An Innovative Approach for Restoring the Mechanical Properties of Thermoplastic-Matrix Nanocomposite by the Use of Partially Polymerized Cyclic Butylene Terephthalate
Abstract
:1. Introduction
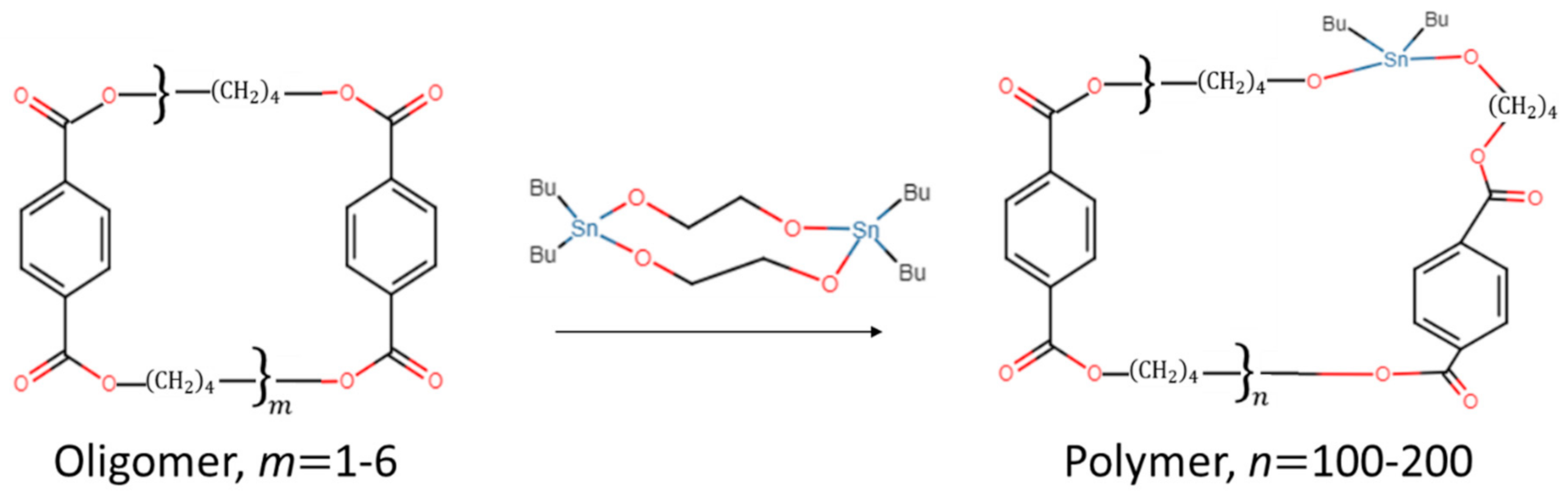
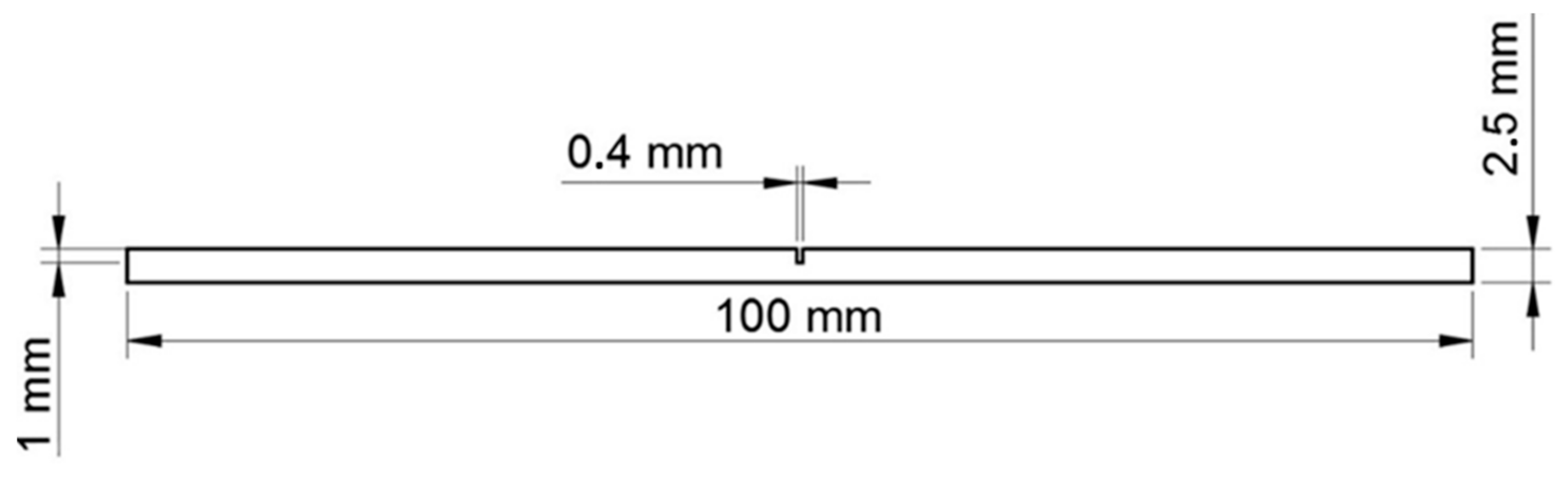
2. Materials and Methods
3. Results and Discussion
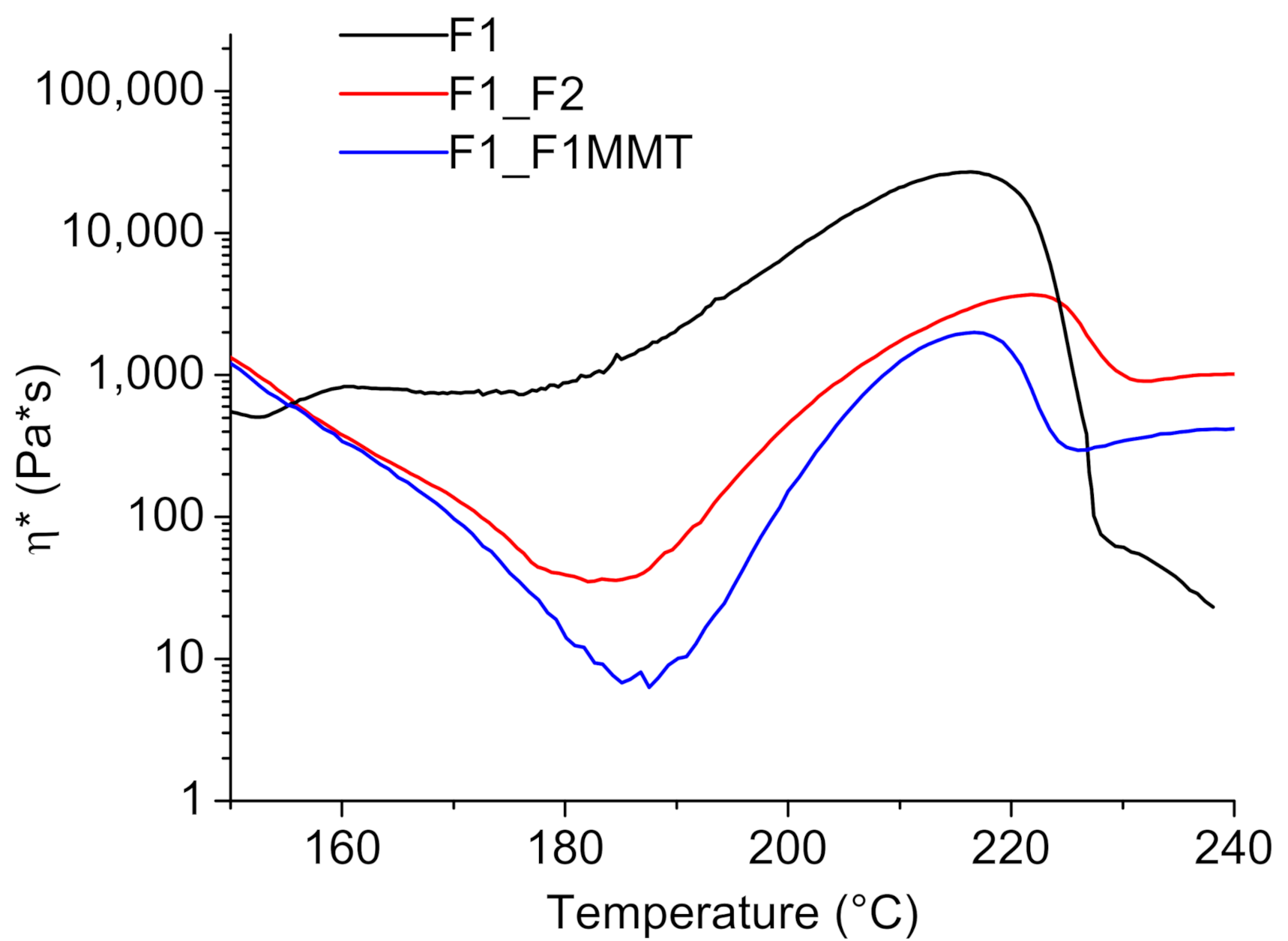
3.1. Viscosity Evolution
3.2. F1_F2 System
3.2.1. Mechanical Characterization of F1_F2 System
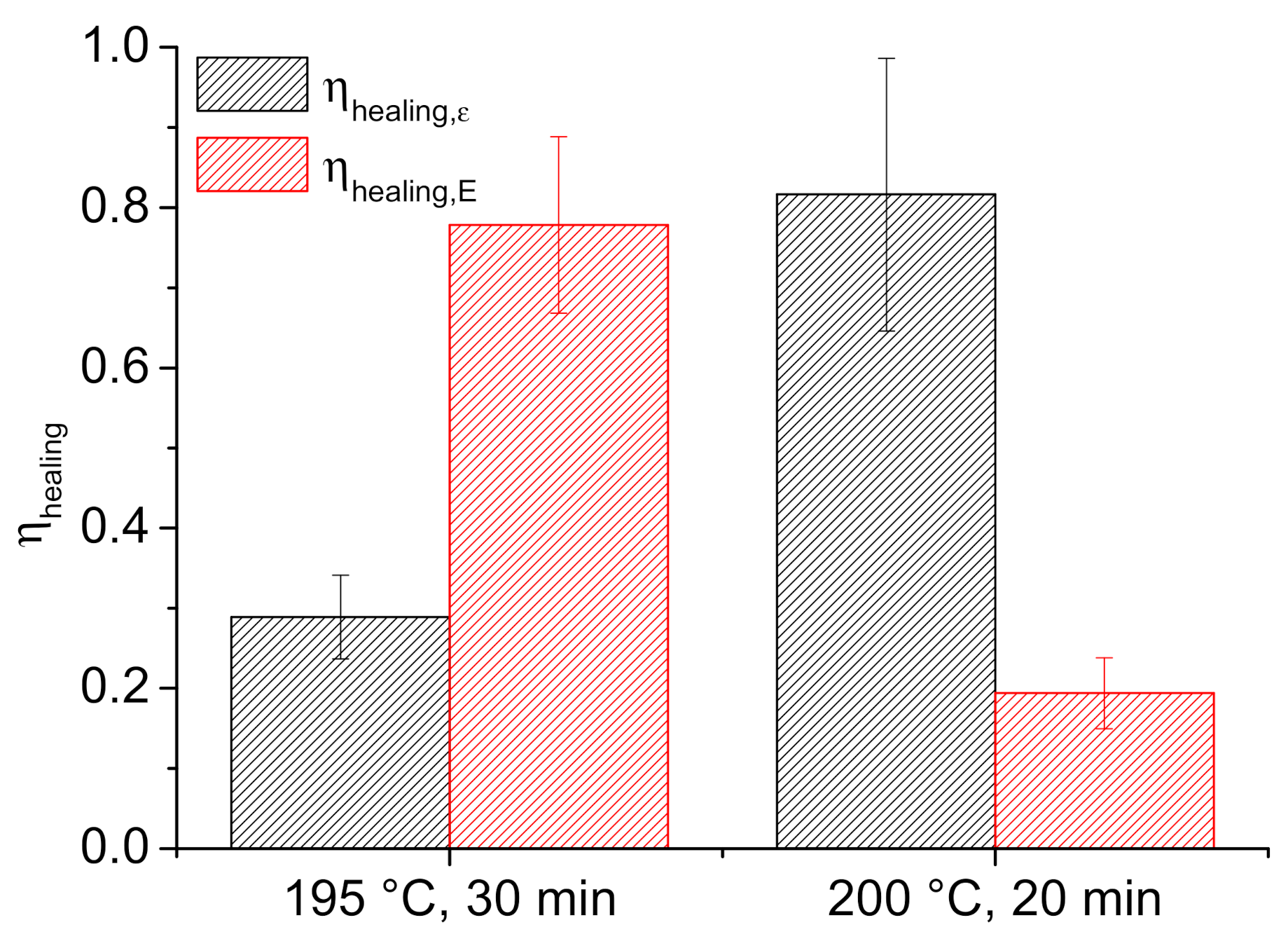
3.2.2. Healing Efficiency of F1_F2 System
3.3. F1_F1MMT System
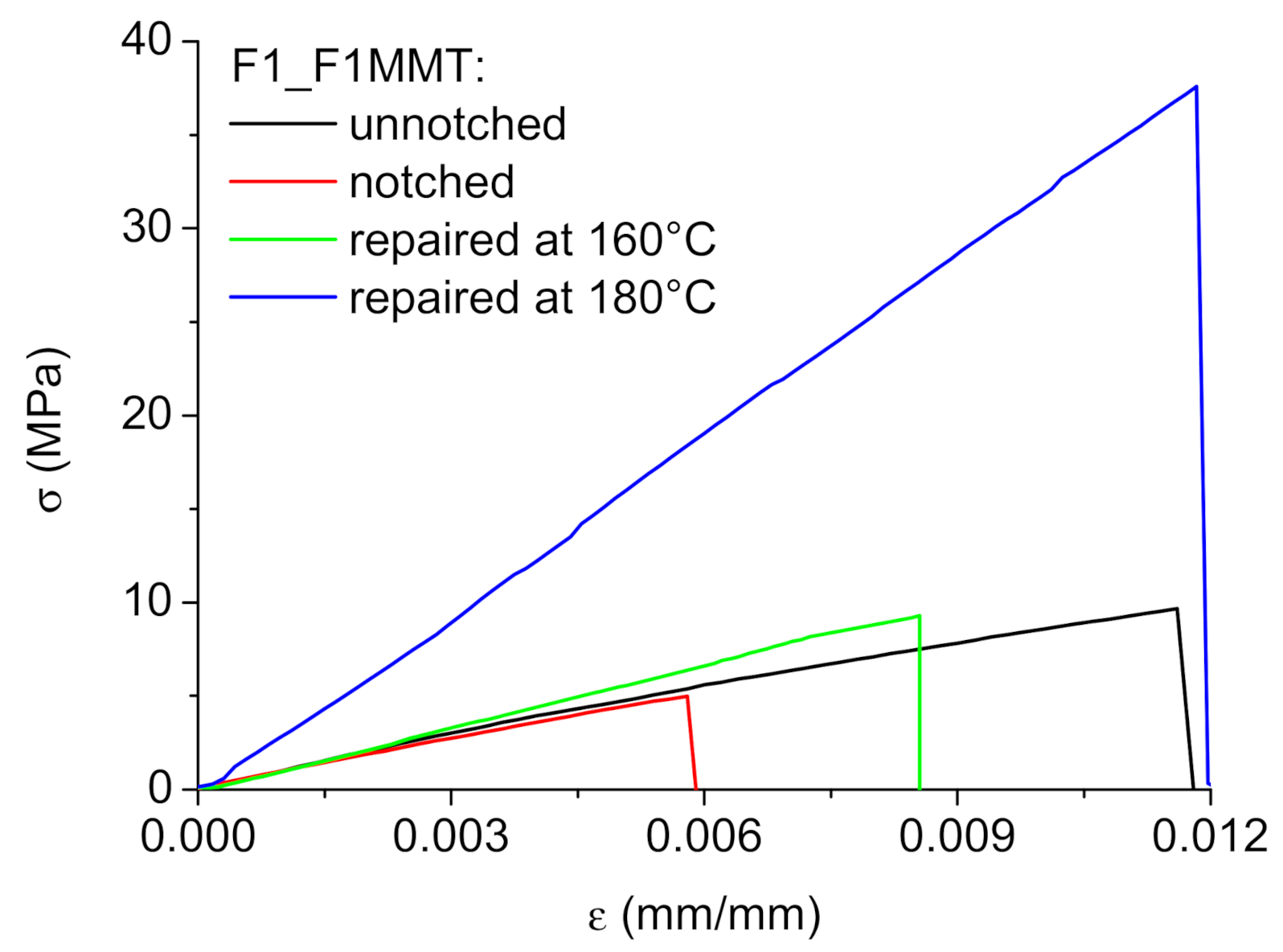
3.3.1. Mechanical Characterization of F1_F1MMT System
- The addition of the nanoclay, which is known to reduce the ductility of the materials;
- The higher amount of residual CBT for the nanofilled system compared to the two-catalyst system, as highlighted by DSC analysis reported in a previous work [13].
3.3.2. Healing Efficiency of F1_F1MMT System
4. Conclusions
- A healing cycle at lower temperatures and times (195 °C for 30 min for F1_F2 system and 160°C for 24 h for F1_MMT system) allows for partial recovery of the elongation to break, still retaining a stiffness comparable to the initial stiffness of the material. This cycle is desirable when the stiffness of the material is of utmost relevance, and any variation must be avoided;
- A healing cycle at higher temperature and times (200 °C for 20 min for F1_F2 system and 180°C for 24 h for F1_MMT system) allows a complete recovery of the elongation at break, despite a significant increase of the modulus. This cycle is desirable when the stiffness of the material is of secondary relevance, and variations of the modulus are acceptable.
Author Contributions
Funding
Conflicts of Interest
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, structures, and advanced applications of nanocomposites from biorenewable resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [PubMed]
- Ghobadi, A. Common Type of Damages in Composites and Their Inspections. World J. Mech. 2017, 7, 24–33. [Google Scholar]
- Wu, D.Y.; Meure, S.; Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 2008, 33, 479–522. [Google Scholar]
- Osswald, T.; Menges, G. (Eds.) Failure and damage of polymers. In Materials Science of Polymers for Engineers; Hanser Publishers: Munich, Germany, 2003; pp. 447–519. [Google Scholar]
- Duchene, P.; Chaki, S.; Ayadi, A.; Krawczak, P. A review of non-destructive techniques used for mechanical damage assessment in polymer composites. J. Mater. Sci. 2018, 53, 7915–7938. [Google Scholar]
- Naebe, M.; Abolhasani, M.M.; Khayyam, H.; Amini, A.; Fox, B. Crack Damage in Polymers and Composites: A Review. Polym. Rev. 2016, 56, 31–69. [Google Scholar]
- Jud, K.; Kausch, H.H.; Williams, J.G. Fracture-mechanics studies of crack healing and welding of polymers. J. Mater. Sci. 1981, 16, 204–210. [Google Scholar]
- Camara, L.A.; Wons, M.; Esteves, I.C.; Medeiros-Junior, R.A. Monitoring the Self-healing of Concrete from the Ultrasonic Pulse Velocity. J. Compos. Sci. 2019, 3, 16. [Google Scholar]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar]
- Almutairi, M.D.; Aria, A.I.; Thakur, V.K.; Khan, M.A. Self-Healing mechanisms for 3D-printed polymeric structures: From lab to reality. Polymers 2020, 12, 1534. [Google Scholar]
- Wool, R.P.; O’Connor, K.M. A theory of crack healing in polymers. J. Appl. Phys. 1981, 52, 5953–5963. [Google Scholar]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Dry, C.M.; Sottos, N.R. Passive smart self-repair in polymer matrix composite materials. In Proceedings of the Conference on Recent Advances in Adaptive and Sensory Materials and Their Applications, Blacksburg, VA, USA, 1–4 February 1993; pp. 438–444. [Google Scholar]
- Outwater, J.O.; Gerry, D.J. On the fracture energy, rehealing velocity and refracture energy of cast epoxy resin. J. Adhes. 1969, 1, 290–298. [Google Scholar] [CrossRef]
- Hegeman, A. Self-Repairing Polymers: Repair Mechanisms and Micromechanical Modelling. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 1997. [Google Scholar]
- Jud, K.; Kausch, H.H. Load transfer through chain molecules after interpenetration at interfaces. Polym. Bull. 1979, 1, 697–707. [Google Scholar] [CrossRef]
- Chung, C.; Roh, Y.; Cho, S.; Kim, J. Crack healing in polymeric materials via photochemical [2+2] cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Brunelle, D.J.; Bradt, J.E.; Serth-Guzzo, J.; Takekoshi, T.; Evans, T.L.; Pearce, E.J.; Wilson, P.R. Semicrystalline polymers via ring-opening polymerization: Preparation and polymerization of alkylene phthalate cyclic oligomers. Macromolecules 1998, 31, 4782–4790. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Liu, L.; Zhu, Y.; Xu, H.; Liu, D. Properties of polymerized cyclic butylene terephthalate and its composites via ring-opening polymerization. J. Thermoplast. Compos. Mater. 2018, 31, 181–201. [Google Scholar] [CrossRef]
- Mohd Ishak, Z.A.; Gatos, K.G.; Karger-Kocsis, J. On the in-situ polymerization of cyclic butylene terephthalate oligomers: DSC and rheological studies. Polym. Eng. Sci. 2006, 46, 743–750. [Google Scholar] [CrossRef]
- Brunelle, D.J. Cyclic Oligomer Chemistry; GE Global Research: Niskayuna, NY, USA, 2008; p. 12309. [Google Scholar]
- Ferrari, F.; Greco, A. Thermal analysis of self-healing thermoplastic-matrix nanocomposite from cyclic butylene terephthalate. J. Therm. Anal. Calorim. 2018, 134, 567–574. [Google Scholar] [CrossRef]
- Abt, T.; Sánchez-Soto, M.; Martínez De Ilarduya, A. Toughening of in situ polymerized cyclic butylene terephthalate by chain extension with a bifunctional epoxy resin. Eur. Polym. J. 2012, 48, 163–171. [Google Scholar] [CrossRef]
- Steeg, M. Prozesstechnologie für Cyclic Butylene Terephthalate im Faser-Kunststoff-Verbund. Ph.D. Thesis, Technische Universität Kaiserslautern, Kaiserslautern, Germany, 2009. [Google Scholar]
- Lanciano, G.; Greco, A.; Maffezzoli, A.; Mascia, L. Effects of thermal history in the ring opening polymerization of CBT and its mixtures with montmorillonite on the crystallization of the resulting poly(butylene terephthalate). Thermochim. Acta 2009, 493 (Suppl. 1–2), 61–67. [Google Scholar] [CrossRef]




| Sample | CBT | Elantech | Fascat 4101 | Fascat 4102 | Intercalated Fascat 4101 | MMT | Healing |
|---|---|---|---|---|---|---|---|
| F1 | 95.5 | 4 | 0.5 | 0 | 0 | 0 | - |
| F1_F2 | 95.5 | 4 | 0.25 | 0.25 | 0 | 0 | 195 °C, 30 min |
| 200 °C, 20 min | |||||||
| F1_F1MMT | 94.5 | 4 | 0.25 | 0 | 0.25 | 1 | 160 °C, 24 h |
| 180 °C, 24 h |
| F1_F2 System | σR (MPa) | εR (mm/mm)*10−2 | E (GPa) |
|---|---|---|---|
| Unnotched | 15.4 ± 6.63 | 1.97 ± 0.122 | 0.91 ± 0.39 |
| Notched | 8.59 ± 0.82 (−44%) | 1.59 ± 0.259 (−19%) | 0.84 ± 0.24 (−8%) |
| repaired, 195 °C 30 min | 12.5 ± 3.48 (−18%) | 1.70 ± 0.604 (−14%) | 0.93 ± 0.37 (+2%) |
| repaired, 200 °C 20 min | 21.4 ± 4.25 (+39%) | 1.902 ± 0.707 (−3%) | 1.20 ± 0.17 (+31%) |
| F1_F2 | ΔHm-CBT (J/g) | Tm-PBT (°C) | ΔHm-PBT (J/g) | xCBT |
|---|---|---|---|---|
| Before healing | 6.85 | 205.7 | 21.3 | 0.32 |
| Healing at 195 °C for 30 min | 1.89 | 212.8 | 44.6 | 0.04 |
| Healing at 200 °C for 20 min | 0 | 219.3 | 44.9 | 0 |
| F1_F1MMT System | σR (MPa) | εR (mm/mm)*10−2 | E (GPa) |
|---|---|---|---|
| Unnotched | 10.9 ± 1.96 | 1.55 ± 0.565 | 0.92 ± 0.27 |
| Notched | 5.59 ± 1.37 (−48%) | 0.667 ± 0.194 (−57%) | 0.87 ± 0.11 (−5%) |
| Repaired at 160 °C | 9.35 ± 2.11 (−14%) | 1.00 ± 0.435 (−35%) | 1.07 ± 0.20 (+16%) |
| Repaired at 180 °C | 34.9 ± 6.01 (+220%) | 1.36 ± 0.305 (−12%) | 2.86 ± 0.16 (+210%) |
| F1_F1MMT | ΔHm-CBT (J/g) | Tm-PBT (°C) | ΔHm-PBT (J/g) | xCBT |
|---|---|---|---|---|
| Before healing | 14.4 | 192.6 | 10.9 | 0.57 |
| Healing at 160 °C for 24 h | 8.94 | 196.0 | 27.7 | 0.32 |
| Healing at 180 °C for 24 h | 0 | 229.7 | 71.9 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.; Greco, A. An Innovative Approach for Restoring the Mechanical Properties of Thermoplastic-Matrix Nanocomposite by the Use of Partially Polymerized Cyclic Butylene Terephthalate. J. Compos. Sci. 2020, 4, 146. https://doi.org/10.3390/jcs4040146
Ferrari F, Greco A. An Innovative Approach for Restoring the Mechanical Properties of Thermoplastic-Matrix Nanocomposite by the Use of Partially Polymerized Cyclic Butylene Terephthalate. Journal of Composites Science. 2020; 4(4):146. https://doi.org/10.3390/jcs4040146
Chicago/Turabian StyleFerrari, Francesca, and Antonio Greco. 2020. "An Innovative Approach for Restoring the Mechanical Properties of Thermoplastic-Matrix Nanocomposite by the Use of Partially Polymerized Cyclic Butylene Terephthalate" Journal of Composites Science 4, no. 4: 146. https://doi.org/10.3390/jcs4040146
APA StyleFerrari, F., & Greco, A. (2020). An Innovative Approach for Restoring the Mechanical Properties of Thermoplastic-Matrix Nanocomposite by the Use of Partially Polymerized Cyclic Butylene Terephthalate. Journal of Composites Science, 4(4), 146. https://doi.org/10.3390/jcs4040146






