Microbial Adhesion on 3D-Printed Composite Polymers Used for Orthodontic Clear Aligners: A Systematic Review and Meta-Analysis of In Vitro Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Information Sources
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
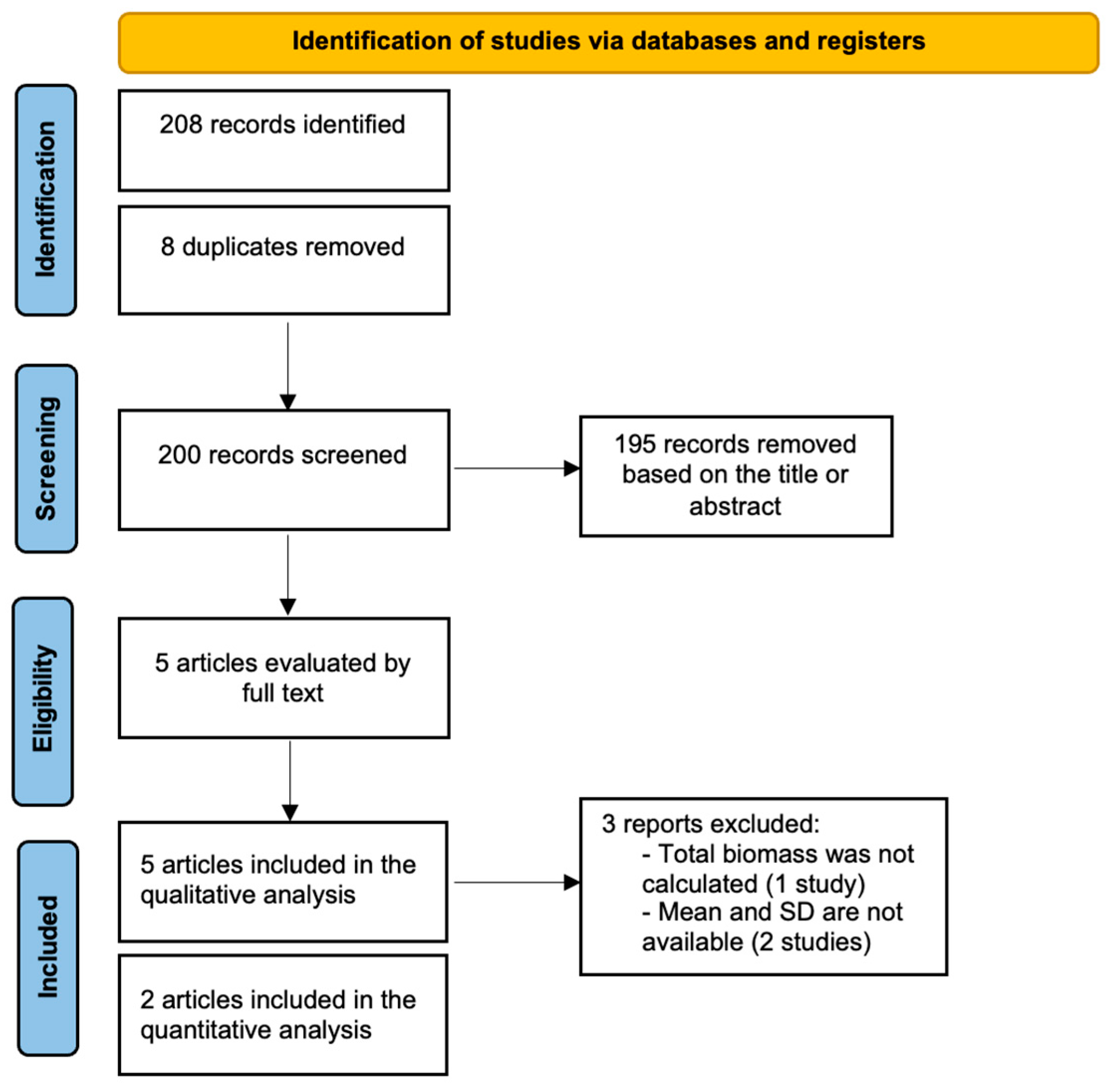
3.1. Search Strategy
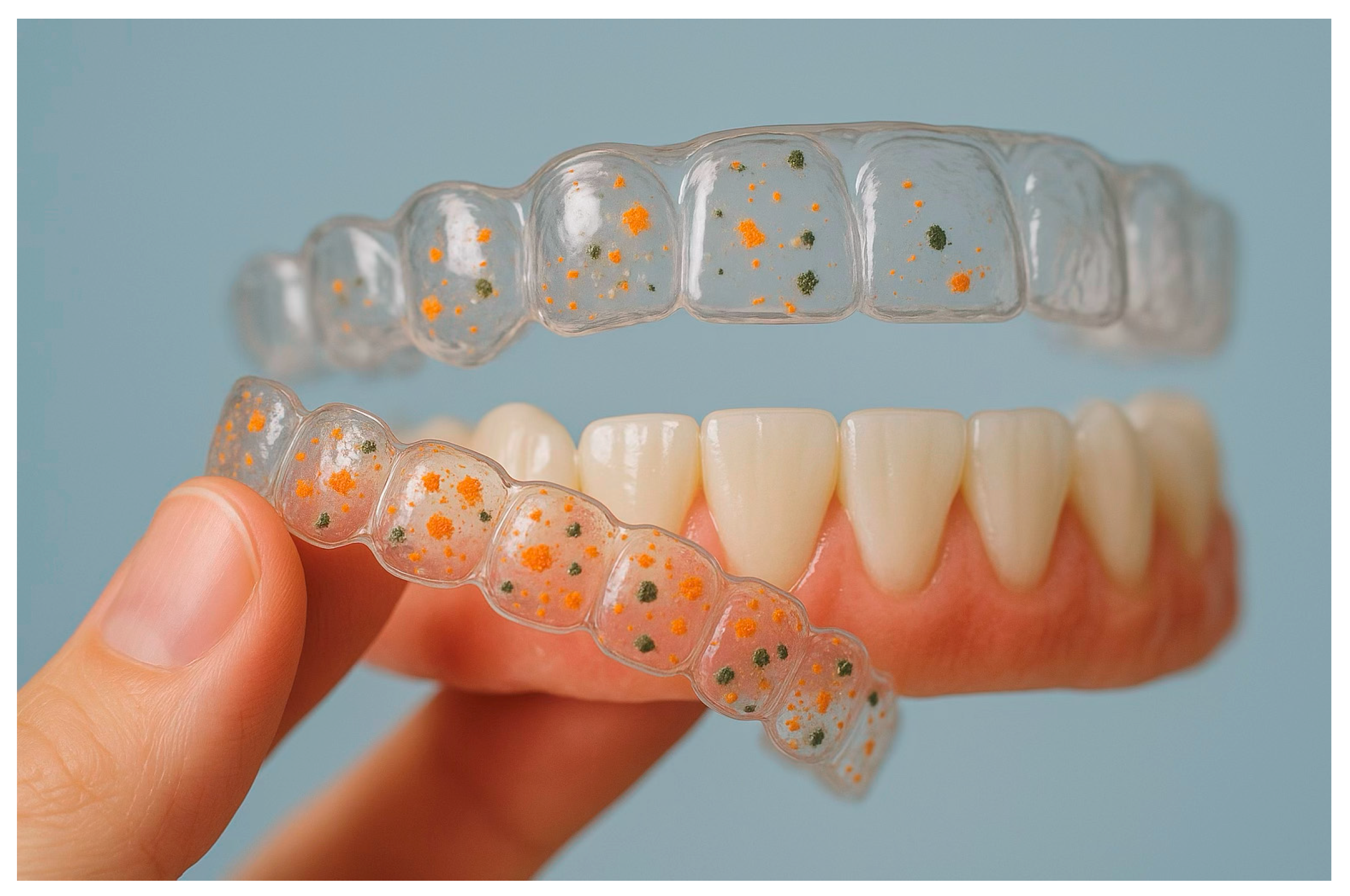
3.2. Main Findings
3.3. Meta-Analysis
3.4. Quality Assessment and Risk of Bias
3.5. Study Heterogeneity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rouzi, M.; Zhang, X.; Jiang, Q.; Long, H.; Lai, W.; Li, X. Impact of clear aligners on oral health and oral microbiome during orthodontic treatment. Int. Dent. J. 2023, 73, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Torkomian, T.; de la Iglesia, F.; Puigdollers, A. 3D-printed clear aligners: An emerging alternative to the conventional thermoformed aligners?—A systematic review. J. Dent. 2025, 155, 105616. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, L.; Čimić, S.; Meštrović, S. Three-Dimensionally Printed Splints in Dentistry: A Comprehensive Review. Dent. J. 2025, 13, 312. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, J.B.; Wang, B.; Zhang, X.; Yin, Y.L.; Bai, H. Alterations of the oral microbiome in patients treated with the Invisalign system or with fixed appliances. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 633–640. [Google Scholar] [CrossRef]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.N.; Xavier, G.M.; Cobourne, M.T.; Eliades, T. Effect of orthodontic treatment on the subgingival microbiota: A systematic review and meta-analysis. Orthod. Craniofacial Res. 2018, 21, 175–185. [Google Scholar] [CrossRef]
- Tektas, S.; Thurnheer, T.; Eliades, T.; Attin, T.; Karygianni, L. Initial bacterial adhesion and biofilm formation on aligner materials. Antibiotics 2020, 9, 908–912. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Yi, L.; Wu, X.; Zhao, Y.; Yu, J.; Liu, H.; Shen, Y.; Guo, J.; Huang, C. Dental plaque-inspired versatile nanosystem for caries prevention and tooth restoration. Bioact. Mater. 2023, 20, 418–433. [Google Scholar] [CrossRef]
- Ionescu, A.; Wutscher, E.; Brambilla, E.; Schneider-Feyrer, S.; Giessibl, F.J.; Hahnel, S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur. J. Oral Sci. 2012, 120, 458–465. [Google Scholar] [CrossRef]
- Park, J.W.; Song, C.W.; Jung, J.H.; Ahn, S.; Ferracane, J. The effects of surface roughness of composite resin on biofilm formation of Streptococcus mutans in the presence of saliva. Oper. Dent. 2012, 37, 532–539. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man: A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Macrì, M.; Murmura, G.; Varvara, G.; Traini, T.; Festa, F. Clinical performances and biological features of clear aligner materials in orthodontics. Front. Mater. 2022, 9, 819121. [Google Scholar] [CrossRef]
- Gold, B.P.; Siva, S.; Duraisamy, S.; Idaayath, A.; Kannan, R. Properties of orthodontic clear aligner materials—A review. J. Evol. Med. Dent. Sci. 2021, 10, 3288–3294. [Google Scholar] [CrossRef]
- Nasef, A.A.; El-Beialy, A.R.; Mostafa, Y.A. Virtual techniques for designing and fabricating a retainer. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 394–398. [Google Scholar] [CrossRef]
- Jindal, P.; Worcester, F.; Siena, F.L.; Forbes, C.; Juneja, M.; Breedon, P. Mechanical behaviour of 3D printed vs thermoformed clear dental aligner materials under non-linear compressive loading using FEM. J. Mech. Behav. Biomed. Mater. 2020, 112, 104045. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L. Evaluation of the mechanical properties and degree of conversion of 3D printed splint material. J. Mech. Behav. Biomed. Mater. 2021, 115, 104254. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Westphal, D.; Stawarczyk, B.; Edelhoff, D.; Kollmuss, M. Surface properties and initial bacterial biofilm growth on 3D-printed oral appliances: A comparative in vitro study. Clin. Oral Investig. 2023, 27, 2667–2677. [Google Scholar] [CrossRef]
- Schubert, A.; Bürgers, R.; Baum, F.; Kurbad, O.; Wassmann, T. Influence of the manufacturing method on the adhesion of Candida albicans and Streptococcus mutans to oral splint resins. Polymers 2021, 13, 1534. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Delgado, A.H.; Sauro, S.; Lima, A.F.; Loguercio, A.D.; Della Bona, A.; Mazzoni, A.; Collares, F.M.; Staxrud, F.; Ferracane, J.; Tsoi, J.; et al. RoBDEMAT: A risk of bias tool and guideline to support reporting of pre-clinical dental materials research and assessment of systematic reviews. J. Dent. 2022, 127, 104350. [Google Scholar] [CrossRef]
- Taher, B.B.; Rasheed, T.A. The impact of adding chitosan nanoparticles on biofilm formation, cytotoxicity, and certain physical and mechanical aspects of directly printed orthodontic clear aligners. Nanomaterials 2023, 13, 2649. [Google Scholar] [CrossRef]
- Moradinezhad, M.; Abbasi Montazeri, E.; Hashemi Ashtiani, A.; Pourlotfi, R.; Rakhshan, V. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: An in vitro study. BMC Oral Health 2024, 24, 1107. [Google Scholar] [CrossRef]
- Bozkurt, A.P.; Demirci, M.; Erdogan, P.; Kayalar, E. Comparison of microbial adhesion and biofilm formation on different orthodontic aligners. Am. J. Orthod. Dentofac. Orthop. 2025, 167, 47–62. [Google Scholar] [CrossRef]
- Faltermeier, A.; Bürgers, R.; Rosentritt, M. Bacterial Adhesion of Streptococcus mutans to Esthetic Bracket Materials. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S99–S103. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Hahnel, S.; König, A.; Brambilla, E. Resin Composite Blocks for Dental CAD/CAM Applications Reduce Biofilm Formation in Vitro. Dent. Mater. 2020, 36, 603–616. [Google Scholar] [CrossRef]
- Ozel, G.S.; Guneser, M.B.; Inan, O.; Eldeniz, A.U. Evaluation of C. albicans and S. mutans Adherence on Different Provisional Crown Materials. J. Adv. Prosthodont. 2017, 9, 335–340. [Google Scholar] [CrossRef]
- Esberg, A.; Sheng, N.; Mårell, L.; Claesson, R.; Persson, K.; Borén, T.; Strömberg, N. Streptococcus mutans Adhesin Biotypes That Match and Predict Individual Caries Development. eBioMedicine 2017, 24, 205–215. [Google Scholar] [CrossRef]
- Brady, L.J.; Maddocks, S.E.; Larson, M.R.; Forsgren, N.; Persson, K.; Deivanayagam, C.C.; Jenkinson, H.F. The Changing Faces of Streptococcus Antigen I/II Polypeptide Family Adhesins. Mol. Microbiol. 2010, 77, 276–286. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Sundstrom, P. Adhesins in Candida albicans. Curr. Opin. Microbiol. 1999, 2, 353–357. [Google Scholar] [CrossRef]
- Li, S.-X.; Wu, H.-T.; Liu, Y.-T.; Jiang, Y.-Y.; Zhang, Y.-S.; Liu, W.-D.; Zhu, K.-J.; Li, D.-M.; Zhang, H. The F1Fo-ATP Synthase β Subunit Is Required for Candida albicans Pathogenicity Due to Its Role in Carbon Flexibility. Front. Microbiol. 2018, 9, 1025. [Google Scholar] [CrossRef]
- Zhao, Y.; Lyu, Y.; Zhang, Y.; Li, S.; Zhang, Y.; Liu, Y.; Tang, C.; Zhang, Z.; Li, D.; Zhang, H. The Fungal-Specific Subunit i/j of F1FO-ATP Synthase Stimulates the Pathogenicity of Candida albicans Independent of Oxidative Phosphorylation. Med. Mycol. 2020, 59, 639–652. [Google Scholar] [CrossRef]
- Albert, L.S.; Brown, D.G. Variation in Bacterial ATP Concentration during Rapid Changes in Extracellular PH and Implications for the Activity of Attached Bacteria. Colloids Surf. B Biointerfaces 2015, 132, 111–116. [Google Scholar] [CrossRef]
- Listgarten, M.A. The role of dental plaque in gingivitis and periodontitis. J. Clin. Periodontol. 1988, 15, 485–487. [Google Scholar] [CrossRef]
- Waerhaug, J. Effect of rough surfaces upon gingival tissue. J. Dent. Res. 1956, 35, 323–325. [Google Scholar] [CrossRef]
- Tjan, A.H.; Chan, C.A. The polishability of posterior composites. J. Prosthet. Dent. 1989, 61, 138–146. [Google Scholar] [CrossRef]
- Mayer, J.; Reymus, M.; Wiedenmann, F.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Temporary 3D printed fixed dental prosthesis materials: Impact of post printing cleaning methods on degree of conversion as well as surface and mechanical properties. Int. J. Prosthodont. 2021, 34, 784–795. [Google Scholar] [CrossRef]
- Reymus, M.; Stawarczyk, B. In vitro study on the influence of postpolymerization and aging on the Martens parameters of 3D-printed occlusal devices. J. Prosthet. Dent. 2021, 125, 817–823. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.J.; Hudson, A.O.; Parthasarathy, A. The Quest for Novel Antimicrobial Compounds: Emerging Trends in Research, Development, and Technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Husseinsyah, S.; Amri, F.; Husin, K.; Ismail, H. Mechanical and Thermal Properties of Chitosan-Filled Polypropylene Composites: The Effect of Acrylic Acid. J. Vinyl. Addit. Technol. 2011, 17, 125–131. [Google Scholar] [CrossRef]
- Woźniak, A.; Biernat, M. Methods for Crosslinking and Stabilization of Chitosan Structures for Potential Medical Applications. J. Bioact. Compat. Polym. 2022, 37, 151–167. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in orthodontic clear aligner materials. Bioact. Mater. 2023, 22, 384–403. [Google Scholar] [CrossRef]
- Yazdi, M.; Daryanavard, H.; Ashtiani, A.H.; Moradinejad, M.; Rakhshan, V. A systematic review of biocompatibility and safety of orthodontic clear aligners and transparent vacuum-formed thermoplastic retainers: Bisphenol—A release, adverse effects, cytotoxicity, and estrogenic effects. Dent. Res. J. 2023, 20, 41. [Google Scholar] [CrossRef]
- Hamdoon, S.M.; AlSamak, S.; Ahmed, M.K.; Gasgoos, S. Evaluation of biofilm formation on different clear orthodontic retainer materials. J. Orthod. Sci. 2022, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Dos Reis, A.C. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Kreve, S.; Dos Reis, A.C. Effect of surface properties of ceramic materials on bacterial adhesion: A systematic review. J. Esthet. Restor. Dent. 2022, 34, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Suter, F.; Zinelis, S.; Patcas, R.; Schätzle, M.; Eliades, G.; Eliades, T. Roughness and wettability of aligner materials. J. Orthod. 2020, 47, 223–231. [Google Scholar] [CrossRef]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in roughness and mechanical properties of Invisalign® appliances after one- and two-weeks use. Materials 2019, 12, 2406. [Google Scholar] [CrossRef]
- Saldarriaga Fernández, I.C.; Busscher, H.J.; Metzger, S.W.; Grainger, D.W.; van der Mei, H.C. Competitive time- and density-dependent adhesion of staphylococci and osteoblasts on crosslinked poly(ethylene glycol)-based polymer coatings in co-culture flow chambers. Biomaterials 2011, 32, 979–984. [Google Scholar] [CrossRef]
- Dittmer, M.P.; Hellemann, C.F.; Grade, S.; Heuer, W.; Stiesch, M.; Schwestka-Polly, R.; Demling, A.P. Comparative three-dimensional analysis of initial biofilm formation on three orthodontic bracket materials. Head Face Med. 2015, 11, 10. [Google Scholar] [CrossRef]
- Velliyagounder, K.; Ardeshna, A.; Koo, J.; Rhee, M.; Fine, D.H. The microflora diversity and profiles in dental plaque biofilms on brackets and tooth surfaces of orthodontic patients. J. Indian Orthod. Soc. 2019, 53, 183–188. [Google Scholar] [CrossRef]
- Bawazir, M.; Dhall, A.; Lee, J.; Kim, B.; Hwang, G. Effect of surface stiffness in initial adhesion of oral microorganisms under various environmental conditions. Colloids Surf. B 2023, 221, 112952. [Google Scholar] [CrossRef]
- Ozer, N.E.; Sahin, Z.; Yikici, C.; Duyan, S.; Kilicarslan, M.A. Bacterial adhesion to composite resins produced by additive and subtractive manufacturing. Odontology 2024, 112, 460–471. [Google Scholar] [CrossRef]
- Baybekov, O.; Stanishevskiy, Y.; Sachivkina, N.; Bobunova, A.; Zhabo, N.; Avdonina, M. Isolation of clinical microbial isolates during orthodontic aligner therapy and their ability to form biofilm. Dent. J. 2023, 11, 13. [Google Scholar] [CrossRef]
- Thurnheer, T.; Bostanci, N.; Belibasakis, G.N. Microbial dynamics during conversion from supragingival to subgingival biofilms in an in vitro model. Mol. Oral Microbiol. 2016, 31, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Eliades, T.; Eliades, G.; Brantley, W.A. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, J.; Liu, J.; Yang, R.; Lu, X.; He, X.; Shi, W.; Guo, L. Interspecies interactions between Streptococcus mutans and Streptococcus agalactiae in vitro. Front. Cell. Infect. Microbiol. 2020, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Lee, S.J.; Lim, B.S.; Ahn, S.J. Surface characteristics of orthodontic materials and their effects on adhesion of mutans streptococci. Angle Orthod. 2009, 79, 353–360. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lim, B.S.; Lee, S.J. Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 489–495. [Google Scholar] [CrossRef]
- Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Flores-Estrada, J.; Uematsu, S.; Yamaguchi, R. Quantitative analysis of S. mutans and S. sobrinus cultivated independently and adhered to polished orthodontic composite resins. J. Appl. Oral Sci. 2012, 20, 544–549. [Google Scholar] [CrossRef]
- Ihssen, B.A.; Willmann, J.H.; Nimer, A.; Drescher, D. Effect of in vitro aging by water immersion and thermocycling on the mechanical properties of PETG aligner material. J. Orofac. Orthop. 2019, 80, 292–303. [Google Scholar] [CrossRef]
- Eslami, S.; Kopp, S.; Goteni, M.; Dahmer, I.; Sayahpour, B. Alterations in the surface roughness and porosity parameters of 3D-printed aligners after intraoral service. Am. J. Orthod. Dentofac. Orthop. 2024, 165, 73–79. [Google Scholar] [CrossRef]
- Šimunović, L.; Pečanić, P.; Marić, A.J.; Haramina, T.; Rakić, I.Š.; Meštrović, S. Impact of various cleaning protocols on the physical and aesthetic properties of 3D-printed orthodontic aligners. Sci. Rep. 2025, 15, 19022. [Google Scholar] [CrossRef] [PubMed]


| Search | Terms |
|---|---|
| 1 (Clear Aligners and 3D Printing) | 3D printed aligners OR clear aligners OR orthodontic aligners OR thermoplastic aligners OR aligner materials OR additive manufacturing OR 3D printing OR rapid prototyping |
| 2 (Microbial Adhesion and Biofilm) | microbial adhesion OR bacterial adhesion OR biofilm formation OR bacterial colonization OR microbial accumulation OR plaque formation |
| 3 (Oral Microorganisms) | Streptococcus mutans OR Lactobacillus OR Candida OR oral microbiota OR oral biofilm OR cariogenic bacteria OR periodontal pathogens |
| 4 | 1 AND 2 AND 3 |
| Author and Year | 3D Printed Material Aligner Tested | Thermoformed Material Aligner Tested | Microorganisms’ Strains Evaluated | Microbiological Assay | Main Results |
|---|---|---|---|---|---|
| Schubert et al., 2021 [21]. | Med610 (PolyJet), V-Print Splint & Freeprint ortho 385 (DLP), Dental LT Clear (SLA) | Erkodur (thermoformed) and pressed acrylic resin | Candida albicans, Streptococcus mutans | ATP-based luminescence assay after 2 h incubation | 3D-printed and milled resins showed significantly higher Candida albicans adhesion than thermoformed and pressed resins. Streptococcus mutans adhesion was not significantly affected by manufacturing method. Surface roughness and surface free energy had no significant correlation with microbial adhesion. |
| Wuersching et al., 2022 [20]. | SHERAprint-Ortho Plus UV, NextDent Ortho Rigid, LuxaPrint Ortho Plus, V-Print Splint, and KeySplint Soft | Erkodur Thermoforming Foil (Erkodent, Pfalzgrafenweiler, Baden-Württemberg, Germany) | Actinomyces naeslundii, Streptococcus gordonii, Streptococcus mutans, Streptococcus oralis, and Streptococcus sanguinis | Multi-species biofilm formation assay for 72 h; biofilm quantified using crystal violet staining and CFU counts. | 3D-printed materials showed smoother surfaces and lower bacterial adhesion than thermoformed and PMMA materials. KeySplint Soft and PMMA exhibited the highest surface roughness and bacterial accumulation. A positive correlation (r = 0.69, p = 0.04) was found between surface roughness and bacterial adhesion. |
| Taher & Rasheed, 2023 [24]. | Dental LT Clear Resin V2 (Formlabs, Somerville, MA, USA) incorporated with 2%, 3%, and 5% (w/w) chitosan nanoparticles | Zendura (Bay Materials, Fremont, CA, USA) thermoformed using Biostar (Scheu-Dental, Iserlohn, Germany) | Streptococcus mutans (ATCC-25175) | Direct cell culture antibiofilm assay; CFU/mL counted after 24 h incubation at 37 °C under anaerobic conditions | Incorporation of 3% and 5% chitosan nanoparticles significantly reduced Streptococcus mutans biofilm formation compared to unmodified resin and thermoformed control. Mechanical and biological properties remained within acceptable limits. |
| Moradinezhad, 2024 [25]. | dx Ortho (DETAX Freeprint 3D, Ettlingen, Germany) | Erkodural (Erkodent, Pfalzgrafenweiler, Baden-Württemberg, Germany); EasyVac (polyethylene); DB (polyester based on terephthalic acid); Clear Tech | Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida albicans | Microtiter plate assay during 24, 72, and 120 h. | There were not significant differences among the clear retained materials tested. |
| Bozkurt et al., 2025 [26]. | Graphy (Tera Harz TC-85, Graphy Inc., Seoul, Republic of Korea) | Invisalign (SmartTrack, Align Technology, San José, CA, USA); Clarity (3M ESPE, St. Paul, MN, USA); ClearCorrect (ClearQuartz, Straumann AG, Basel, Switzerland); Smartee (Erkodur foil, Erkodent, Pfalzgrafenweiler, Baden-Württemberg, Germany); Orthero (ABA 3-layer copolyester/elastomer, Orthero, Istanbul, Turkey) | Streptococcus mutans (ATCC 25175) and Lactobacillus acidophilus (ATCC 4356) | Crystal violet microtiter plate biofilm assay measured spectrophotometrically at 540 nm after 0, 24, 48, 72, 96, 120, 168, and 240 h incubation at 37 °C | Time-dependent biofilm accumulation was observed across all aligners. Streptococcus mutans biofilm was significantly higher on ClearCorrect at 120–168 h and on Graphy at 168 h compared with Smartee (p < 0.05). Mixed Streptococcus mutans + Lactobacillus acidophilus biofilms formed more on Graphy than Invisalign at 120 and 168 h (p < 0.05). All materials showed increased biofilm over time, with Graphy and ClearCorrect exhibiting greater bacterial adhesion potential. |
| Study | D1. Bias in Planning and Allocation | D2. Bias in Sample/Specimen Preparation | D3. Bias in Outcome Assessment | D4. Bias in Data Treatment and Outcome Reporting | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 1.3 | 2.1 | 2.2 | 3.1 | 3.2 | 4.1 | 4.2 | |
| Schubert et al., 2021 [21] | R | R | NR | R | R | R | NR | R | R |
| Wuersching et al., 2022 [20] | R | R | NR | R | R | R | NR | R | R |
| Taher & Rasheed, 2023 [24] | R | IR | NR | R | R | R | NR | R | R |
| Moradinezhad, 2024 [25] | R | R | R | R | R | R | R | R | R |
| Bozkurt et al., 2025 [26] | R | NR | NR | R | R | R | R | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hazko, S.; Holiel, A.A.; Bourgi, R.; Cuevas-Suárez, C.E.; Kmeid, R.; Hardan, L.; Osman, A.; Flores-Ledesma, A.; Kharouf, N.; Nassar, N. Microbial Adhesion on 3D-Printed Composite Polymers Used for Orthodontic Clear Aligners: A Systematic Review and Meta-Analysis of In Vitro Evidence. J. Compos. Sci. 2026, 10, 26. https://doi.org/10.3390/jcs10010026
Hazko S, Holiel AA, Bourgi R, Cuevas-Suárez CE, Kmeid R, Hardan L, Osman A, Flores-Ledesma A, Kharouf N, Nassar N. Microbial Adhesion on 3D-Printed Composite Polymers Used for Orthodontic Clear Aligners: A Systematic Review and Meta-Analysis of In Vitro Evidence. Journal of Composites Science. 2026; 10(1):26. https://doi.org/10.3390/jcs10010026
Chicago/Turabian StyleHazko, Sandy, Ahmed A. Holiel, Rim Bourgi, Carlos Enrique Cuevas-Suárez, Roland Kmeid, Louis Hardan, Aly Osman, Abigailt Flores-Ledesma, Naji Kharouf, and Nicolas Nassar. 2026. "Microbial Adhesion on 3D-Printed Composite Polymers Used for Orthodontic Clear Aligners: A Systematic Review and Meta-Analysis of In Vitro Evidence" Journal of Composites Science 10, no. 1: 26. https://doi.org/10.3390/jcs10010026
APA StyleHazko, S., Holiel, A. A., Bourgi, R., Cuevas-Suárez, C. E., Kmeid, R., Hardan, L., Osman, A., Flores-Ledesma, A., Kharouf, N., & Nassar, N. (2026). Microbial Adhesion on 3D-Printed Composite Polymers Used for Orthodontic Clear Aligners: A Systematic Review and Meta-Analysis of In Vitro Evidence. Journal of Composites Science, 10(1), 26. https://doi.org/10.3390/jcs10010026












