Chitin
2.4. Characterization of Cricket Chitin and Nanochitin
2.4.1. 13C CP/MAS-Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4.2. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.4.3. Scanning Electron Microscopy (SEM) Observation
2.4.4. Atomic Force Microscopy (AFM) Observation
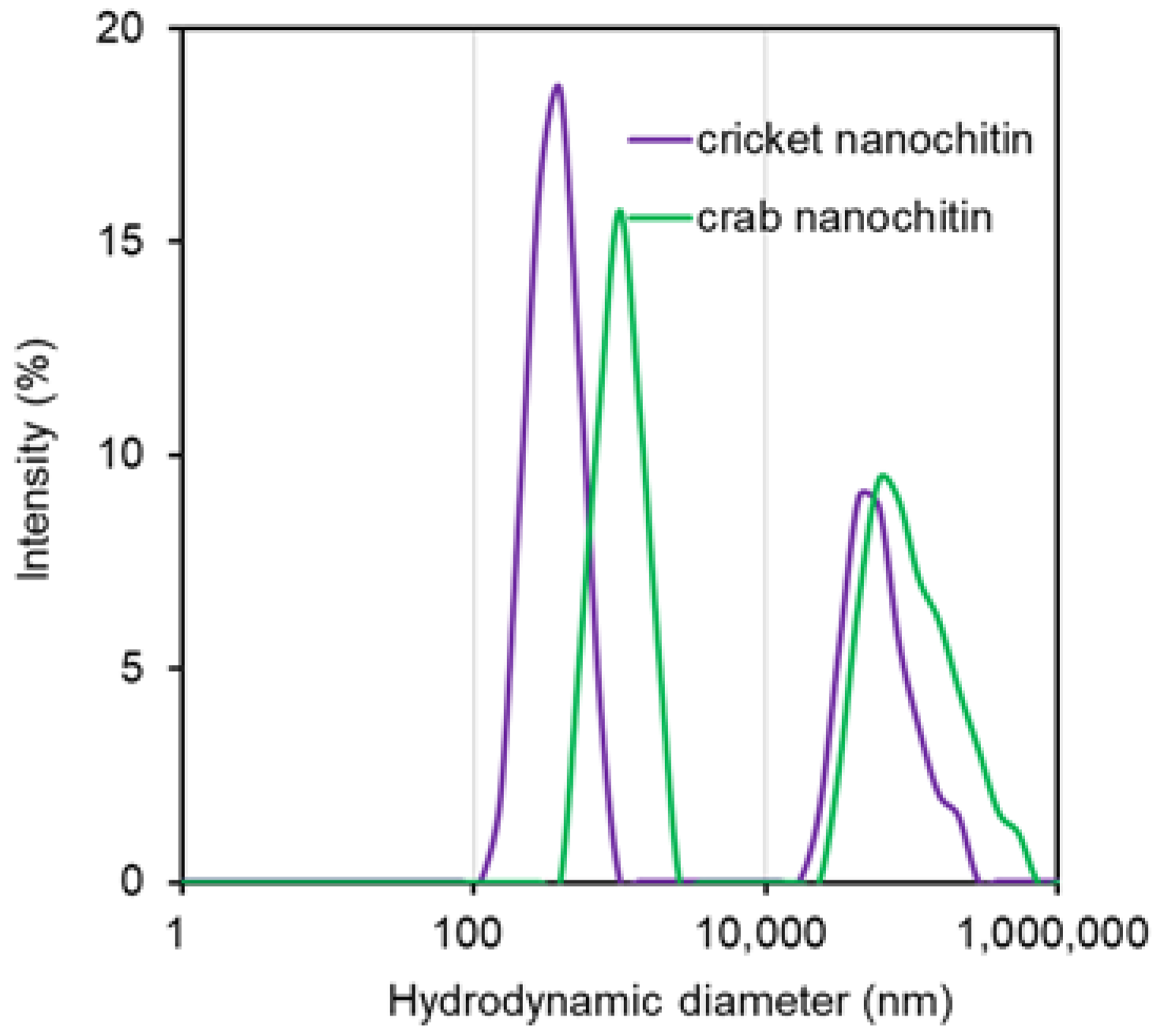
2.4.5. Particle Size Measurement
2.4.6. X-ray Diffraction (XRD) Profile
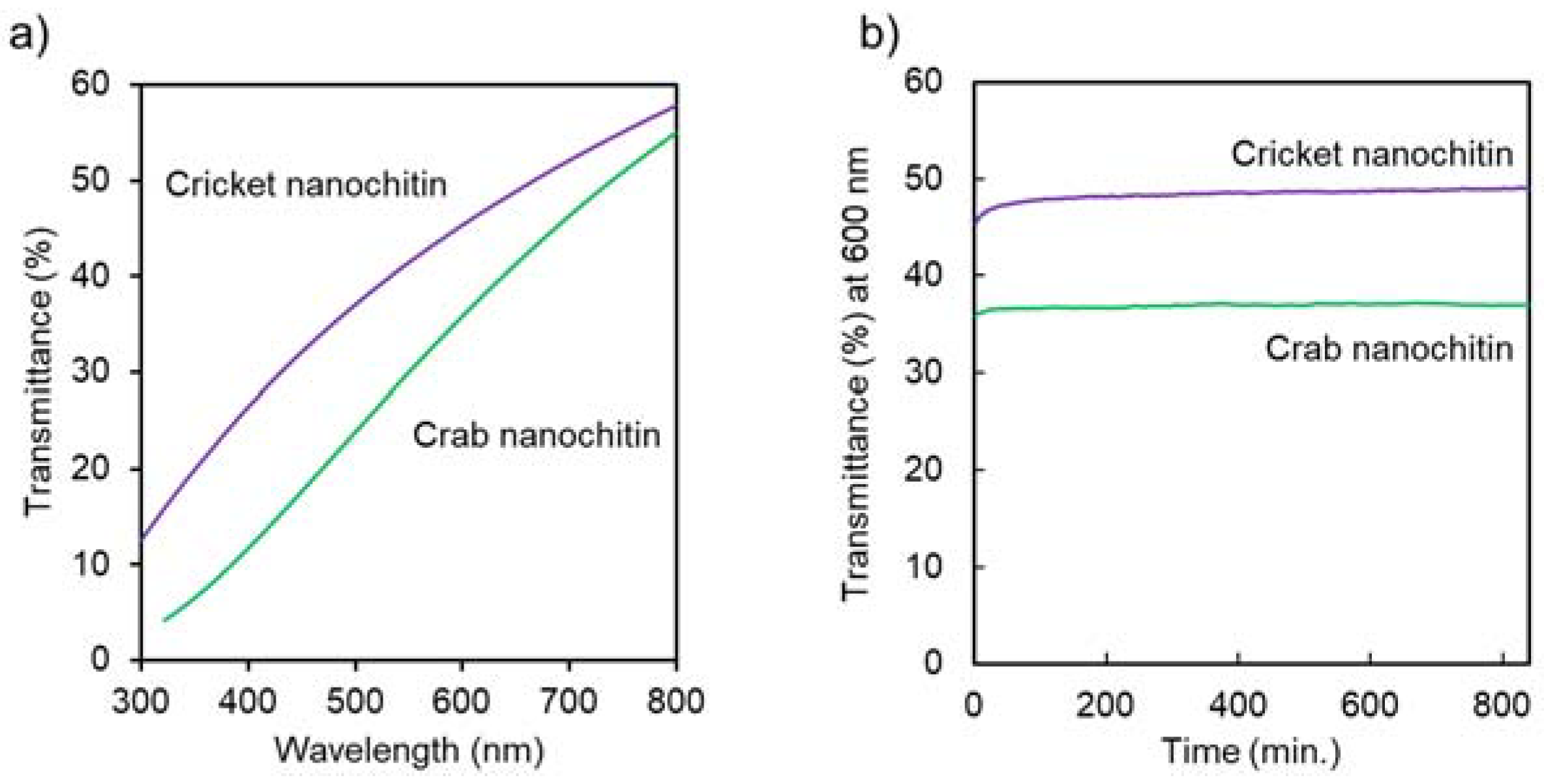
2.4.7. Ultraviolet-Visible (UV–Vis) Transmittance
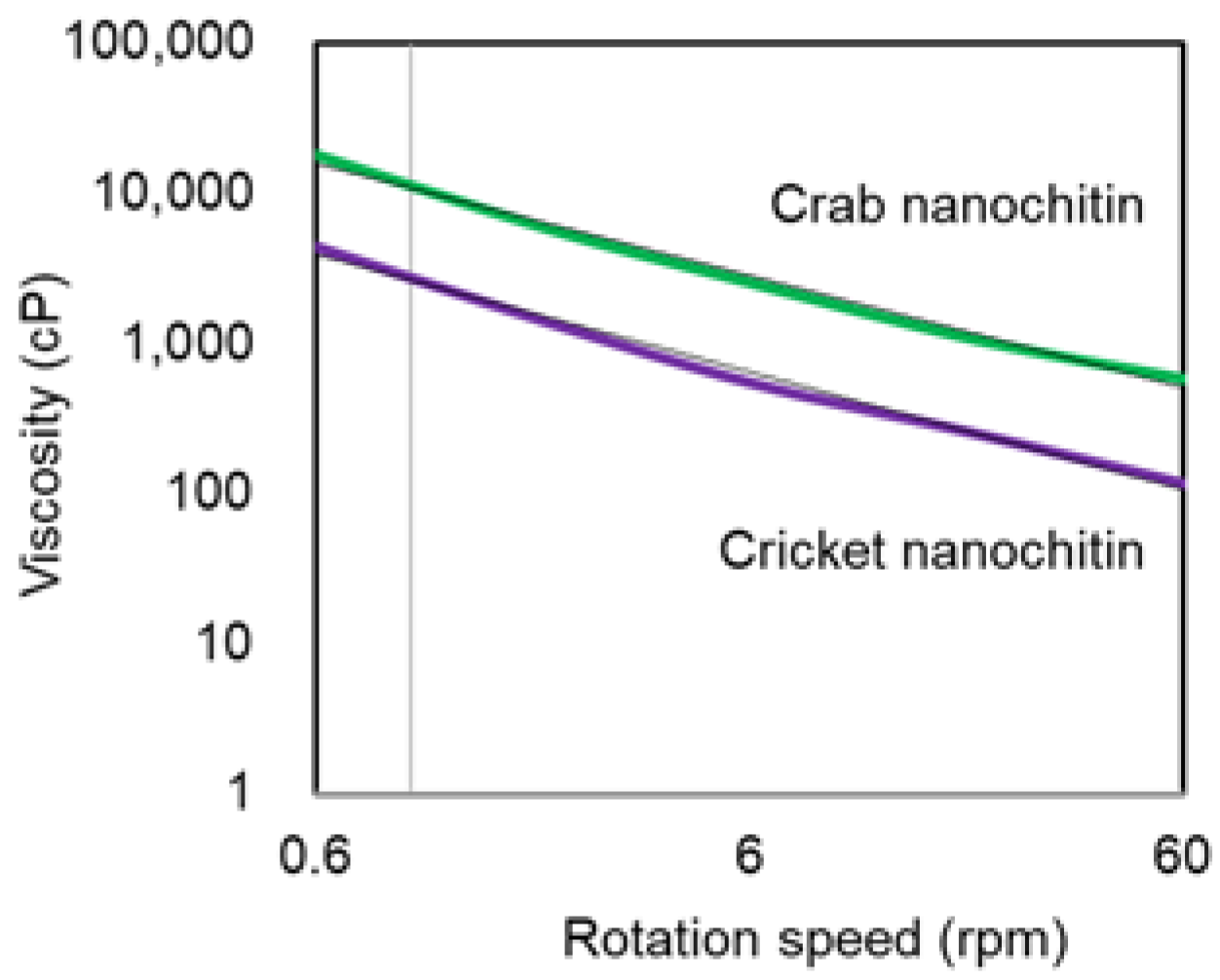
2.4.8. Viscosity Measurement
2.4.9. Specific Surface Area Measurement by Brunauer-Emmett-Teller (BET) Method
2.4.10. Tensile Test of Cricket Nanochitin Sheet
3. Results and Discussions
3.1. Isolation of Chitin from Crickets
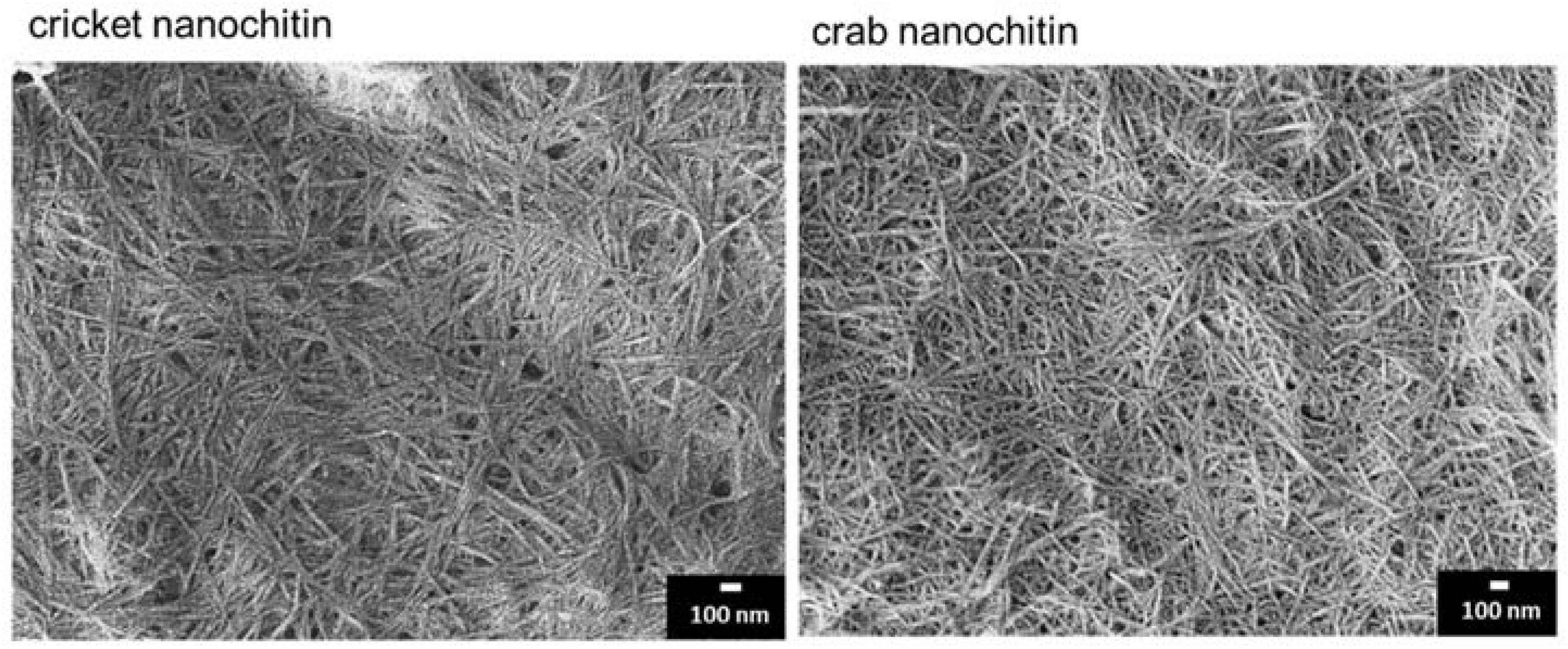
3.2. Preparation and Characterization of Cricket Nanochitin
3.3. Preparation and Mechanical Properties of Cricket Nanochitin Sheet
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Progress Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Das, P.; Heuser, T.; Wolf, A.; Zhu, B.; Demco, D.E.; Ifuku, S.; Walther, A. Tough and catalytically active hybrid biofibers wet-spun from nanochitin hydrogels. Biomacromolecules 2012, 13, 4205–4212. [Google Scholar] [CrossRef]
- Izumi, R.; Komada, S.; Kosuke, O.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef]
- Azuma, K.; Koizumi, R.; Izawa, H.; Morimoto, M.; Saimoto, H.; Osaki, T.; Ito, N.; Yamashita, M.; Tsuka, T.; Imagawa, T.; et al. Hair growth-promoting activities of chitosan and surface-deacetylated chitin nanofibers. Int. J. Biolog. Macromol. 2019, 126, 11–17. [Google Scholar] [CrossRef]
- Goto, M.; Iohara, D.; Michihara, A.; Ifuku, S.; Azuma, K.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Hirayama, F.; Anraku, M. Effects of surface-deacetylated chitin nanofibers on non-alcoholic steatohepatitis model rats and their gut microbiota. Int. J. Biolog. Macromol. 2020, 164, 659–666. [Google Scholar] [CrossRef]
- Anraku, M.; Tabuchi, R.; Ifuku, S.; Nagae, T.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Miyamura, S.; Hirayama, F.; et al. An oral absorbent, surface-deacetylated chitin nano-fiber amelioratesrenal injury and oxidative stress in 5/6 nephrectomized rats. Carbohydr. Polym. 2017, 161, 21–25. [Google Scholar] [CrossRef]
- Egusa, M.; Matsui, H.; Urakami, T.; Okuda, S.; Ifuku, S.; Nakagami, H.; Kaminaka, H. Chitin nanofiber elucidates the elicitor activity of polymeric chitin in plants. Front. Plant. Sci. 2015, 6, 1098. [Google Scholar] [CrossRef]
- Parada, R.Y.; Egusa, M.; Aklog, Y.F.; Miura, C.; Ifuku, S.; Kaminaka, H. Optimization of nanofibrillation degree of chitin for induction of plant disease resistance: Elicitor activity and systemic resistance induced by chitin nanofiber in cabbage and strawberry. Int. J. Biolog. Macromol. 2018, 118, 2185–2192. [Google Scholar] [CrossRef]
- Wada, M.; Saito, Y. Lateral thermal expansion of chitin crystals. J. Polym. Sci., Part. B Polym. Phys. 2001, 39, 168–174. [Google Scholar] [CrossRef]
- Ifuku, S.; Morooka, S.; Nakagaito, A.N.; Morimoto, M.; Saimoto, H. Preparation and characterization of optically transparent chitin nanofiber/(meth)acrylic resin composites. Green Chem. 2011, 13, 1708–1711. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of high-strength transparent chitosan film reinforced with surface-deacetylated chitin nanofibers. Carbohydr. Polym. 2013, 98, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.; Joost, I.; Harmke, K.; Esther, M.; Afton, H.; Giulia, M.; Paul, V. EDIBLE INSECTS Future Prospects of Food and Feed Security, FAO Forestry Paper 171; Food and Agriculture Organization of the United Nations, FAO: Rome, Italy; Wageningen, UR: Wageningen, The Netherlands, 2013. [Google Scholar]
- Arnold, H. Potential of insects as food and feed in assuring food security. Ann. Rev. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. BioScience 2004, 54, 909–918. [Google Scholar] [CrossRef]
- Thomas, H.; Elena, T.; Aman, P.; Rosanna, S.; Patrizia, F.; Susanne, Z. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Ohno, M.; Miyazaki, M.; Kimura, M.; Minowa, Y.; Sakaguchi, M.; Oyama, F.; Yamashita, T. Characterization of mouse di-N-acetylchitobiase that can degrade chitin-oligosaccharides. Biosci. Biotechnol. Biochem. 2020, 84, 1499–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, C.; Xue, Y.; Gao, R.; Zhang, X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr. Res. 2005, 340, 1914–1917. [Google Scholar] [CrossRef]
- Yanchao, W.; Yaoguang, C.; Long, Y.; Cuiyu, Z.; Xiaoqi, X.; Yong, X.; Zhaojie, L.; Changhu, X. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Res. 2013, 92, 90–97. [Google Scholar] [CrossRef]
- Yao, H.; Yan, F.; Lingyun, C.; Ang, L.; Lina, Z. One-step synthesis of size-tunable gold nanoparticles immobilized on chitin nanofibrils via green pathway and their potential applications. Chem. Eng. J. 2017, 315, 573–582. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Phil. Trans. R. Soc. 2022, A379, 20200331. [Google Scholar] [CrossRef]
- Kono, H. Two-dimensional magic angle spinning NMR investigation of naturally occurring chitins: Precise 1H and 13C resonance assignment of α- and β-chitin. Biopolymer 2004, 75, 2555-263. [Google Scholar] [CrossRef] [PubMed]
- Gustavo, C.; Cristian, G.; Aleksandra, N.; Kelly, P.; Oscar, G.; Cédric, D.; Oscar, V.; Heng, Y.; Gaston, B.; Juan, C. Utilization of marine waste to obtain β-chitin nanofibers and films from giant humboldt squid Dosidicus gigas. Marine Drugs 2021, 19, 184. [Google Scholar] [CrossRef]
- El Montassir, D.; Moha, T.; Nadia, E.; Mohammed, R. Extraction and Characterization of Chitin and Chitosan from Parapenaeus longirostris from Moroccan Local Sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar]
- Chen, B.; Wang, J.; Peng, X.; Cai, C.; Wu, X. Nanometer chitin fiber and layup of the chafer cuticle. Int. J. Nanosci. 2004, 3, 707–714. [Google Scholar] [CrossRef]
- Ifuku, S.; Yamada, K.; Morimoto, M.; Saimoto, H. Nanofibrillation of dry chitin powder by star burst system. J. Nanomater. 2012, 2012, 645624. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated a-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Wang, J. Acidic ethanol/water casting approach to improve chitin nanofibril dispersion and properties of propionylated chitin biocomposites. ACS Sustain. Chem. Eng. 2021, 9, 3289–3299. [Google Scholar] [CrossRef]
- Dutta, A.K.; Yamada, K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers from dry chitin powder by star burst system: Dependence on number of passes. J. Chitin Chitosan Sci. 2013, 1, 59–64. [Google Scholar] [CrossRef]
- Sakuma, W.; Yamasaki, S.; Fujisawa, S.; Kodama, T.; Shiomi, J.; Kanamori, K.; Saito, T. Mechanically strong, scalable, Mesoporous xerogels of nanocellulose featuring light permeability, thermal insulation, and flame self-extinction. ACS Nano 2021, 15, 1436–1444. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xu, F. Chemical characteristics of wood cell wall with an emphasis on ultrastructure: A mini-review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]



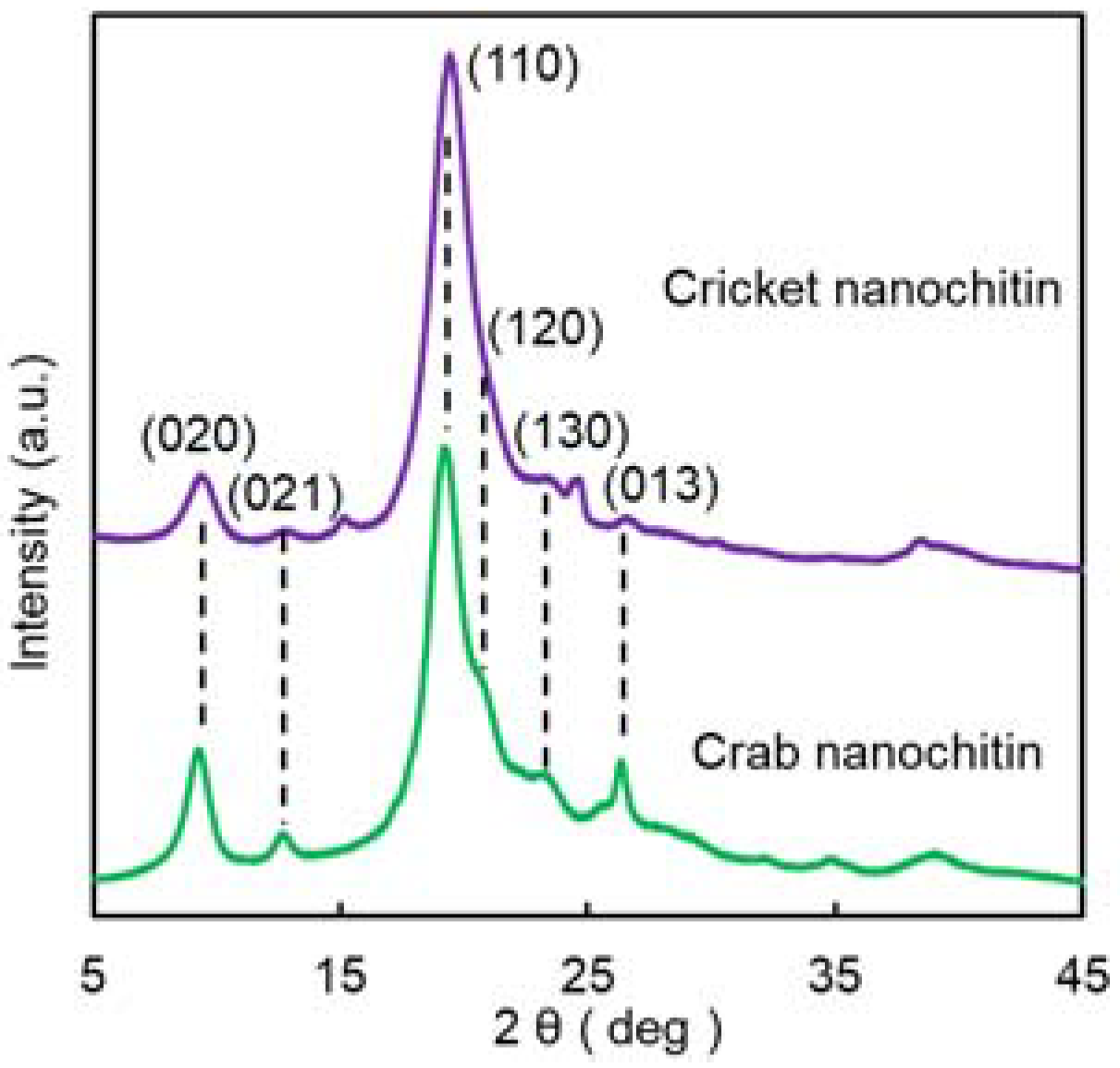

| Sample | Relative Crystalline Index (%) | Crystalline Size (nm) | |
|---|---|---|---|
| 020 | 110 | ||
| Cricket nanochitin | 86.3 | 6.1 | 4.3 |
| Crab nanochitin | 87.6 | 8.0 | 4.6 |
| Density (g m−3) | Young’s Modulus (MPa) | Fracture Strength (MPa) | Fracture Strain (%) | |
|---|---|---|---|---|
| Cricket nanochitin | 0.587 | 15.0 (±8.9) | 6.60 (±3.3) | 0.39 (±0.05) |
| Crab nanochitin | 0.453 | 9.6 (±3.9) | 5.89 (±2.3) | 0.62 (±0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishida, K.; Mizuta, T.; Izawa, H.; Ifuku, S. Preparation of Nanochitin from Crickets and Comparison with That from Crab Shells. J. Compos. Sci. 2022, 6, 280. https://doi.org/10.3390/jcs6100280
Kishida K, Mizuta T, Izawa H, Ifuku S. Preparation of Nanochitin from Crickets and Comparison with That from Crab Shells. Journal of Composites Science. 2022; 6(10):280. https://doi.org/10.3390/jcs6100280
Chicago/Turabian StyleKishida, Kana, Toshifumi Mizuta, Hironori Izawa, and Shinsuke Ifuku. 2022. "Preparation of Nanochitin from Crickets and Comparison with That from Crab Shells" Journal of Composites Science 6, no. 10: 280. https://doi.org/10.3390/jcs6100280
APA StyleKishida, K., Mizuta, T., Izawa, H., & Ifuku, S. (2022). Preparation of Nanochitin from Crickets and Comparison with That from Crab Shells. Journal of Composites Science, 6(10), 280. https://doi.org/10.3390/jcs6100280






