Study on the Layered Structure of Ceramic-Side Bonding Area and the Mechanical Property of Al2O3–Kovar Brazed Joint with Ag-Cu-Ti Filler
Abstract
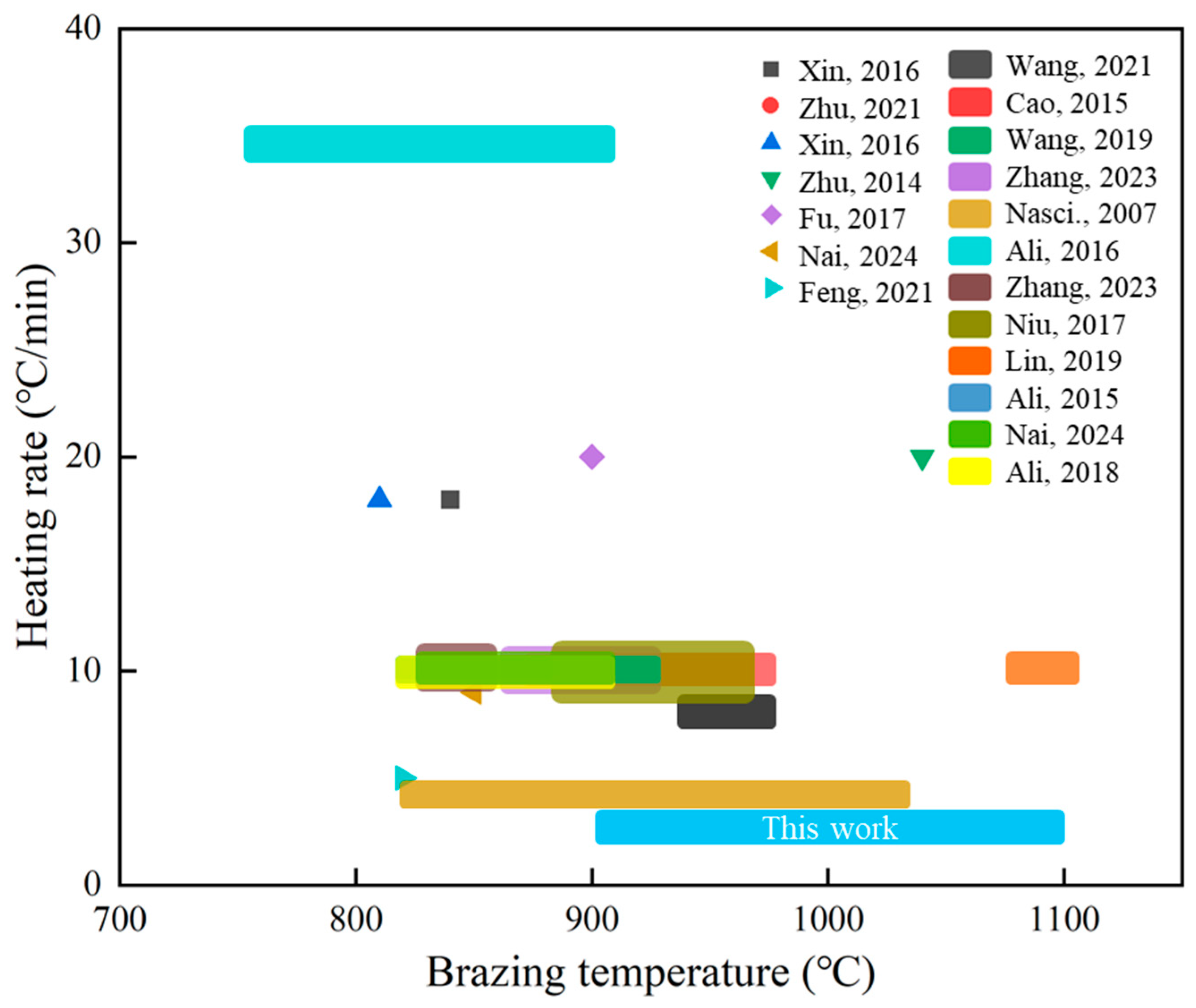
1. Introduction
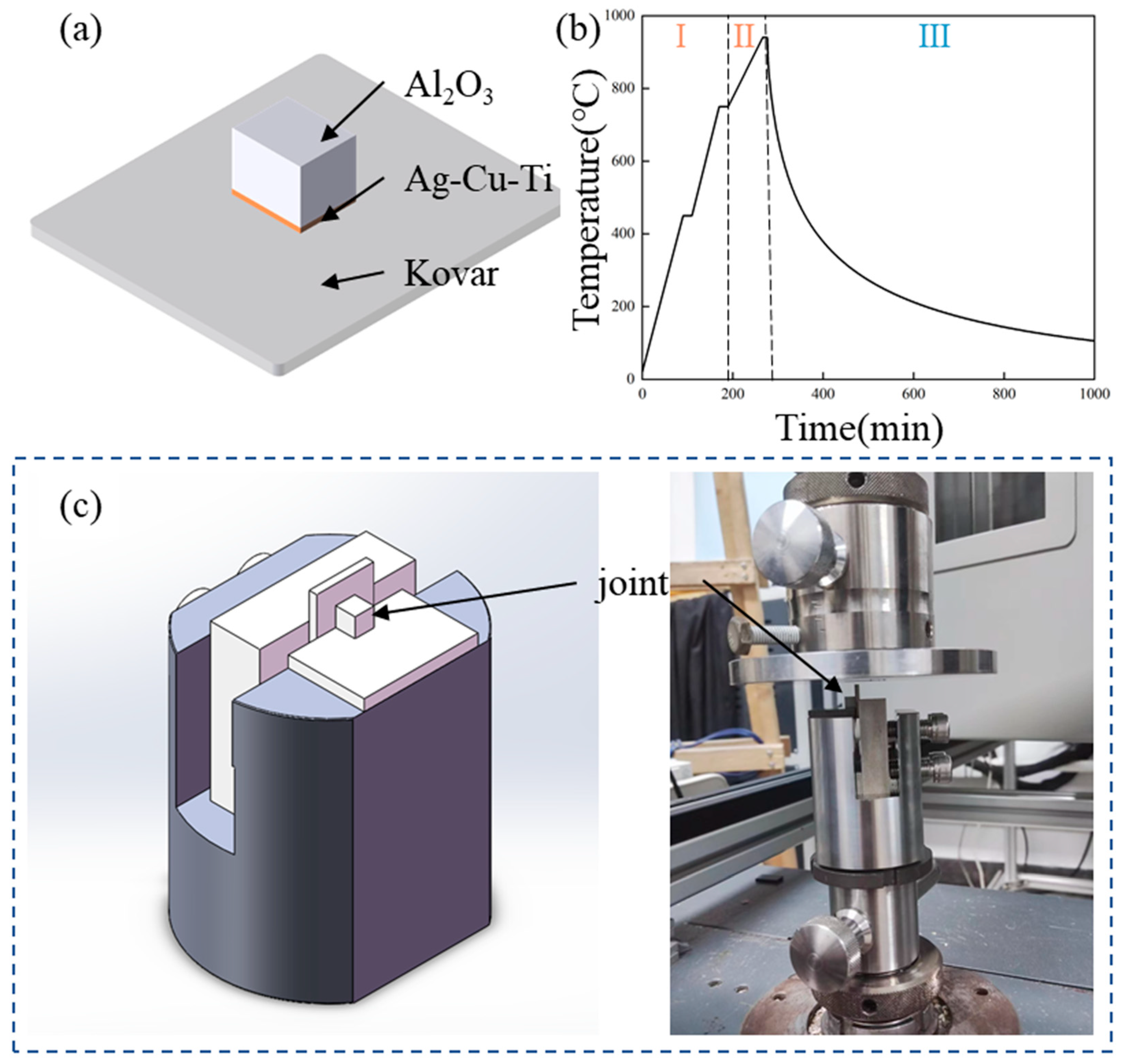
2. Materials and Methods
3. Results
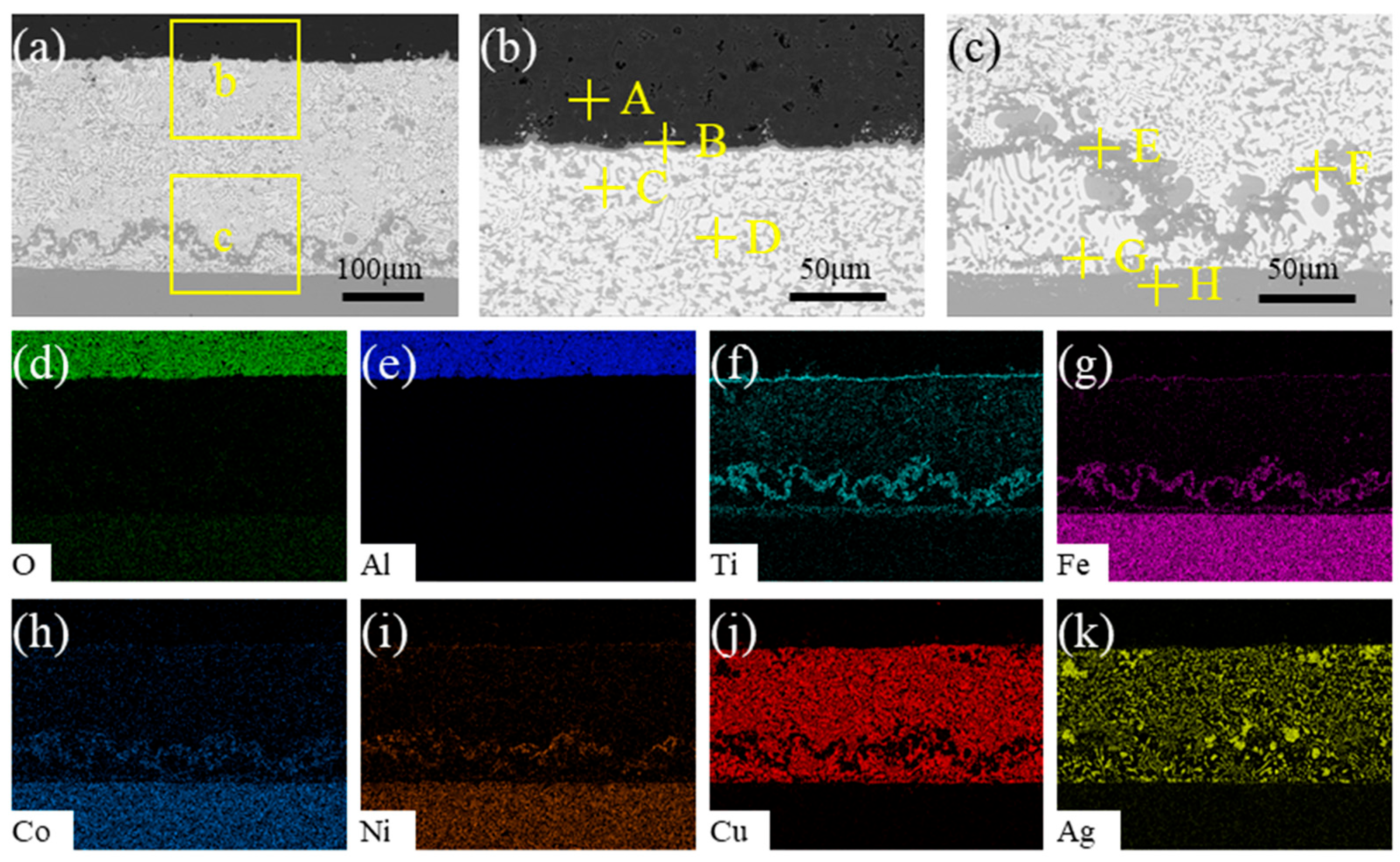
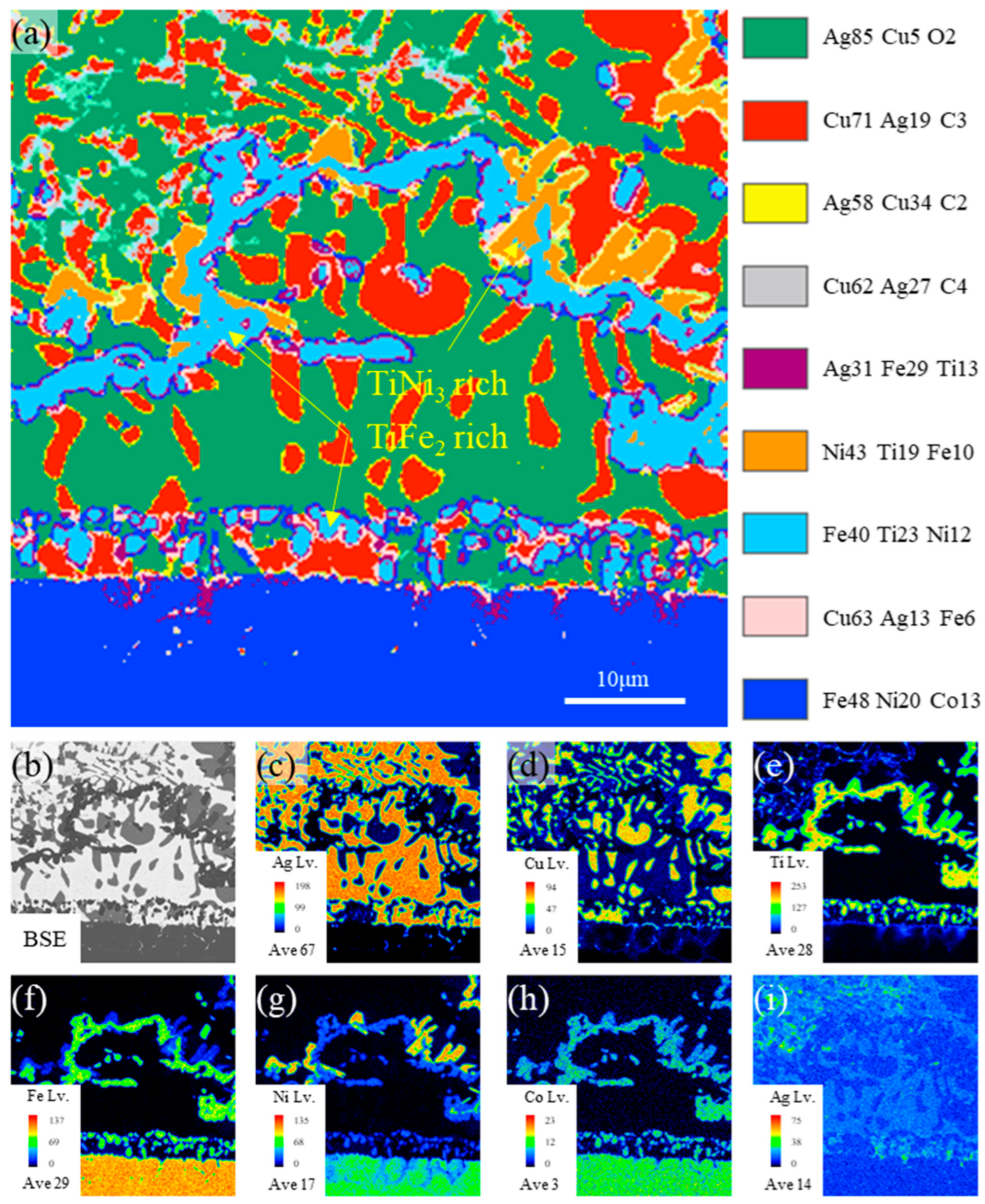
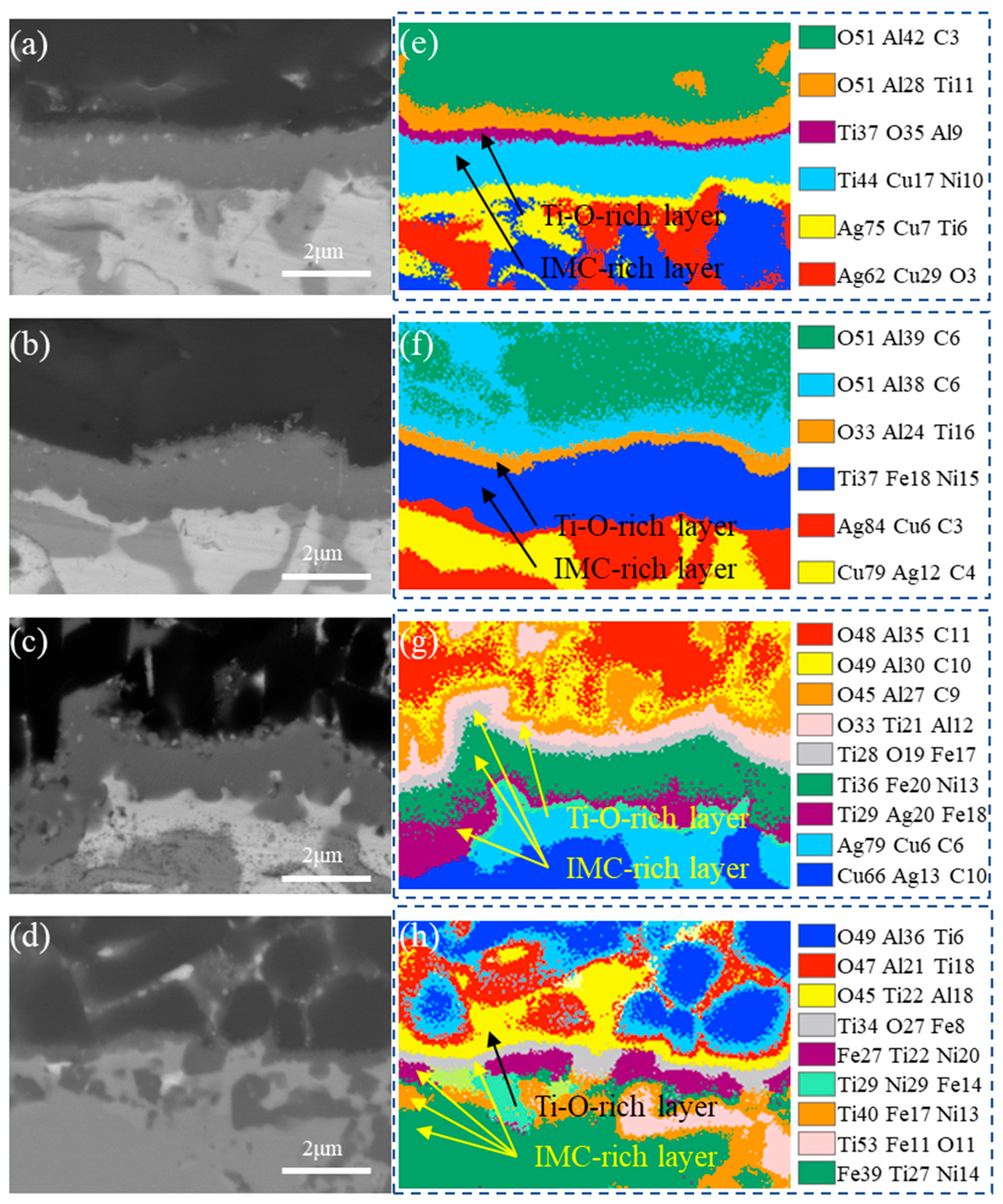
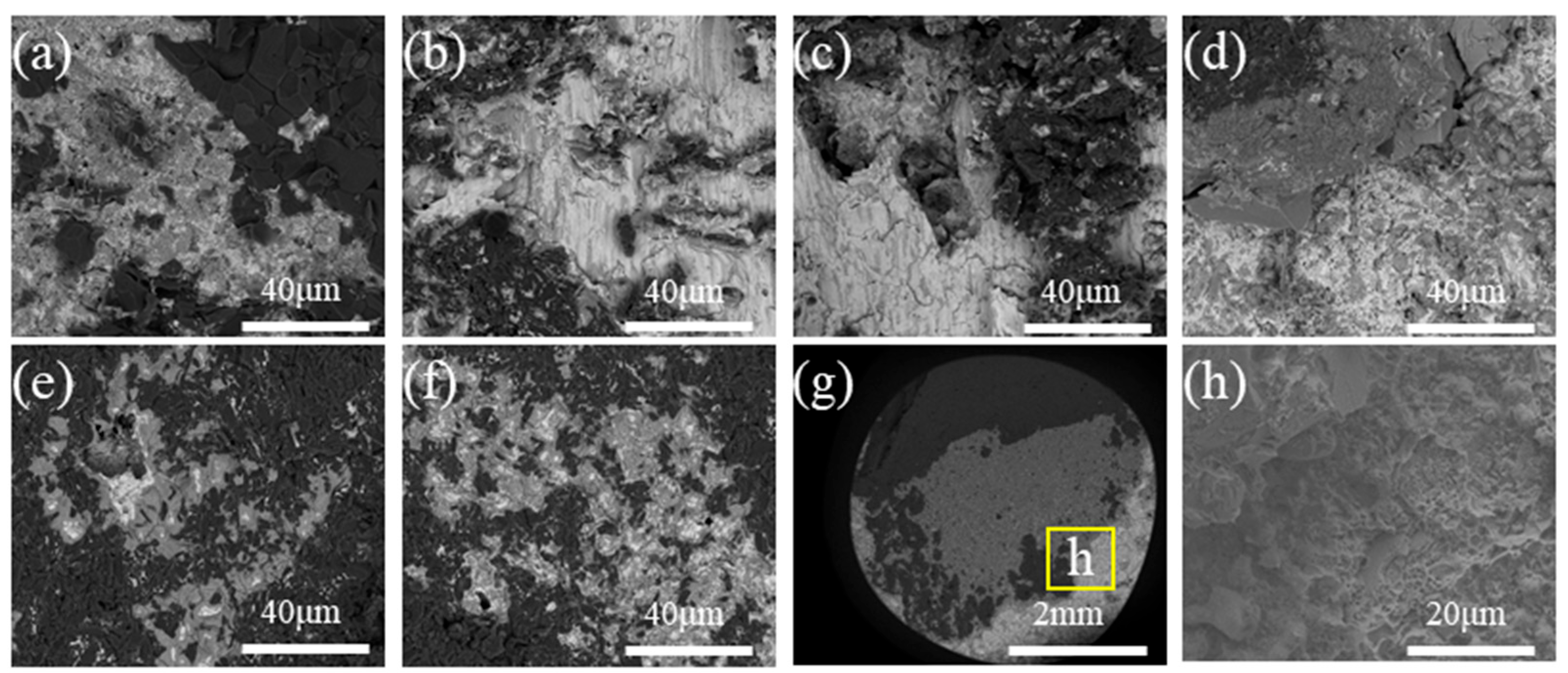
3.1. Typical Microstructure of the Brazing Joint
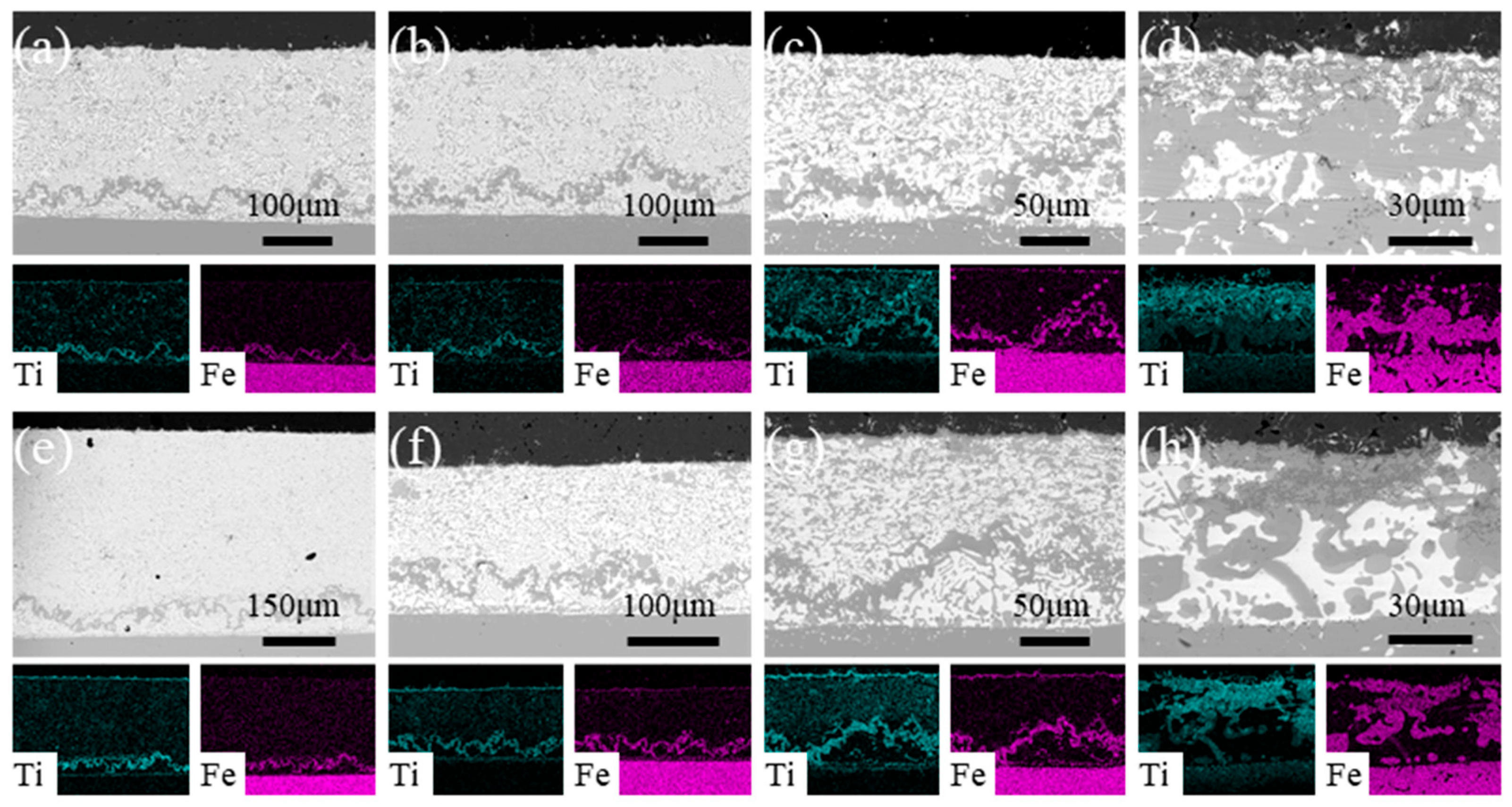
3.2. Effect of the Brazing Temperature and Heating Rate on the Microstructure of Al2O3–Kovar Joint
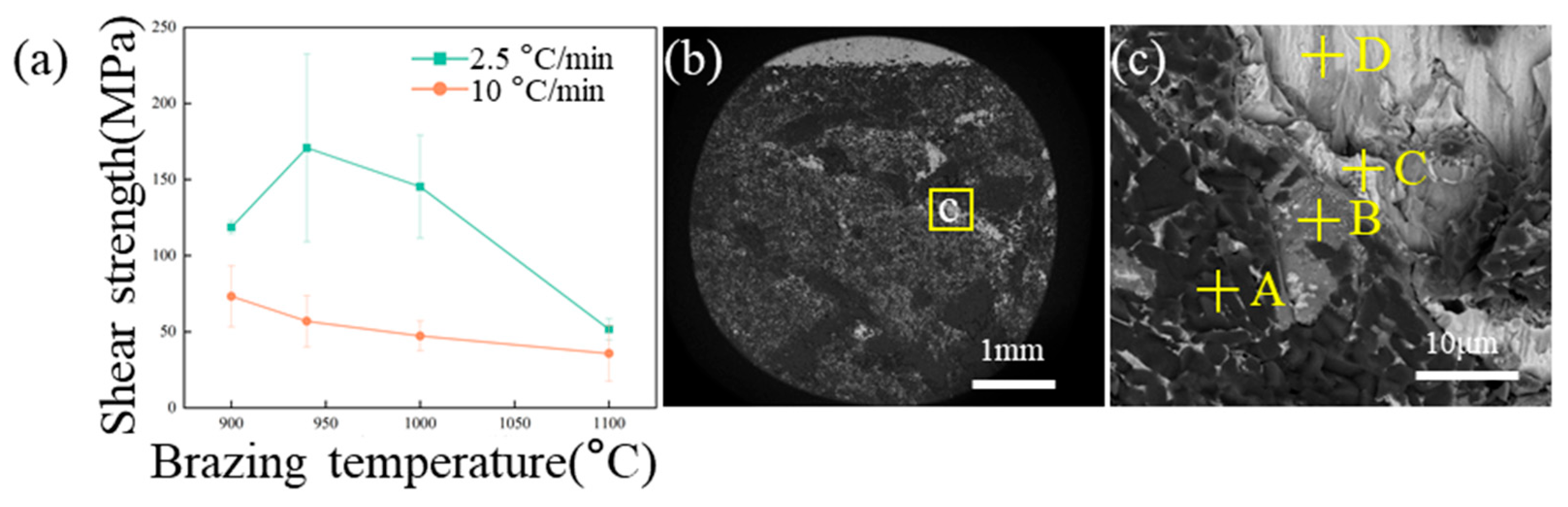
3.3. Effect of Brazing Temperature and Heating Rate on Mechanical Properties
4. Discussion
5. Conclusions
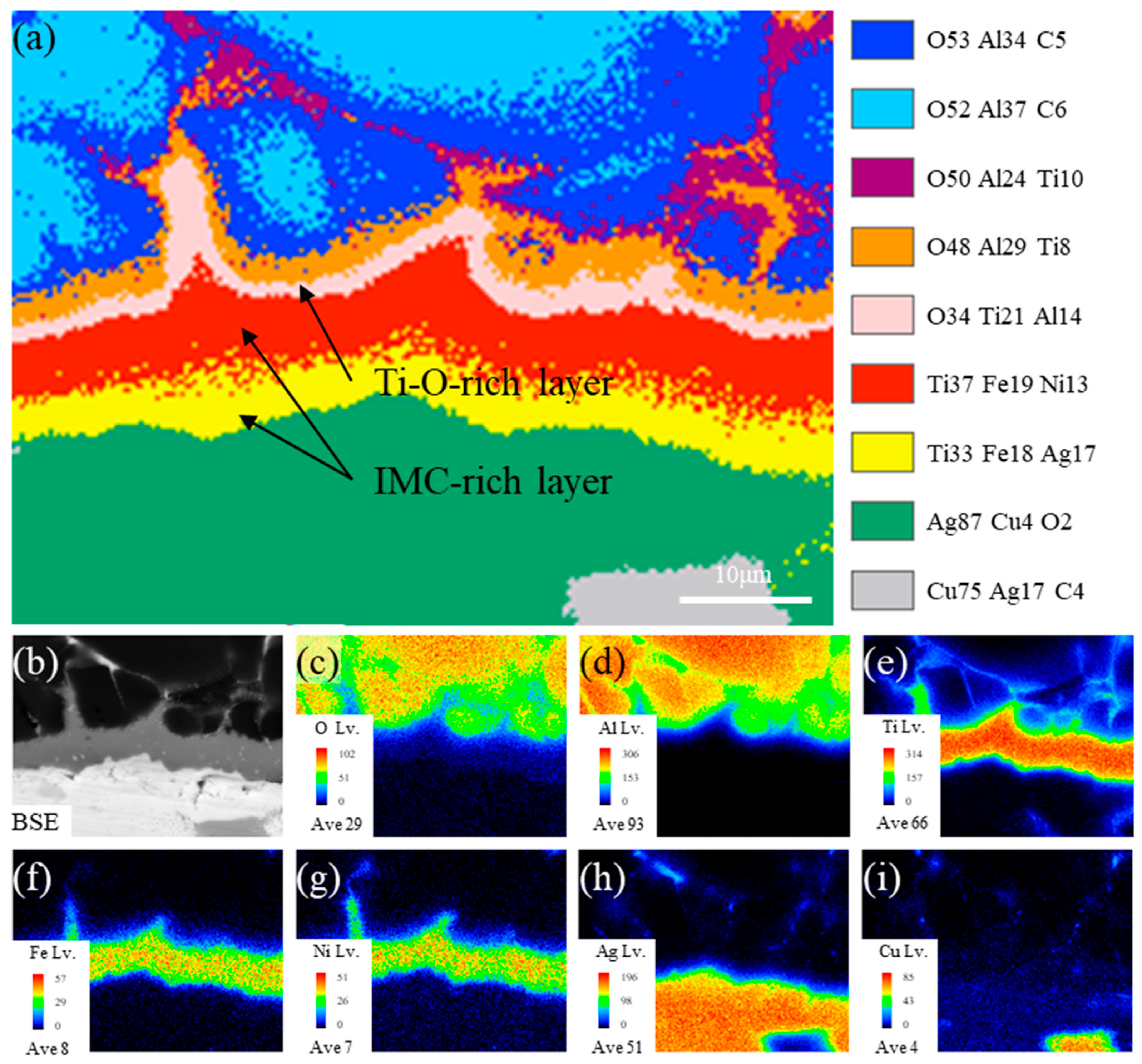
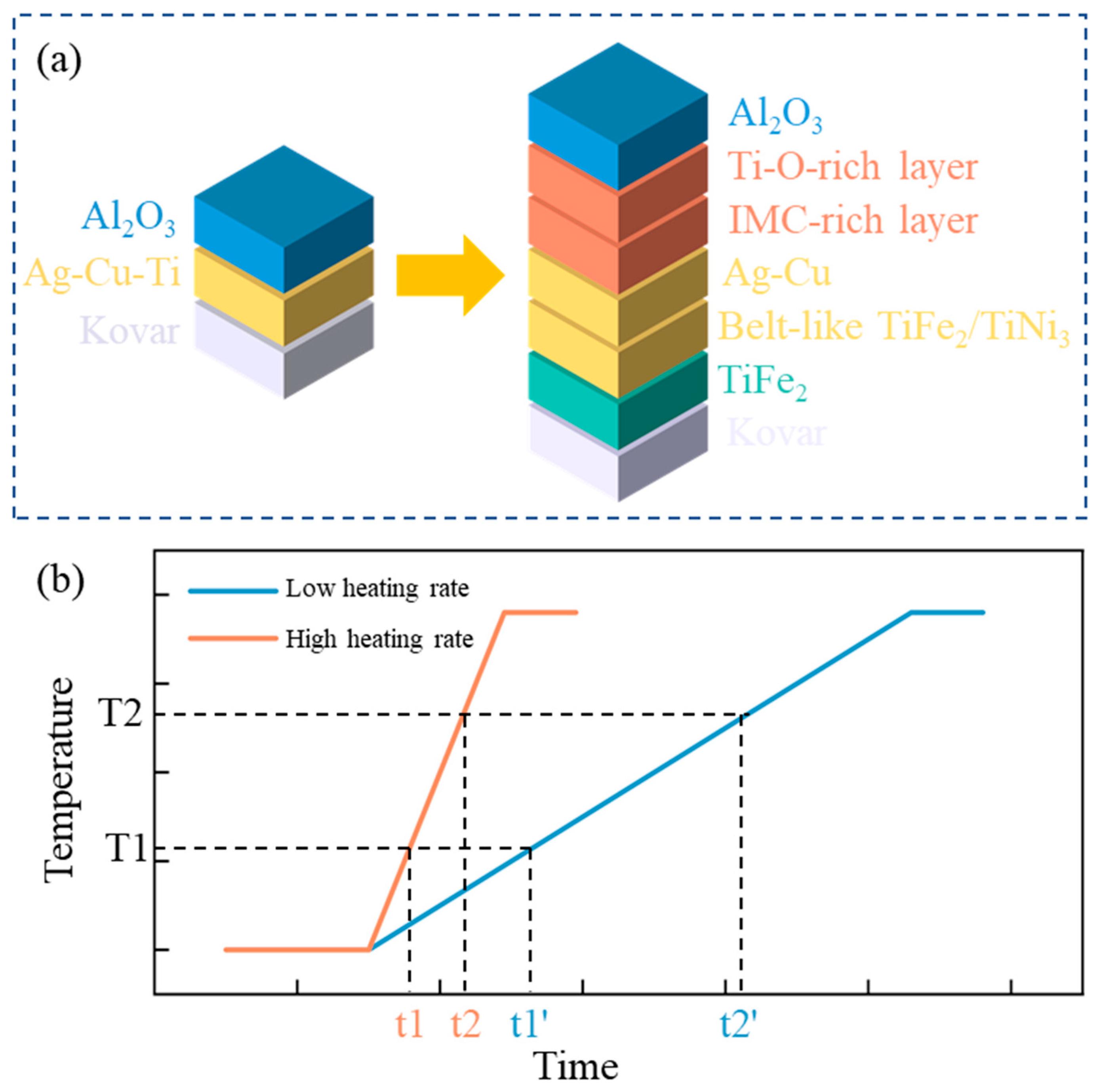
- The typical microstructure of the brazed joint could be divided into five layers as follows: Al2O3/ceramic-side reaction layer/filler layer/Kovar-side reaction layer/Kovar. The ceramic-side reaction layer can be further divided into a Ti-O-rich layer and IMC-rich layer, and the filler layer is composed of a Ag-Cu eutectic with a TiFe2 + TiNi3 belt-like IMC embedded. The Kovar-side reaction layer consists of TiFe2 IMC particles, Ag-Cu eutectic, and the remaining Kovar base metal.
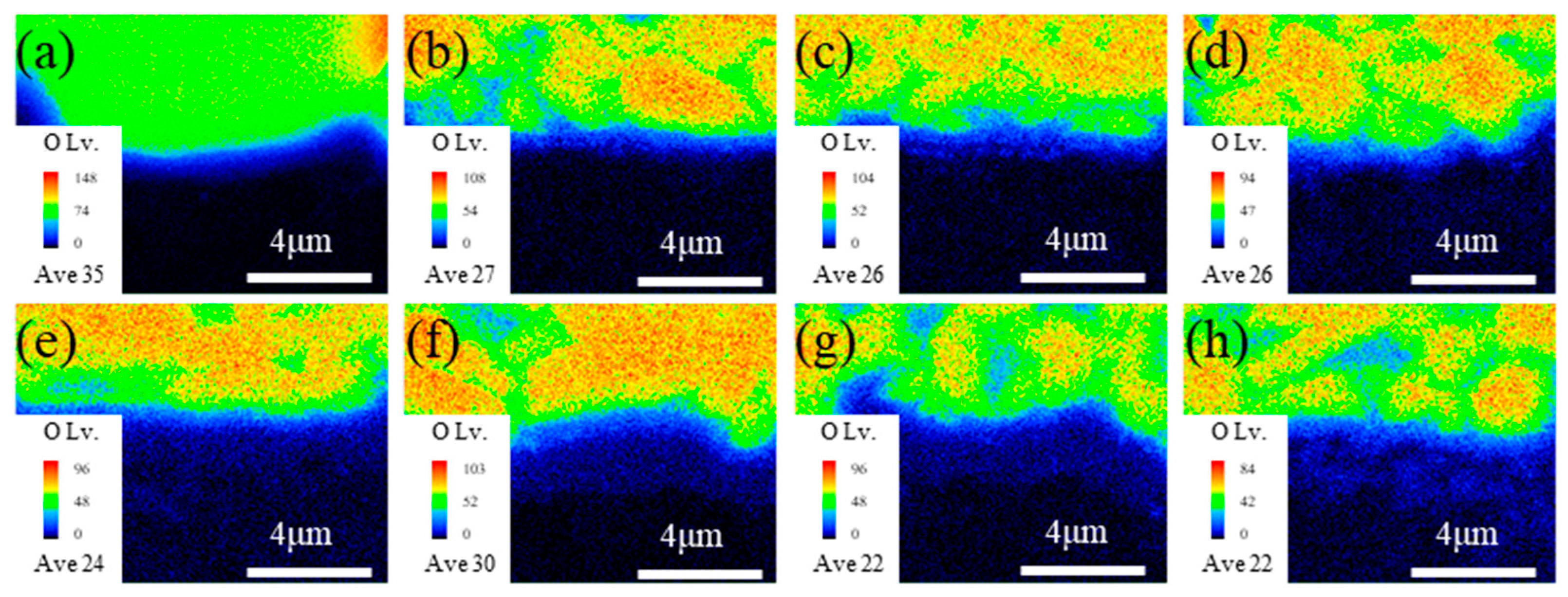
- For both heating rates, as the brazing temperature increases, the size of the TiFe2 + TiNi3 belt in the filler layer and the thickness of the IMC-rich layer in the ceramic-side reaction layer increase while the Ti-O-rich layer in the ceramic-side reaction layer remains relatively constant. For the same brazing temperature, using a higher heating rate (10 °C/min) results in a much thicker IMC-rich layer in the ceramic-side reaction layer.
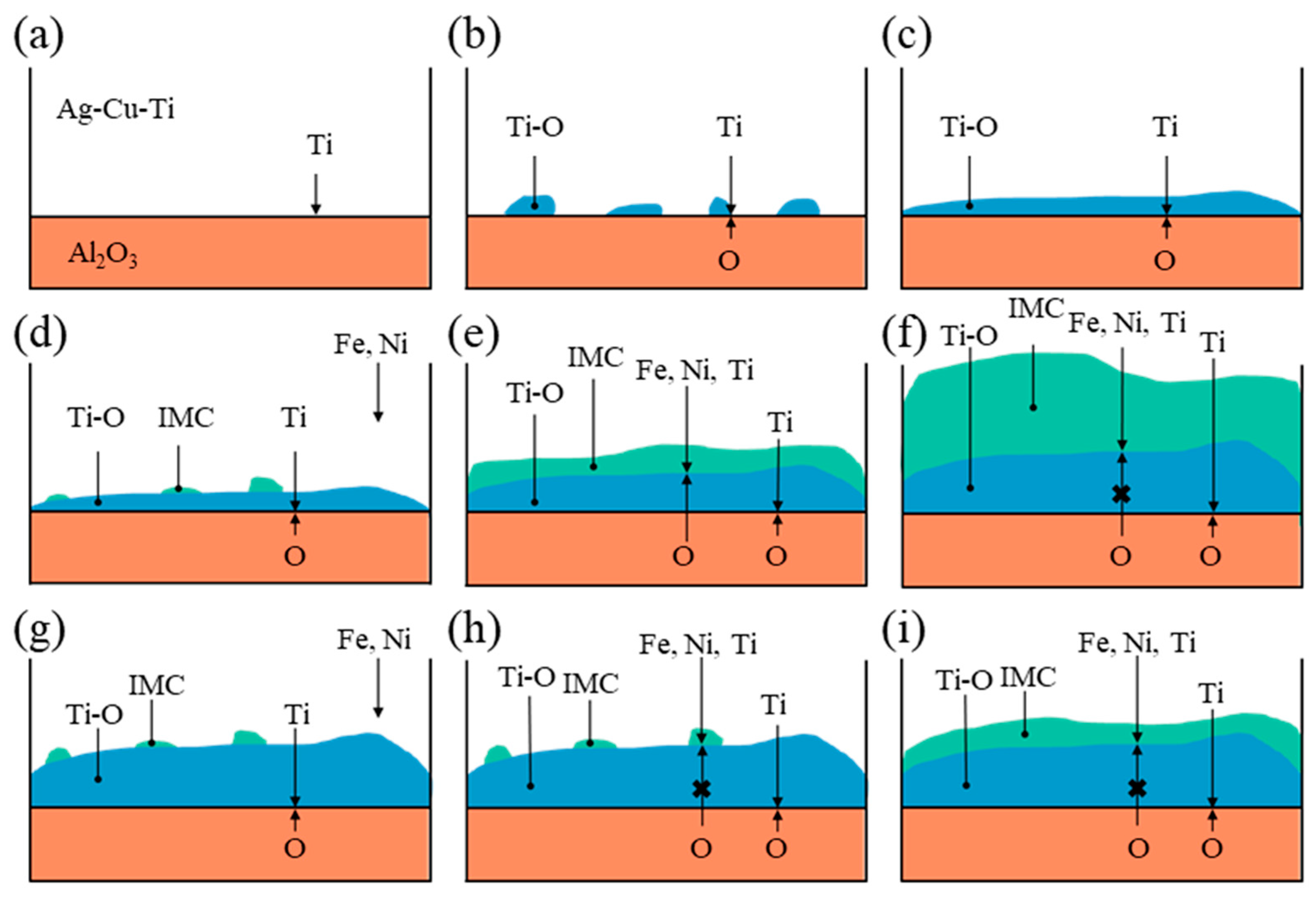
- The formation of the ceramic-side reaction layer can be divided into two steps. Firstly, Ti in the brazing filler metal reacts with O from Al2O3 to form the Ti-O-rich layer at a relatively low temperature in the heating process. Secondly, as the temperature increases, the IMC-rich layer forms between the Ti-O-rich layer and the filler layer. The thickness of the formed Ti-O-rich layer will affect the diffusion rate of O, thereby affecting the thickness of the IMC-rich layer.
- A lower heating rate of 2.5 °C/min consistently yielded higher shear strengths than 10 °C/min. The optimal parameters were 940 °C with the 2.5 °C/min rate, producing a peak strength of 224 MPa (average 170 ± 61 MPa). At this slower rate, the strength initially increased with the temperature (900–940 °C) then decreased, whereas it only decreased at the faster rate.
- The brazing parameters should be optimized to obtain an appropriate thickness of the ceramic-side IMC-rich layer, so as to shift the fracture position from the ceramic-side reaction layer to the filler layer in shear tests, and increase the overall shear strength of the brazed joints.
- Future work should systematically investigate a wider range of brazing temperature and heating rate combinations to identify the optimal processing window. Concurrently, finite element analysis (FEA) should be employed to study the residual stress distribution within the brazed joints and to explore strategies for mitigating these stresses, thereby further enhancing mechanical performance. Furthermore, the mechanical characterization of the joints should be augmented by including other testing methods, such as tensile strength and toughness measurements. The percentage of the ceramic region in the fracture surface analysis can be used to evaluate changes in the bonding degree between the ceramic and the filler metal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Lin, J. Improvement of microstructure and mechanical properties of Sapphire/Kovar alloy joints brazed with Ag-Cu-Ti composite filler by adding B powders. J. Manuf. Process. 2023, 99, 243–253. [Google Scholar] [CrossRef]
- Yang, M.; Lin, T. In situ synthesis of TiB whisker reinforcements in the joints of Al2O3/TC4 during brazing. Mater. Sci. Eng. A 2011, 528, 3520–3525. [Google Scholar] [CrossRef]
- Niu, G.B.; Wang, D.P. Microstructure and mechanical properties of Al2O3/TiAl joints brazed with B powders reinforced Ag-Cu-Ti based composite fillers. Ceram. Int. 2017, 43, 439–450. [Google Scholar] [CrossRef]
- Mandal, S.; Ray, A.K. Correlation between the mechanical properties and the microstructural behaviour of Al2O3-(Ag-Cu-Ti) brazed joints. Mater. Sci. Eng. A 2004, 383, 235–244. [Google Scholar] [CrossRef]
- Fu, W.; Hu, S.P. Effect of Ti content on the metallization layer and copper/alumina brazed joint. Ceram. Int. 2017, 43, 13206–13213. [Google Scholar] [CrossRef]
- Xin, C.; Yan, J. Microstructural evolution during the brazing of Al2O3 ceramic to kovar alloy by sputtering Ti/Mo films on the ceramic surface. Ceram. Int. 2016, 42, 12586–12593. [Google Scholar] [CrossRef]
- Xin, C.; Liu, W. Metallization of Al2O3 ceramic by magnetron sputtering Ti/Mo bilayer thin films for robust brazing to Kovar alloy. Ceram. Int. 2016, 42, 9599–9604. [Google Scholar] [CrossRef]
- Nascimento, R.M.; Martinelli, A.E. Interface microstructure of alumina mechanically metallized with Ti brazed to Fe-Ni-Co using different fillers. Mater. Sci. Eng. A 2007, 466, 195–200. [Google Scholar] [CrossRef]
- Lin, P.; Lin, T. Microstructure evolution and mechanical properties of a vacuum-brazed Al2O3/Ti joint with Mo-coating on Al2O3 and Ti surfaces. Ceram. Int. 2019, 45, 11195–11203. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M. Microstructural evolution and characterisation of interfacial phases in Al2O3/Ag-Cu-Ti/Al2O3 braze joints. Acta Mater. 2015, 96, 143–158. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M. Interfacial reactions between sapphire and Ag-Cu-Ti-based active braze alloys. Acta Mater. 2016, 103, 859–869. [Google Scholar] [CrossRef]
- Li, P.; Yan, Y. The regulation strategy for releasing residual stress in ceramic-metal brazed joints. J. Manuf. Process. 2023, 85, 935–947. [Google Scholar] [CrossRef]
- Nai, X.; Wang, P. Unveiling solid-state transformation toughening mechanism of yttria stabilized zirconia on brazed joint by in-situ observation. J. Manuf. Process. 2024, 127, 211–217. [Google Scholar] [CrossRef]
- Nai, X.; Zhang, H. Strengthening Ti3SiC2/Cu brazed joint assisted with cold spray additive manufacturing: Lower brazing temperature through interdiffusion and graded reinforcement for stress relaxation. J. Mater. Process. Technol. 2024, 331, 118530. [Google Scholar] [CrossRef]
- Way, M.; Luo, D. A new high entropy alloy brazing filler metal design for joining skutterudite thermoelectrics to copper. J. Alloys Compd. 2021, 858, 157750. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, P. Controllable distribution of reinforcements for reducing the strain energy in dissimilar ceramic/metal joints. J. Eur. Ceram. Soc. 2021, 41, 1076–1087. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J. High shear strength Kovar/AlN joints brazed with AgCuTi/Cu/AgCuTi sandwich composite filler. Mater. Sci. Eng. A 2023, 862, 144435. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. Compound connection mechanism of Al2O3 ceramic and TC4 Ti alloy with different joining modes. Sci. Rep. 2021, 11, 21251. [Google Scholar] [CrossRef]
- Ali, M.; Knowles, K.M.; Mallinson, P.M.; Fernie, J.A. Active metal brazing of Al2O3 to Kovar® (Fe-29Ni-17Co wt.%) using Copper ABA® (Cu-3.0Si-2.3Ti-2.0Al wt.%). Philos. Mag. 2018, 98, 182–202. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, C. Effects of Cu interlayers on the microstructure and mechanical properties of Al2O3/AgCuTi/Kovar brazed joints. Int. J. Appl. Ceram. Technol. 2019, 16, 896–906. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, J. Effects of Ti thickness on microstructure and mechanical properties of alumina-Kovar joints brazed with Ag-Pd/Ti filler. Ceram. Int. 2014, 40, 5699–5705. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C. Microstructure of alumina ceramic/Ag-Cu-Ti brazing alloy/Kovar alloy joint. Mater. Sci. Technol. 2007, 23, 320–323. [Google Scholar] [CrossRef]
- Yu, Z.S.; Yang, P. Crack formation mechanisms in Al2O3/Kovar brazed joint. Mater. Sci. Technol. 2006, 22, 864–866. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C. The brazing of Al2O3 ceramic and other materials. Int. J. Adv. Manuf. Technol. 2022, 120, 59–84. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, J. Effects of brazing temperature on microstructure and mechanical performance of Al2O3/AgCuTi/Fe-Ni-Co brazed joints. J. Alloys Compd. 2015, 650, 30–36. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, Z.W. Microstructural stability and mechanical properties of Al2O3/Kovar 4 J34 joint vacuum brazed using Ag-5Cu-1Al-1.25Ti (wt%) filler metal. J. Manuf. Process. 2021, 72, 553–564. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, S. Enhanced mechanical properties and thermal cycling stability of Al2O3-4J42 joints brazed using Ag-Cu-Ti/Cu/Ag-Cu composite filler. Ceram. Int. 2021, 47, 30247–30255. [Google Scholar] [CrossRef]



| Element | Fe | Ni | Co | Mn | Si | C | Other |
|---|---|---|---|---|---|---|---|
| Percentage (%) | Bal. | 32.5 | 14.4 | 0.27 | 0.22 | 0.009 | <0.01 |
| Content | Al2O3 | SiO2 | CaO | Other |
|---|---|---|---|---|
| Percentage (%) | 94.84 | 3.03 | 1.92 | 0.21 |
| Point | O | Al | Ti | Fe | Co | Ni | Cu | Ag | Possible Phase |
|---|---|---|---|---|---|---|---|---|---|
| A | 37.49 | 59.96 | - | - | - | 0.08 | - | 2.47 | Al2O3 |
| B | 11.37 | 5.36 | 48.72 | 16.29 | 2.14 | 15.65 | - | 0.48 | Ti-Fe-Ni-O |
| C | 1.95 | - | - | - | - | 1.94 | 3.10 | 93.01 | Ag |
| D | - | 2.62 | 3.63 | - | 1.64 | - | 86.93 | 5.19 | Cu |
| E | - | - | 28.66 | 46.38 | 12.10 | 11.31 | 0.43 | 1.12 | TiFe2+TiNi3 |
| F | 2.04 | 0.20 | 28.26 | 11.19 | 3.69 | 54.62 | - | - | TiNi3+TiFe2 |
| G | - | 0.41 | 25.98 | 48.11 | 13.98 | 9.98 | - | 1.54 | TiFe2 |
| H | - | 1.27 | 1.20 | 51.32 | 18.28 | 26.76 | - | 1.17 | Kovar |
| Point | O | Al | Ti | Fe | Co | Ni | Cu | Ag | Possible Phase | Fracture Position |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 57.59 | 40.53 | 0.71 | - | - | - | 0.37 | 0.80 | Al2O3 | Ceramics |
| B | 27.04 | 3.46 | 32.42 | 16.24 | 4.79 | 10.00 | 3.59 | 2.47 | Ti-O-Fe-Ni | Reaction layer |
| C | - | - | 5.41 | - | - | - | 12.19 | 82.41 | Ag | Filler layer |
| D | - | - | 3.03 | - | - | - | 91.36 | 5.62 | Cu | Filler layer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Du, D.; Zhang, D.; Xue, S.; Zhang, J.; Yi, J.; You, H.; Chang, B. Study on the Layered Structure of Ceramic-Side Bonding Area and the Mechanical Property of Al2O3–Kovar Brazed Joint with Ag-Cu-Ti Filler. J. Manuf. Mater. Process. 2025, 9, 355. https://doi.org/10.3390/jmmp9110355
Qi J, Du D, Zhang D, Xue S, Zhang J, Yi J, You H, Chang B. Study on the Layered Structure of Ceramic-Side Bonding Area and the Mechanical Property of Al2O3–Kovar Brazed Joint with Ag-Cu-Ti Filler. Journal of Manufacturing and Materials Processing. 2025; 9(11):355. https://doi.org/10.3390/jmmp9110355
Chicago/Turabian StyleQi, Junjie, Dong Du, Dongqi Zhang, Shuai Xue, Jiaming Zhang, Jiamin Yi, Haifei You, and Baohua Chang. 2025. "Study on the Layered Structure of Ceramic-Side Bonding Area and the Mechanical Property of Al2O3–Kovar Brazed Joint with Ag-Cu-Ti Filler" Journal of Manufacturing and Materials Processing 9, no. 11: 355. https://doi.org/10.3390/jmmp9110355
APA StyleQi, J., Du, D., Zhang, D., Xue, S., Zhang, J., Yi, J., You, H., & Chang, B. (2025). Study on the Layered Structure of Ceramic-Side Bonding Area and the Mechanical Property of Al2O3–Kovar Brazed Joint with Ag-Cu-Ti Filler. Journal of Manufacturing and Materials Processing, 9(11), 355. https://doi.org/10.3390/jmmp9110355






