Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review
Abstract
1. Introduction
2. Heavy Metals as Pollutants
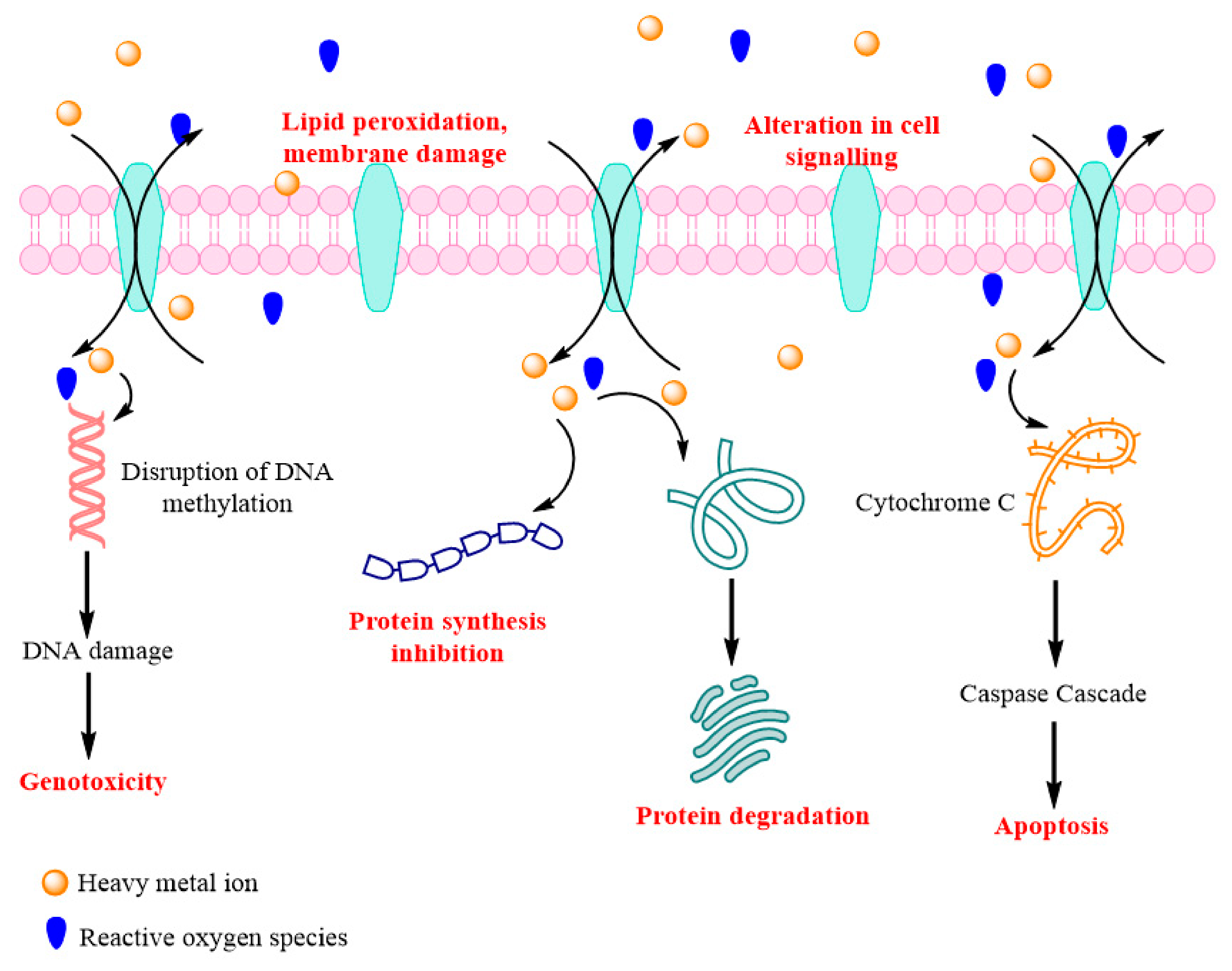
Toxicity of Heavy Metals on Human Health
3. Cyanobacteria: An Outlook for Environmental Remediation
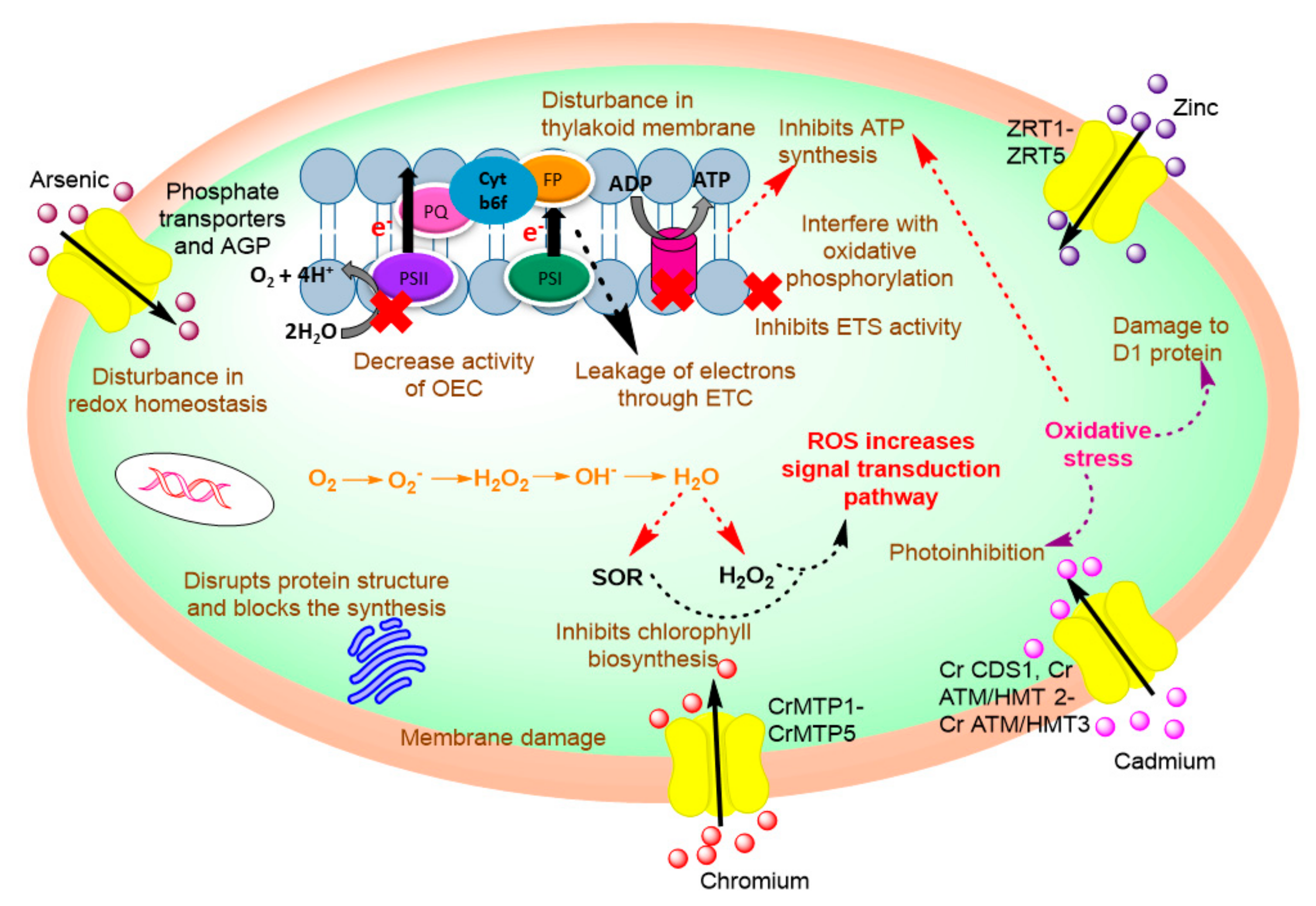
Effect of Heavy Metal on Cyanobacterial Physiological and Biochemical Processes
4. Heavy Metal Removal by Cyanobacteria
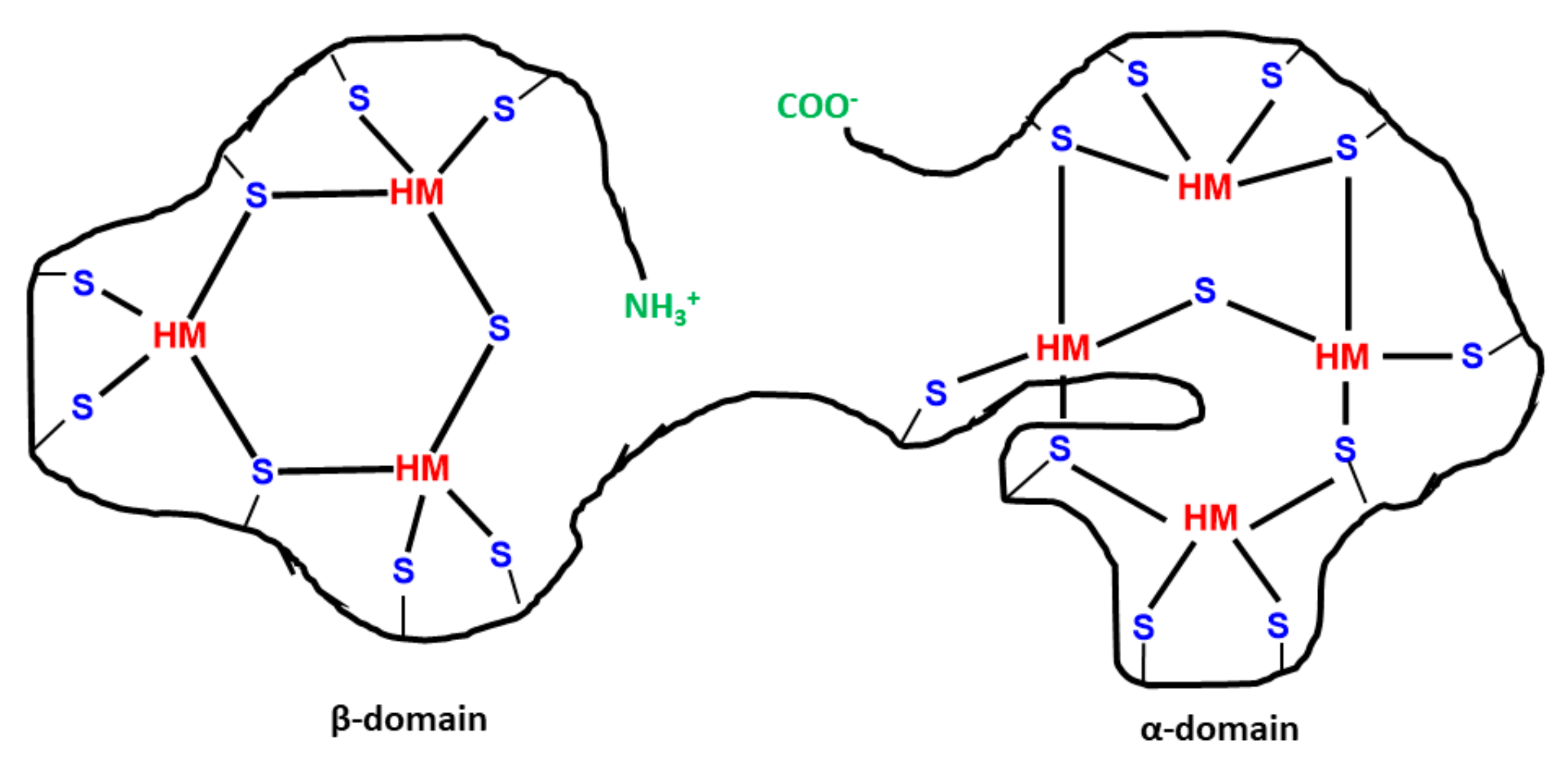
Cyanobacterial EPS: Structure and Occurrence and Role of Metallothionein
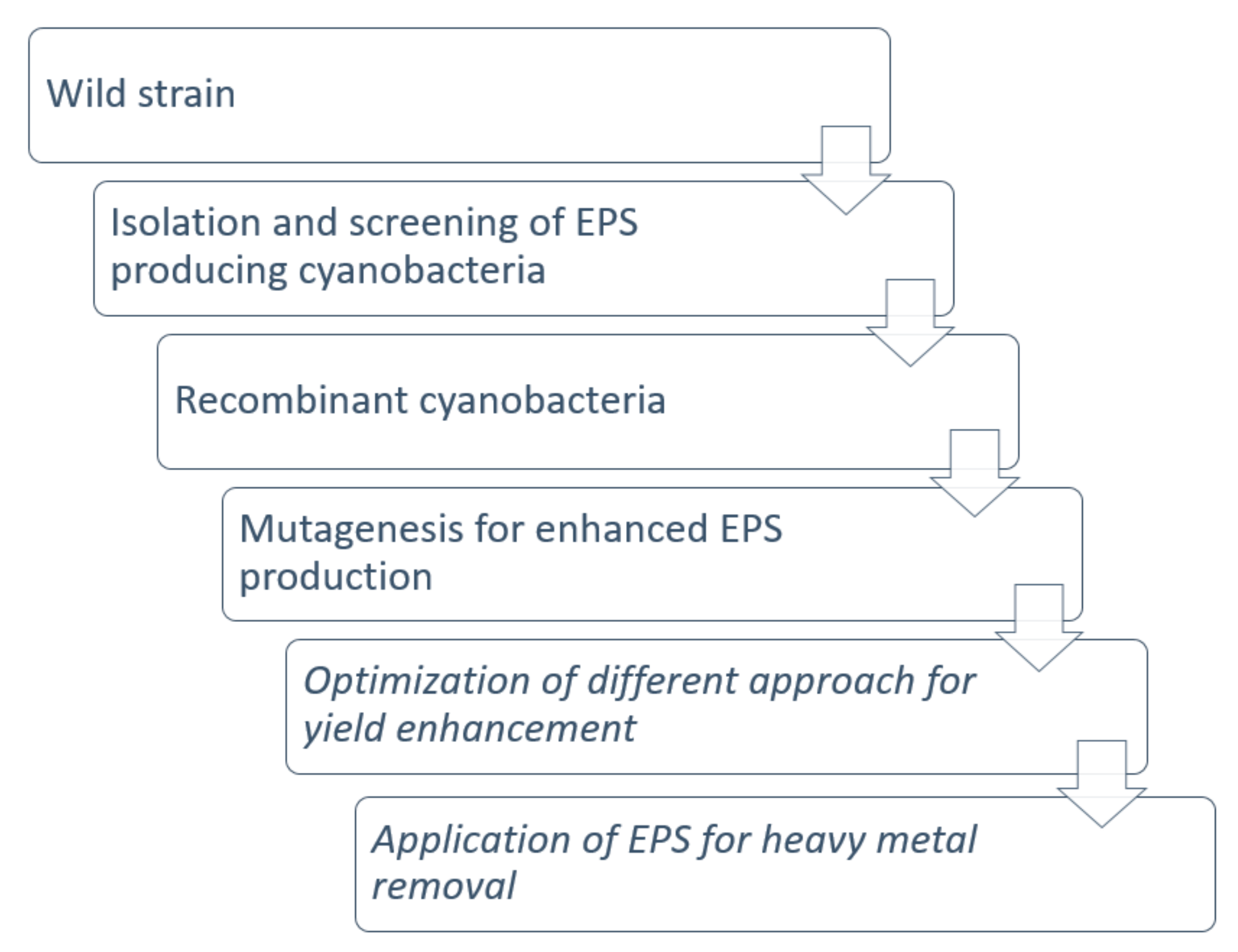
5. Strategy for Enhancing the EPS Production
5.1. Wild Strain
5.2. Isolation and Screening of EPS Producing Cyanobacteria
5.3. Recombinant Cyanobacteria
5.4. Mutagenesis for Enhanced EPS Production
5.5. Optimization of a Different Approach for Yield Enhancement
5.6. Application of EPS for Heavy Metal Removal
6. Different Factors Assessment for the Wastewater Cultivated Cyanobacteria
6.1. Life-Cycle Assessment and Risk Assessment Analyses for Industrial Application
6.2. Limitations of Wastewater-Cultivated Cyanobacteria
7. Conclusions and Future Perspectives
- ⮚
- In spite of the fact that the methods of poisonous metal biosorption and microbial biosorbents have been broadly examined in labs, the improvement of reasonable biosorption advances and their usage in different divisions delivering substantial metals have not increased as desired. Nonetheless, a predetermined number of organizations have created and popularized microorganism based bioremediation advances.
- ⮚
- Assessment and improvement of biosorbent materials from microbial biomass is a quickly expanding thirst area in academia and in industries.
- ⮚
- Cyanobacteria are a captivating and extraordinary class of microorganism with wonderful versatility, omnipresence, and assorted variety. In spite of the fact that the presence of cyanobacteria in the terrestrial and in the aquatic environment are easily available, screening and determination of the promising cyanobacterial species/strains (isolation and selection) with a high metal sorption limit is a demanding task.
Author Contributions
Funding
Conflicts of Interest
References
- Goswami, L.; Kumar, R.V.; Borah, S.N.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: A review. J. Water Process Eng. 2018, 26, 314–328. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S. Mechanism of soil-metal-microbe interactions and their implication on microbial bioremediation and phytoremediation. Environ. Sci. Eng. 2017, 8. [Google Scholar]
- Kushwaha, A.; Rani, R.; Agarwal, V. Environmental Fate and Eco-toxicity of Engineered Nano-particles: Current Trends and Future Perspective. In Advanced Nanomaterials for Wastewater Remediation; Taylor & Francis Group: Abingdon, UK, 2016; pp. 387–404. [Google Scholar]
- Goswami, L.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Simultaneous heavy metal removal and anthracene biodegradation by the oleaginous bacteria Rhodococcus opacus. 3 Biotech 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Kumar, R.V.; Pakshirajan, K.; Pugazhenthi, G. A novel integrated biodegradation—Microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard. Mater. 2019, 365, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Rani, R.; Kumar, S.; Thomas, T.; David, A.A.; Ahmed, M. A new insight to adsorption and accumulation of high lead concentration by exopolymer and whole cells of lead-resistant bacterium Acinetobacter junii L. Pb1 isolated from coal mine dump. Environ. Sci. Pollut. Res. 2017, 24, 10652–10661. [Google Scholar] [CrossRef]
- Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of biomass gasification wastewater using hydrocarbonoclastic bacterium Rhodococcus opacus in an up-flow packed bed bioreactor with a novel waste-derived nano-biochar based bio-support material. J. Clean. Prod. 2020, 256, 120253. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Bind, A.; Kushwaha, A.; Devi, G.; Goswami, S.; Sen, B.; Prakash, V. Biosorption valorization of floating and submerged macrophytes for heavy-metal removal in a multi-component system. Appl. Water Sci. 2019, 9, 95. [Google Scholar] [CrossRef]
- Sathe, S.S.; Goswami, L.; Mahanta, C.; Devi, L.M. Integrated factors controlling arsenic mobilization in an alluvial floodplain. Environ. Technol. Innov. 2020, 17, 100525. [Google Scholar] [CrossRef]
- Dutta, M.; Kushwaha, A.; Kalita, S.; Devi, G.; Bhuyan, M. Assessment of bioaccumulation and detoxification of cadmium in soil-plant-insect food chain. Bioresour. Technol. Rep. 2019, 7, 100242. [Google Scholar] [CrossRef]
- Goswami, L.; Manikandan, N.A.; Dolman, B.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of wastewater containing a mixture of polycyclic aromatic hydrocarbons using the oleaginous bacterium Rhodococcus opacus. J. Clean. Prod. 2018, 196, 1282–1291. [Google Scholar] [CrossRef]
- Bind, A.; Goswami, L.; Prakash, V. Comparative analysis of floating and submerged macrophytes for heavy metal (copper, chromium, arsenic and lead) removal: Sorbent preparation, characterization, regeneration and cost estimation. Geol. Ecol. Landsc. 2018, 2, 61–72. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Patra, J.K. Adsorption kinetics and molecular interactions of lead [Pb(II)] with natural clay and humic acid. Int. J. Environ. Sci. Technol. 2020, 17, 1325–1336. [Google Scholar] [CrossRef]
- Singh, P.K.; Kushwaha, A.; Hans, N.; Gautam, A.; Rani, R. Evaluation of the cytotoxicity and interaction of lead with lead resistant bacterium Acinetobacter junii Pb1. Braz. J. Microbiol. 2019, 50, 223–230. [Google Scholar] [CrossRef]
- Gupt, C.B.; Kushwaha, A.; Prakash, A.; Chandra, A.; Goswami, L.; Sekharan, S. Mitigation of Groundwater Pollution: Heavy Metal Retention Characteristics of Fly Ash Based Liner Materials, Fate and Transport of Subsurface Pollutants; Springer: Singapore, 2020. [Google Scholar]
- Gupta, P.K.; Kumar, A.; Goswami, L.; Yadav, B. Rhizospheric Treatment of Hydrocarbons Containing Wastewater. In Microbial Technology for Health and Environment; Springer: Singapore, 2020; pp. 289–301. [Google Scholar]
- Kushwaha, A.; Goswami, S.; Hans, N.; Goswami, L.; Devi, G.; Deshavath, N.N.; Yadav, M.K.; Lall, A.M. An Insight into Biological and Chemical Technologies for Micropollutant Removal from Wastewater, Fate and Transport of Subsurface Pollutants; Springer: Singapore, 2020. [Google Scholar]
- Bulat, Z.P.; Đukić-Ćosić, D.; Đokić, M.; Bulat, P.; Matović, V. Blood and urine cadmium and bioelements profile in nickel-cadmium battery workers in Serbia. Toxicol. Ind. Health 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Chaumont, A.; De Winter, F.; Dumont, X.; Haufroid, V.; Bernard, A. The threshold level of urinary cadmium associated with increased urinary excretion of retinol-binding protein and β2-microglobulin: A re-assessment in a large cohort of nickel-cadmium battery workers. Occup. Environ. Med. 2011, 68, 257–264. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Thijssen, S.; Cuypers, A.; Maringwa, J.; Smeets, K.; Horemans, N.; Lambrichts, I.; Van Kerkhove, E. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 2007, 236, 29–41. [Google Scholar] [CrossRef]
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [Google Scholar]
- Yang, Z.-Y.; He, C.; Shen, C.; Guo, J.; Mubeen, S.; Yuan, J.; Yang, Z.-Y. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Cadmium and cardiovascular diseases: Cell biology, pathophysiology, and epidemiological relevance. BioMetals 2010, 23, 811–822. [Google Scholar] [CrossRef]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Chromium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2000. Available online: http://www.atsdr.cdc.gov/toprofiles/tp7.html (accessed on 20 October 2020).
- Martin, S.; Griswold, W. Human Health and effects of heavy metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar] [CrossRef]
- Huy, T.B.; Tuyet-Hanh, T.T.; Johnston, R.B.; Nguyen-Viet, H. Assessing Health Risk due to Exposure to Arsenic in Drinking Water in Hanam Province, Vietnam. Int. J. Environ. Res. Public Health 2014, 11, 7575–7591. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, H. Thermophiles harbored in Armenian geothermal springs and their potential in biotechnology. In Proceedings of the Industrial Biotechnology Congress, Birmingham, UK, 10–11 August 2015. [Google Scholar]
- Hedges, S.B.; Chen, H.; Kumar, S.; Wang, D.Y.; Thompson, A.S.; Watanabe, H. A genomic timescale for the origin of eukaryotes. BMC Evol. Biol. 2001, 1, 4. [Google Scholar] [CrossRef]
- Gupta, A.; Bhagwat, S.G.; Sainis, J.K. Synechococcus elongatus PCC 7942 is more tolerant to chromate as compared to Synechocystis sp. PCC 6803. BioMetals 2013, 26, 309–319. [Google Scholar] [CrossRef]
- Katırcıoğlu, H.; Aslım, B.; Türker, A.R.; Atıcı, T.; Beyatlı, Y. Removal of cadmium (II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour. Technol. 2008, 99, 4185–4191. [Google Scholar]
- Al-Sherif, E.A.; Abd El-Hameed, M.S.; Mahmoud, M.A.; Ahmed, H.S. Use of cyanobacteria and organic fertilizer mixture as soil bioremediation. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 794–799. [Google Scholar]
- Ashworth, H.; Abrahamsen, L.G.; Bryan, N.; Foster, L.; Lloyd, J.; Kellet, S.; Heath, S.L.; Abrahamsen, L. Effect of humic acid & bacterial exudates on sorption–desorption interactions of 90Sr with brucite. Environ. Sci. Process. Impacts 2018, 20, 956–964. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Wajda, Ł.; Tarko, T. The immobilization of Arthrospira platensis biomass in different matrices—A practical application for lead biosorption. J. Environ. Sci. Health 2013, 48, 509–517. [Google Scholar] [CrossRef]
- Heidari, F.; Riahi, H.; Aghamiri, M.R.; Shariatmadari, Z.; Zakeri, F. Isolation of an efficient biosorbent of radionuclides (226Ra, 238U): Green algae from high-background radiation areas in Iran. J. Appl. Phycol. 2017, 29, 2887–2898. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.-H.; Liu, J.-W.; Chen, M.-L.; Wang, J.-H. Cyanobacterium metallothionein decorated graphene oxide nanosheets for highly selective adsorption of ultra-trace cadmium. J. Mater. Chem. 2012, 22, 21909–21916. [Google Scholar] [CrossRef]
- Yang, T.; Ma, L.-Y.; Chen, M.-L.; Wang, J.-H. Metallothionein isoforms for selective biosorption and preconcentration of cadmium at ultra-trace levels. J. Anal. At. Spectrom. 2015, 30, 929–935. [Google Scholar] [CrossRef]
- Warjri, S.M.; Syiem, M.B. Analysis of Biosorption Parameters, Equilibrium Isotherms and Thermodynamic Studies of Chromium (VI) Uptake by a Nostoc sp. Isolated from a Coal Mining Site in Meghalaya, India. Mine Water Environ. 2018, 37, 713–723. [Google Scholar] [CrossRef]
- Singh, A.; Mehta, S.K.; Gaur, J.P. Removal of heavy metals from aqueous solution by common freshwater filamentous algae. World J. Microbiol. Biotechnol. 2007, 23, 1115–1120. [Google Scholar] [CrossRef]
- Prasad, B.B.; Banerjee, S.; Lakshmi, D. An AlgaSORB Column for the Quantitative Sorption of Arsenic (III) from Water Samples. Water Qual. Res. J. 2006, 41, 190–197. [Google Scholar] [CrossRef]
- Miranda, J.; Krishnakumar, G.; Gonsalves, R. Lead sorption by living biomass of Chroococcus multicoloratus and Oscillatoria trichoides: Kinetics and equilibrium studies. Ann. Microbiol. 2013, 63, 591–605. [Google Scholar] [CrossRef]
- Kiran, B.; Kaushik, A. Cyanobacterial biosorption of Cr(VI): Application of two parameter and Bohart Adams models for batch and column studies. Chem. Eng. J. 2008, 144, 391–399. [Google Scholar] [CrossRef]
- Heidari, F.; Riahi, H.; Aghamiri, M.R.; Zakeri, F.; Shariatmadari, Z.; Hauer, T. 226Ra, 238U and Cd adsorption kinetics and binding capacity of two cyanobacterial strains isolated from highly radioactive springs and optimal conditions for maximal removal effects in contaminated water. Int. J. Phytoremediat. 2018, 20, 369–377. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Duca, G.; Rudic, V.; Cepoi, L.; Chiriac, T.; Frontasyeva, M.V.; Pavlov, S.; Gundorina, S.F. Spirulina platensis as biosorbent of zinc in water. In Proceedings of the International Conference Dedicated to the 55th Anniversary from the Foundation of the Institute of Chemistry of the Academy of Sciences of Moldova, Chişinău, Republic Moldova, 28–30 May 2014; pp. 191–192. [Google Scholar]
- Foster, L.; Muhamadali, H.; Boothman, C.; Sigee, D.; Pittman, J.K.; Goodacre, R.; Lloyd, J.R. Radiation Tolerance of Pseudanabaena catenata, a Cyanobacterium Relevant to the First Generation Magnox Storage Pond. Front. Microbiol. 2020, 11, 515. [Google Scholar] [CrossRef]
- Bishop, W.M.; Rodgers, J.H. Responses of Lyngbya wollei to Exposures of Copper-Based Algaecides: The Critical Burden Concept. Arch. Environ. Contam. Toxicol. 2012, 62, 403–410. [Google Scholar] [CrossRef]
- Cain, A.; Vannela, R.; Woo, L.K. Cyanobacteria as a biosorbent for mercuric ion. Bioresour. Technol. 2008, 99, 6578–6586. [Google Scholar] [CrossRef] [PubMed]
- Carro, L.; Barriada, J.L.; Herrero, R.; De Vicente, M.S. Adsorptive behaviour of mercury on algal biomass: Competition with divalent cations and organic compounds. J. Hazard. Mater. 2011, 192, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.C.A.; De França, F.P. Cadmium Interaction with Microalgal Cells, Cyanobacterial Cells, and Seaweeds; Toxicology and Biotechnological Potential for Wastewater Treatment. Mar. Biotechnol. 2003, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A. Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008, 154, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Pandey, L.K.; Gaur, J. Evaluation of various isotherm models, and metal sorption potential of cyanobacterial mats in single and multi-metal systems. Colloids Surf. B Biointerfaces 2010, 81, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Alessi, D.; Owttrim, G.; Petrash, D.; Mloszewska, A.; LaLonde, S.; Martinez, R.; Zhou, Q.; Konhauser, K.O. Cell surface reactivity of Synechococcus sp. PCC 7002: Implications for metal sorption from seawater. Geochim. Cosmochim. Acta 2015, 169, 30–44. [Google Scholar] [CrossRef]
- Micheletti, E.; Colica, G.; Viti, C.; Tamagnini, P.; De Philippis, R. Selectivity in the heavy metal removal by exopolysaccharide-producing cyanobacteria. J. Appl. Microbiol. 2008, 105, 88–94. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, P.; Kumar, S.; Hashmi, A.A. Structural and antimicrobial studies of potassium hydrotris(2-mercaptobenzathiazolyl)borate and its organotin(IV) derivatives. J. Coord. Chem. 2008, 61, 2437–2448. [Google Scholar] [CrossRef]
- Kumar, S.; Habib, K.; Fatma, T. Endosulfan induced biochemical changes in nitrogen-fixing cyanobacteria. Sci. Total Environ. 2008, 403, 130–138. [Google Scholar] [CrossRef]
- Patel, A.; Tiwari, S.; Prasad, S.M. Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in Nostoc muscorum. Ecotoxicol. Environ. Saf. 2018, 157, 369–379. [Google Scholar] [CrossRef]
- Eshanmugam, V.; Lo, J.-C.; Wu, C.-L.; Wang, S.-L.; Lai, C.-C.; Connolly, E.L.; Huang, J.-L.; Yeh, K.-C. Differential expression and regulation of iron-regulated metal transporters in Arabidopsis halleri and Arabidopsis thaliana—The role in zinc tolerance. New Phytol. 2011, 190, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, S.; Singh, S. Hexavalent chromium toxicity to cyanobacterium Spirulina platensis. Int. Res. J. Pharm. 2014, 5, 910–914. [Google Scholar] [CrossRef]
- Mota, R.; Pereira, S.B.; Meazzini, M.; Fernandes, R.; Santos, A.; Evans, C.A.; De Philippis, R.; Wright, P.C.; Tamagnini, P. Differential proteomes of the cyanobacterium Cyanothece sp. CCY 0110 upon exposure to heavy metals. Data Brief 2015, 4, 152–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tottey, S.; Patterson, C.J.; Banci, L.; Bertini, I.; Felli, I.C.; Pavelkova, A.; Dainty, S.J.; Pernil, R.; Waldron, K.J.; Foster, A.W.; et al. Cyanobacterial metallochaperone inhibits deleterious side reactions of copper. Proc. Natl. Acad. Sci. USA 2012, 109, 95–100. [Google Scholar] [CrossRef]
- Choudhary, M.; Jetley, U.K.; Khan, M.A.; Zutshi, S.; Fatma, T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol. Environ. Saf. 2007, 66, 204–209. [Google Scholar] [CrossRef]
- Al-Mousawi, N.J.M. Growth response of Cyanobacterium: Chroococcus sp. To copper (Cu+ 2) and Nickel (Ni+ 2) ions, singly and in dual combination. Marsh Bull. 2010, 5, 141–152. [Google Scholar]
- Kaňa, R.; Prášil, O.; Mullineaux, C.W. Immobility of phycobilins in the thylakoid lumen of a cryptophyte suggests that protein diffusion in the lumen is very restricted. FEBS Lett. 2009, 583, 670–674. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Tiwari, S.; Patel, A.; Prasad, S.M. Kinetin alleviates chromium toxicity on growth and PS II photochemistry in Nostoc muscorum by regulating antioxidant system. Ecotoxicol. Environ. Saf. 2018, 161, 296–304. [Google Scholar] [CrossRef]
- Prasad, S.M.; Singh, J.B.; Rai, L.C.; Kumar, H.D. Metal-induced inhibition of photosynthetic electron transport chain of the cyanobacterium Nostoc muscorum. FEMS Microbiol. Lett. 1991, 82, 95–100. [Google Scholar] [CrossRef]
- Perreault, F.; Ali, N.A.; Saison, C.; Popovic, R.; Juneau, P. Dichromate effect on energy dissipation of photosystem II and photosystem I in Chlamydomonas reinhardtii. J. Photochem. Photobiol. B Biol. 2009, 96, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Ed.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Wang, S.; Zhang, D.; Pan, X. Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2012, 84, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, F.; Zhu, X.; Zhu, J.; Rong, J.; Zhan, J.; Chen, H.; He, C.; Wang, Q. The Acceptor Side of Photosystem II Is the Initial Target of Nitrite Stress in Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, O.; Allakhverdiev, S.I.; Higashi, S.; Watanabe, M.; Nishiyama, Y.; Murata, N. Very strong UV-A light temporally separates the photoinhibition of photosystem II into light-induced inactivation and repair. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cox, A. Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Front. Microbiol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.M.; Garbayo, I.; Domínguez, M.J.; Vigara, J. Effect of abiotic stress on photosynthesis and respiration in Chlamydomonas reinhardtii: Induction of oxidative stress. Enzym. Microb. Technol. 2006, 40, 163–167. [Google Scholar] [CrossRef]
- Ananya, A.K.; Ahmad, I.Z. Cyanobacteria “the blue green algae” and its novel applications: A brief review. Int. J. Innov. Appl. Stud. 2014, 7, 251. [Google Scholar]
- Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Technol. 2012, 3, 299–304. [Google Scholar]
- Turner, J.S.; Robinson, N.J. Cyanobacterial metallothioneins: Biochemistry and molecular genetics. J. Ind. Microbiol. Biotechnol. 1995, 14, 119–125. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Harrison, M.D.; Robinson, A.K.; Parkinson, J.A.; Bowness, P.W.; Sadler, P.J.; Robinson, N.J. Multiple bacteria encode metallothioneins and SmtA-like zinc fingers. Mol. Microbiol. 2002, 45, 1421–1432. [Google Scholar] [CrossRef]
- Ybarra, G.R.; Webb, R. Effects of divalent metal cations and resistance mechanisms of the cyanobacterium Synechococcus sp. strain PCC 7942. J. Hazard. Subst. Res. 1999, 2, 1. [Google Scholar] [CrossRef]
- El-Enany, A.; Issa, A. Cyanobacteria as a biosorbent of heavy metals in sewage water. Environ. Toxicol. Pharmacol. 2000, 8, 95–101. [Google Scholar] [CrossRef]
- Mallick, N.; Rai, L. Characterization of Cd-induced low molecular weight protein in a N-fixing cyanobacterium Anabaena doliolum with special reference to co-/multiple tolerance. BioMetals 1998, 11, 55–61. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; De Philippis, R. Exocellular Polysaccharides in Microalgae and Cyanobacteria: Chemical Features, Role and Enzymes and Genes Involved in Their Biosynthesis. In The Physiology of Microalgae; Springer: Cham, Switzerland, 2016; pp. 565–590. [Google Scholar]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Gupta, P.; Diwan, B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. 2017, 13, 58–71. [Google Scholar] [CrossRef]
- Kehr, J.-C.; Dittmann, E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Algal biotechnology products and processes—Matching science and economics. J. Appl. Phycol. 1992, 4, 267–279. [Google Scholar] [CrossRef]
- Strieth, D.; Schwing, J.; Kuhne, S.; Lakatos, M.; Muffler, K.; Ulber, R. A semi-continuous process based on an ePBR for the production of EPS using Trichocoleus sociatus. J. Biotechnol. 2017, 256, 6–12. [Google Scholar] [CrossRef]
- Wieland, A.; Zopfi, J.; Benthien, M.; Kühl, M. Biogeochemistry of an Iron-Rich Hypersaline Microbial Mat (Camargue, France). Microb. Ecol. 2005, 49, 34–49. [Google Scholar] [CrossRef]
- Bhunia, B.; Uday, U.S.P.; Oinam, G.; Mondal, A.; Bandyopadhyay, T.K.; Tiwari, O.N. Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal. Carbohydr. Polym. 2018, 179, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007; Volume 11. [Google Scholar]
- Yoshimura, H.; Kotake, T.; Aohara, T.; Tsumuraya, Y.; Ikeuchi, M.; Ohmori, M. The role of extracellular polysaccharides produced by the terrestrial cyanobacterium Nostoc sp. strain HK-01 in NaCl tolerance. J. Appl. Phycol. 2012, 24, 237–243. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ben Ouada, H.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, J.; Zhou, X.; Xia, L.; Hu, C. Effects of light intensity on components and topographical structures of extracellular polymeric substances fromMicrocoleus vaginatus(Cyanophyceae). Phycologia 2014, 53, 167–173. [Google Scholar] [CrossRef]
- Diengdoh, O.L.; Syiem, M.B.; Pakshirajan, K.; Rai, A.N. Zn2+ sequestration by Nostoc muscorum: Study of thermodynamics, equilibrium isotherms, and biosorption parameters for the metal. Environ. Monit. Assess. 2017, 189, 314. [Google Scholar] [CrossRef]
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S.B.; De Philippis, R.; Tamagnini, P. Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: Interactions between metals and RPS binding sites. Appl. Microbiol. Biotechnol. 2016, 100, 7765–7775. [Google Scholar] [CrossRef]
- Borah, S.N.; Sen, S.; Goswami, L.; Bora, A.; Pakshirajan, K.; Deka, S. Rice based distillers dried grains with solubles as a low cost substrate for the production of a novel rhamnolipid biosurfactant having anti-biofilm activity against Candida tropicalis. Colloids Surf. B Biointerfaces 2019, 182, 110358. [Google Scholar] [CrossRef]
- Sukumaran, N.P.; Gopi, S. Overview of biopolymers: Resources, demands, sustainability, and life cycle assessment modeling and simulation. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–19. [Google Scholar]
- Kumar, R.V.; Moorthy, I.G.; Goswami, L.; Pugazhenthi, G.; Pakshirajan, K.; Silva, A.M.T.; Morales-Torres, S. Analytical Methods in Biodiesel Production. In Biomass Valorization to Bioenergy; Springer: Singapore, 2019; pp. 197–219. [Google Scholar]
- Rizvi, S.; Goswami, L.; Gupta, S.K. A holistic approach for melanoidin removal via Fe-impregnated activated carbon prepared from Mangifera indica leaves biomass. Bioresour. Technol. Rep. 2020, 12, 100591. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, L.; Singh, A.K.; Sikandar, M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon 2019, 5, e02562. [Google Scholar] [CrossRef]
- Mu, D.; Min, M.; Krohn, B.; Mullins, K.A.; Ruan, R.; Hill, J. Life Cycle Environmental Impacts of Wastewater-Based Algal Biofuels. Environ. Sci. Technol. 2014, 48, 11696–11704. [Google Scholar] [CrossRef]
- Bussa, M.; Zollfrank, C.; Röder, H. Comparative Life Cycle Assessment Study on Cyanobacteria and Maize as Feedstock for Polylactic Acid. In Progress in Life Cycle Assessment 2018; Springer: Cham, Switzerland, 2019; pp. 117–127. [Google Scholar]
- Sander, K.; Murthy, G.S. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar] [CrossRef]
- Shirvani, T.; Yan, X.; Inderwildi, O.R.; Edwards, P.P.; King, D.A. Life cycle energy and greenhouse gas analysis for algae-derived biodiesel. Energy Environ. Sci. 2011, 4, 3773. [Google Scholar] [CrossRef]
- Bussa, M.; Zollfrank, C.; Röder, H. Life-cycle assessment and geospatial analysis of integrating microalgae cultivation into a regional economy. J. Clean. Prod. 2020, 243, 118630. [Google Scholar] [CrossRef]
- Quiroz-Arita, C.; Sheehan, J.J.; Bradley, T.H. Life cycle net energy and greenhouse gas emissions of photosynthetic cyanobacterial biorefineries: Challenges for industrial production of biofuels. Algal Res. 2017, 26, 445–452. [Google Scholar] [CrossRef]
- Lewandowska, A.U.; Śliwińska-Wilczewska, S.; Woźniczka, D. Identification of cyanobacteria and microalgae in aerosols of various sizes in the air over the Southern Baltic Sea. Mar. Pollut. Bull. 2017, 125, 30–38. [Google Scholar] [CrossRef]
- Thuy, H.T.T.; Loan, T.T.C.; Phuong, T.H. The potential accumulation of polycyclic aromatic hydrocarbons in phytoplankton and bivalves in Can Gio coastal wetland, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 17240–17249. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Lewandowska, A.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment—Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
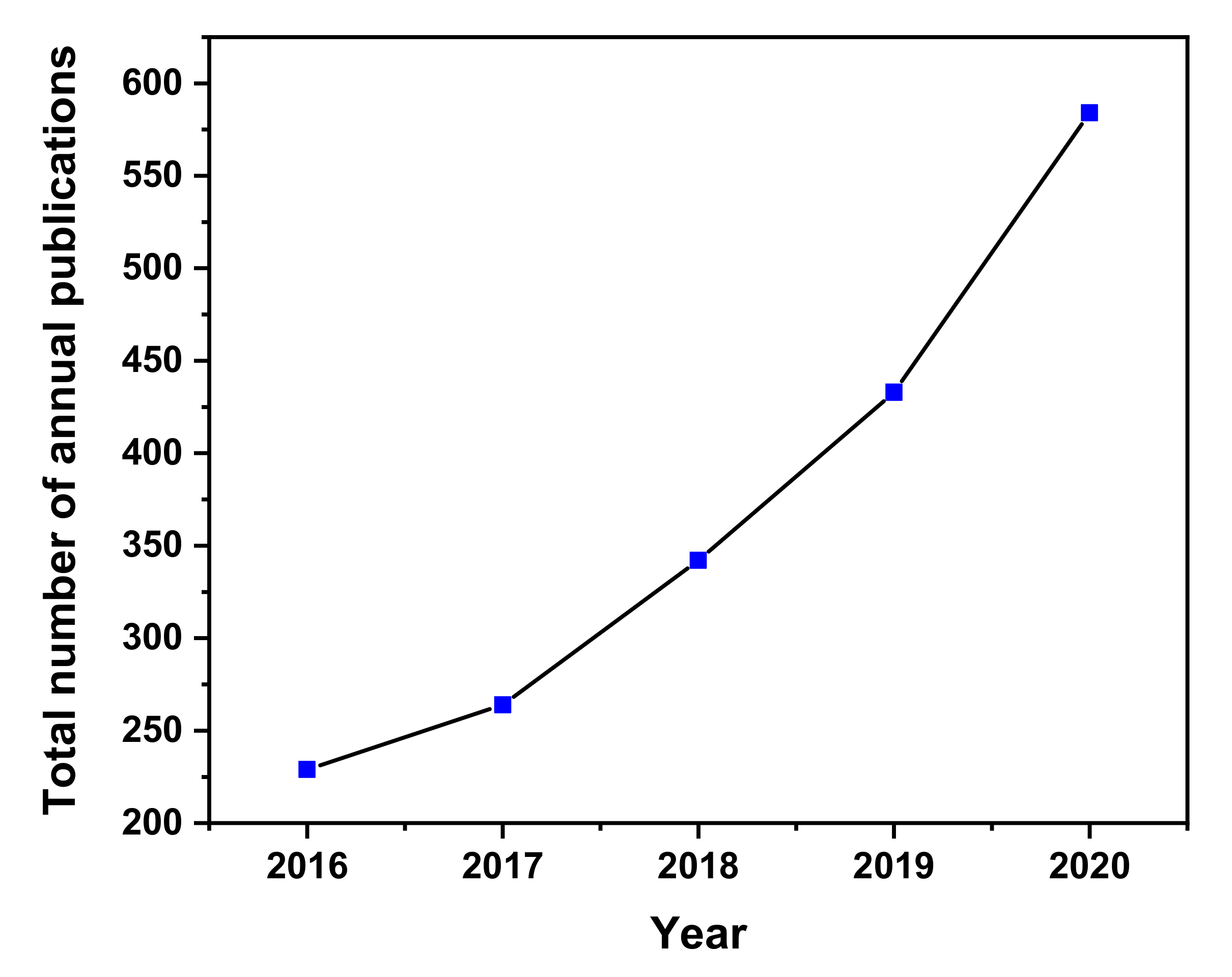

| S. No. | Cyanobacteria | Modified Support | Adsorbate | Aqueous Phase (pH) | References |
|---|---|---|---|---|---|
| 1. | Oscillatoria | Immobilized Ca-alginate beads | Cd | 6 | [33] |
| 2. | Nostoc minutum and Anabaena spiroides | - | Pb, Cd, Ni | - | [34] |
| 3. | Pseudanabaena catenata | - | Sr | 10.5–11.5 | [35] |
| 4. | Arthrospira platensis | Immobilization alginate, silica gel, or agarose | Pb | 4–5 | [36] |
| 5. | Graesiella emersonii | - | U, Ra | 4 | [37] |
| 6. | Cyanobacterium Metallothionein | Immobilized with graphene oxide and SiO2 | Cd | 8 and 6 | [38,39] |
| 7. | Nostoc sp. | - | Cr | 6 | [40] |
| 8. | Pithophora oedogonia and Spirogyra neglecta | - | Pb, Cu | 5 | [41] |
| 9. | Scytonema | Dimethylformamide slurry | As | 6.9 | [42] |
| 10. | Chroococcus multicoloratus and O. trichoides | - | Pb | 5 and 5.14 | [43] |
| 11. | Lyngbya putealis HH-15 | Sodium alginate, calcium chloride | Cr | 2 | [44] |
| 12. | Nostoc punctiforme A. S/S4 and Chroococcidiopsis thermalis S.M/S9 | - | U, Cd, Ra | 4, 2, and 7 | [45] |
| 13. | Spirulina platensis | - | Zn | - | [46] |
| 14. | Pseudanabaena catenate | - | Sr | 10.5, 11, or 11.5 | [47] |
| 15. | Lyngbya wollei | - | Cu | - | [48] |
| 16. | S. platensis and Aphanothece flocculosa | - | Hg | 6 | [49] |
| 17. | S. muticum | - | Hg | 5 | [50] |
| 18. | Tetraselmis chuii and Spirulina maxima | - | Cd | - | [51] |
| 19. | Arthrospira platensis | Immobilization in alginate, silica gel, or agarose | Pb | 4–5.5 | [36] |
| 20. | N. muscorum | - | Cr | 3 | [52] |
| 21. | Oscillatoria sp., Phormidium sp., Lyngbya sp., Aulosira sp., and Scytonema sp | - | Cu, Cd, Pb | 5 | [53] |
| 22. | Synechococcus sp. | - | Cd | 8 | [54] |
| 23. | Cyanothece | - | Cu, Cr, Ni | 5 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.P.S.; Dwivedi, V.; Kumar, S.; Kushwaha, A.; Goswami, L.; Reddy, B.S. Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review. J. Compos. Sci. 2021, 5, 1. https://doi.org/10.3390/jcs5010001
Yadav APS, Dwivedi V, Kumar S, Kushwaha A, Goswami L, Reddy BS. Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review. Journal of Composites Science. 2021; 5(1):1. https://doi.org/10.3390/jcs5010001
Chicago/Turabian StyleYadav, Ajit Pratap Singh, Vinay Dwivedi, Satyendra Kumar, Anamika Kushwaha, Lalit Goswami, and Bezawada Sridhar Reddy. 2021. "Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review" Journal of Composites Science 5, no. 1: 1. https://doi.org/10.3390/jcs5010001
APA StyleYadav, A. P. S., Dwivedi, V., Kumar, S., Kushwaha, A., Goswami, L., & Reddy, B. S. (2021). Cyanobacterial Extracellular Polymeric Substances for Heavy Metal Removal: A Mini Review. Journal of Composites Science, 5(1), 1. https://doi.org/10.3390/jcs5010001






