Evaluation of Processing Conditions in the Performance of Purging Compounds for Polypropylene Injection Molding
Abstract
1. Introduction
- Type of polymer to be purged;
- Process temperature range;
- Polarity;
- Type of plastic conversion process.
2. Research Methods and Equipment
2.1. Materials
2.2. Characterization of Purging Compounds
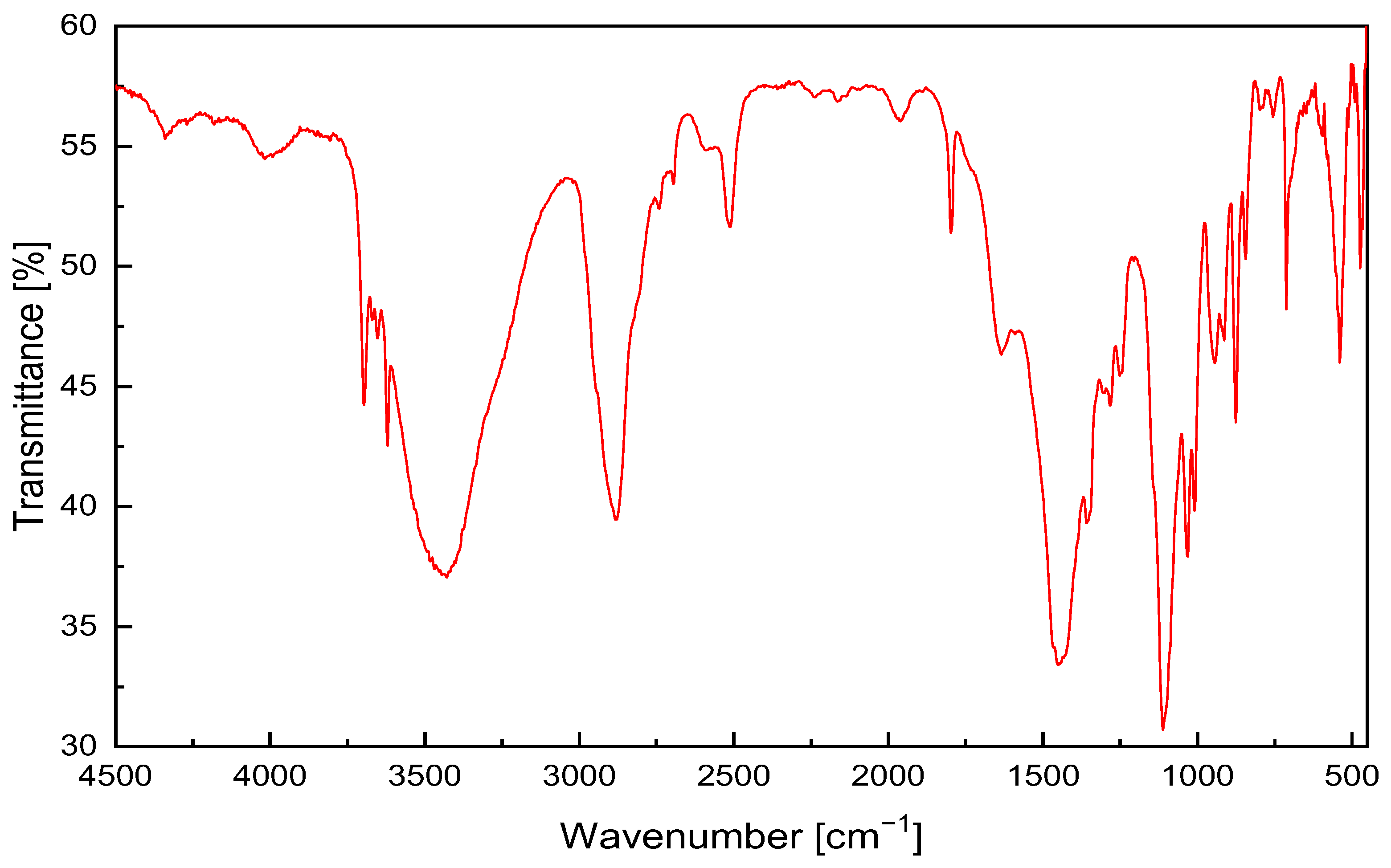
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. X-ray Diffraction (XRD)
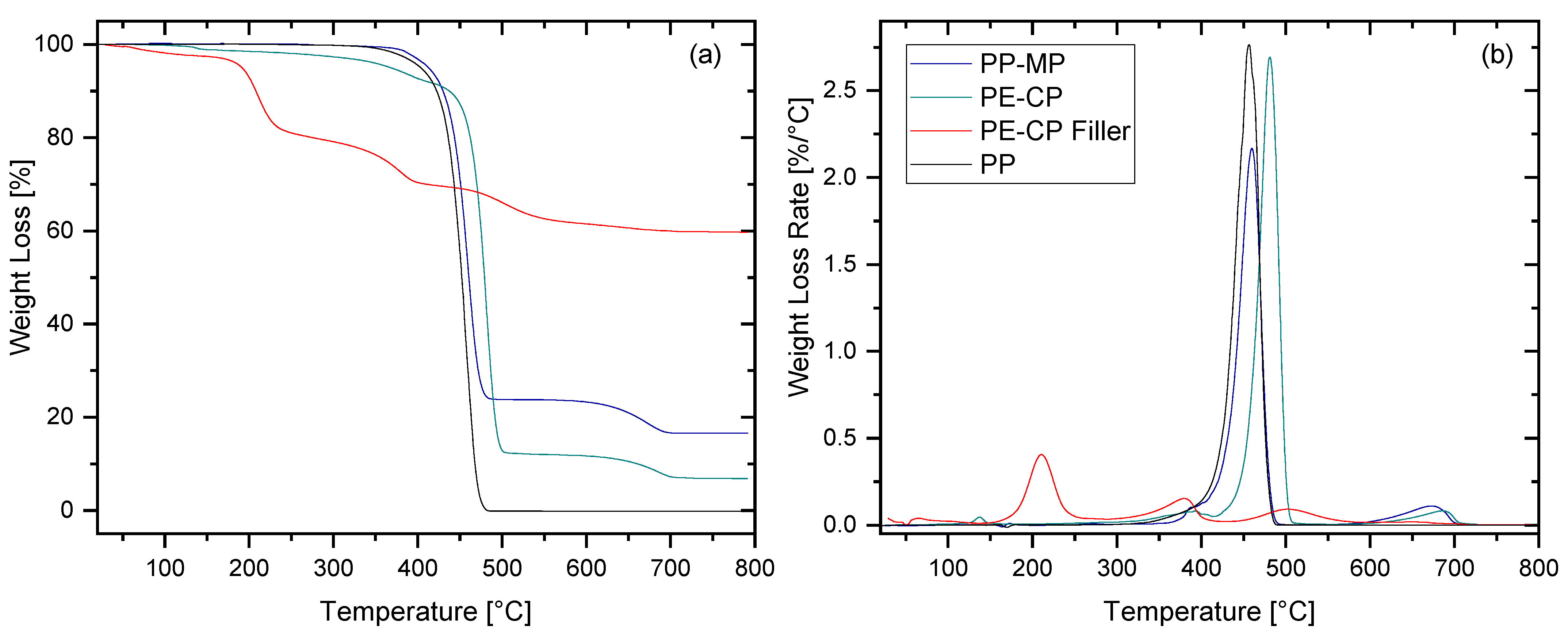
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Differential Scanning Calorimetry (DSC)
2.3. Rheological Analysis
2.4. Purging Methodology
2.5. Specific Energy Consumption
3. Results and Discussion
3.1. Characterization of Purging Materials
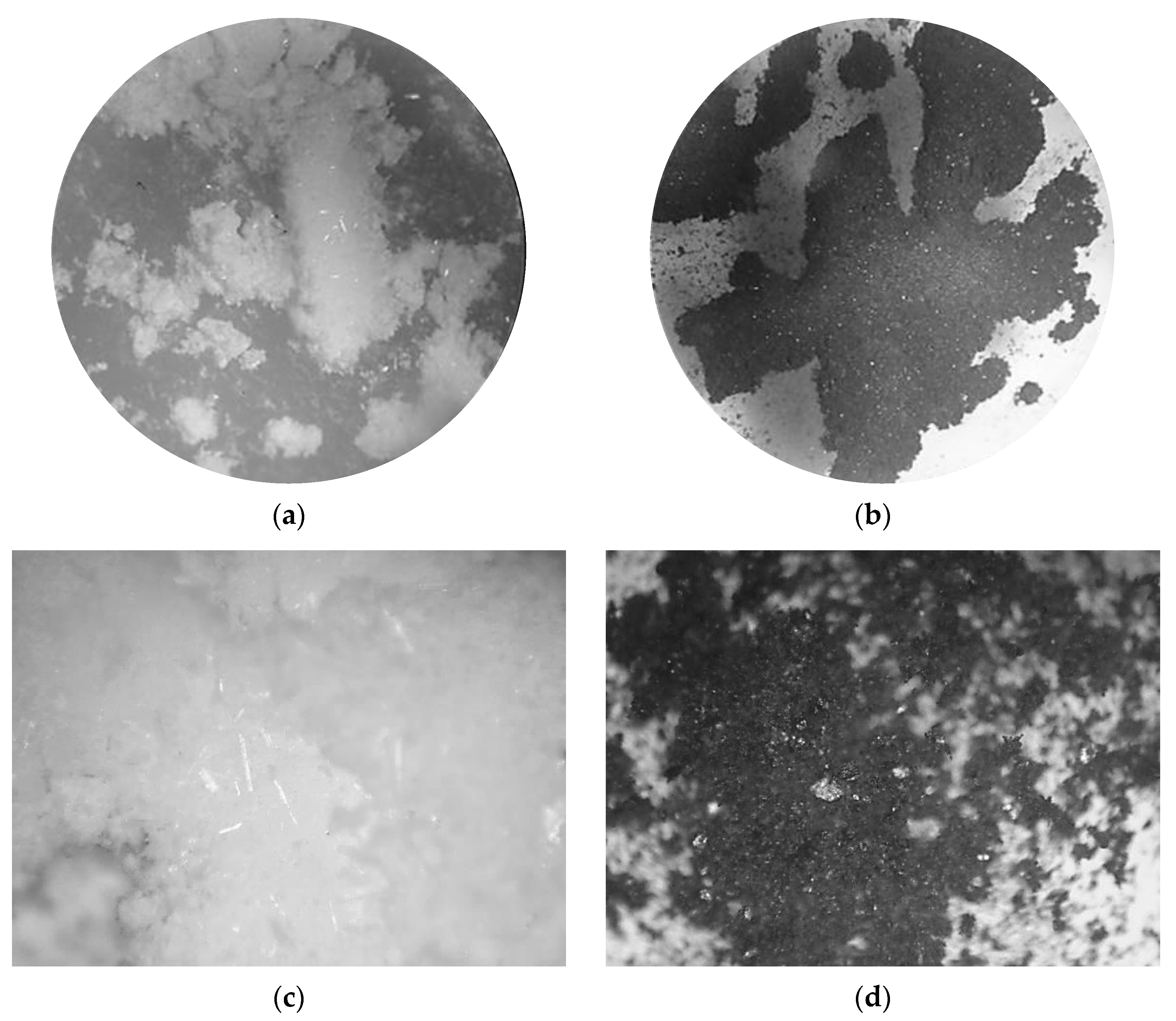
3.1.1. FTIR and XRD Analysis of PE-CP Filler
3.1.2. Thermogravimetric Analysis
3.1.3. Differential Scanning Calorimetry
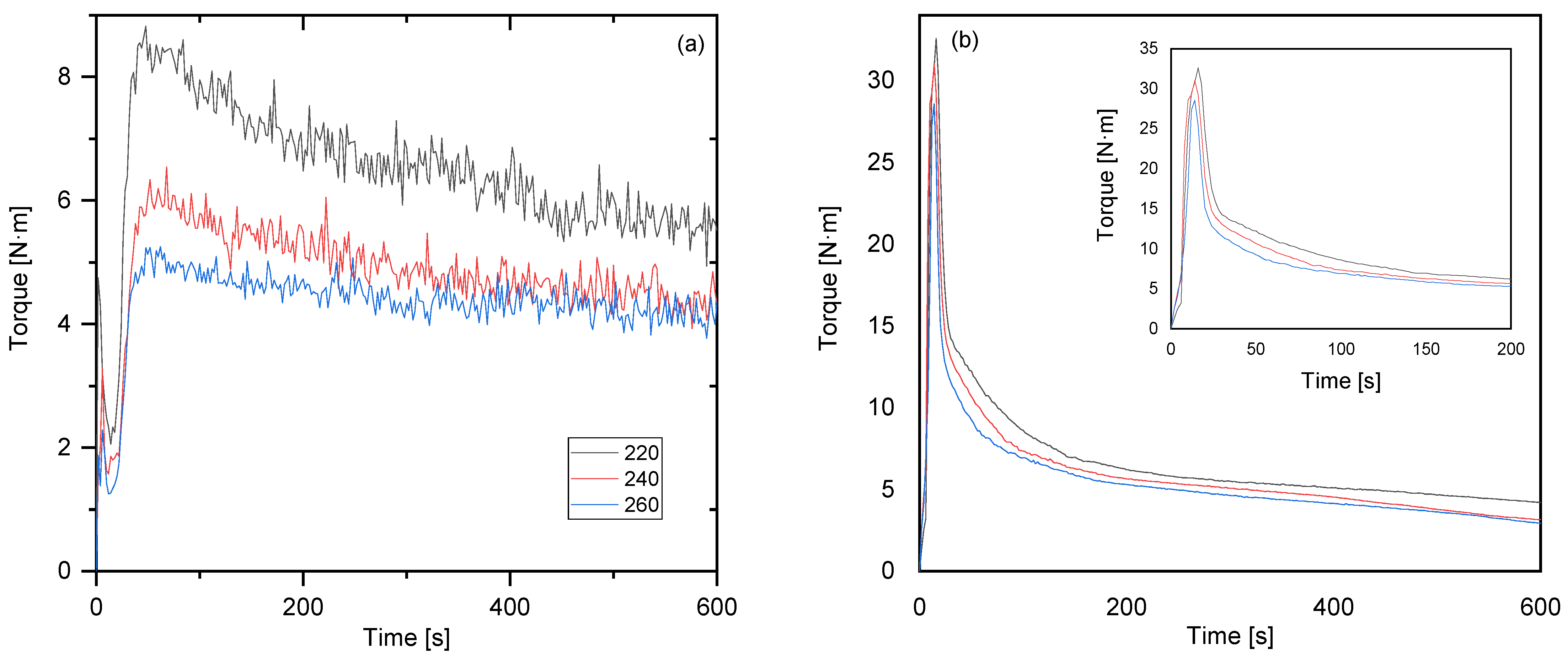
3.2. Rheological Analysis
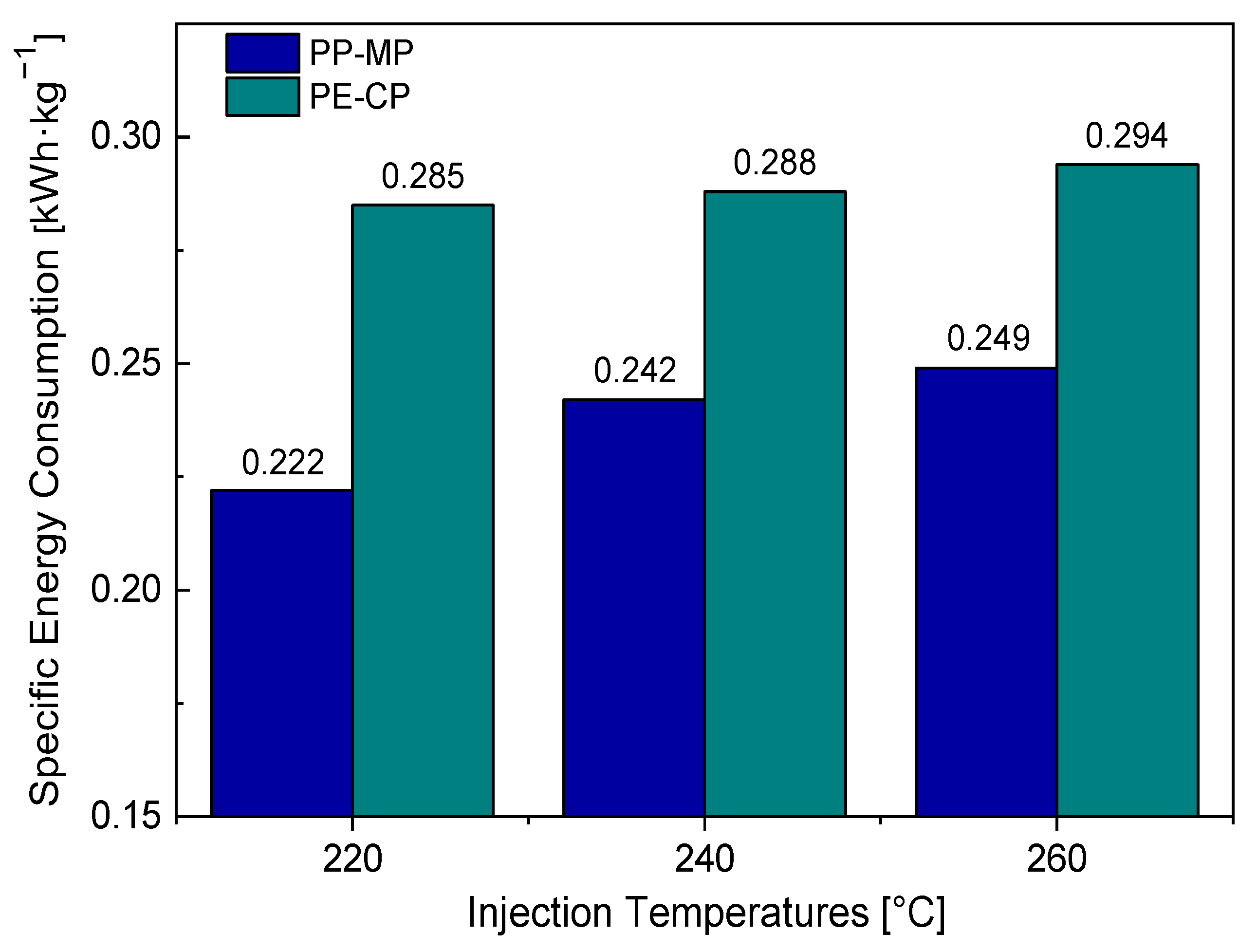
3.3. Statistical and Specific Energy Consumption Analysis in Injection Molding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahani, S.; Khade, V.; Basu, S.; Pilla, S. A Data-Driven Predictive Maintenance Framework for Injection Molding Process. J. Manuf. Process. 2022, 80, 887–897. [Google Scholar] [CrossRef]
- Colella, M. Try a “complete” Method to Clean Screws, Barrels. Plast. Technol. 2016, 62, 58–60. [Google Scholar]
- Govender, M.; Focke, W.; Tolmay, A.; Graeffe, D. Evaluation of Commercial Plastics Purging Compounds on a Laboratory Film Blower. S. Afr. J. Chem. Eng. 2002, 14, 15–26. [Google Scholar] [CrossRef]
- Komarmi, J. Purging Compounds Reduce Machine Downtime and Increase Productivity for Compounders. Plast. Addit. Compd. 2002, 4, 14–16. [Google Scholar] [CrossRef]
- Wagner, J.R.; Mount, E.M.; Giles, H.F. Screw Cleaning and Purge Compounds. In Extrusion; Elsevier: Amsterdam, The Netherlands, 2014; pp. 439–448. [Google Scholar]
- Campo, E.A. The Complete Part Design Handbook for Injection Molding of Thermoplastics; Hanser Gardner Publications: Cincinnati, OH, USA, 2006; ISBN 9781569903759. [Google Scholar]
- Plastics Additives at K 2007. Plast. Addit. Compd. 2007, 9, 10–23. [CrossRef]
- Tolinski, M. Additives Annual. Plast. Eng. 2011, 67, 14–25. [Google Scholar] [CrossRef]
- Denzel, D. Follow These Tips to Maximize Purging Efficiency. Plast. Technol. 2022, 68, 40–43. [Google Scholar]
- Schmiederer, D.; Sriseubsai, W.; Schott, N.R. Molding via Rheological Control during Plastication and Purging. J. Macromol. Sci. Part A 2006, 43, 2019–2030. [Google Scholar] [CrossRef]
- Asaclean Purging Compound. Hoja de Datos Técnicos Grado UP; Asaclean Purging Compound: Parsippany, NJ, USA, 2019; Volume 1. [Google Scholar]
- Eberle, J.; Schroots, R. Purging Compounds. European Patent EP0937567A1, 25 August 1999. [Google Scholar]
- Roy, S.D.; Triokekar, V.; D’Uva, S.; Hindy, E. Compound for Purging Residual Polyamides from an Extruder. U.S. Patent US8492473B2, 23 July 2013. [Google Scholar]
- Botros, M.G. Purging Compound for Polyamides and Ethylene Vinyl Alcohol Copolymers. J. Plast. Film Sheeting 1996, 12, 212–224. [Google Scholar] [CrossRef]
- Chem-Trend, L.P. Lusin Purging Agents. Available online: https://chemtrend.com/wp-content/uploads/2019/03/chemtrend_thermoplastics_purge_compounds_flyer.pdf (accessed on 18 January 2023).
- Murphy, O. Processors of Thermoplastics: The Quest for Improved Operations. Available online: https://purgexonline.com/wp-content/uploads/2020/10/White-Paper_Comparison-of-Purgex-457-to-Natural-Resin.pdf (accessed on 18 January 2023).
- Chem-Trend, L.P. Purging Compounds. Available online: https://chemtrend.com/wp-content/uploads/2019/03/chem-trend-faq-documents-purging-compounds-color-f-210308.pdf (accessed on 18 January 2023).
- Hong, J.S.; Shim, H.S.; Lee, J.-H.; Kwon, M.-K.; Chung, D.-I.; Kim, S.K. Characterization of Color Change in Injection Molding Process Using Hot Runner. Trans. Korean Soc. Mech. Eng. A 2015, 39, 111–115. [Google Scholar] [CrossRef]
- Sudsawat, S.; Sriseubsai, W. Warpage Reduction through Optimized Process Parameters and Annealed Process of Injection-Molded Plastic Parts. J. Mech. Sci. Technol. 2018, 32, 4787–4799. [Google Scholar] [CrossRef]
- Rigail-Cedeño, A.; Lazo, M.; Gaona, J.; Delgado, J.; Tapia-Bastidas, C.V.; Rivas, A.L.; Adrián, E.; Perugachi, R. Processability and Physical Properties of Compatibilized Recycled HDPE/Rice Husk Biocomposites. J. Manuf. Mater. Process. 2022, 6, 67. [Google Scholar] [CrossRef]
- Tiseo, I. Plastic Converters Demand in the European Union (EU-27) in 2021, by Polymer Type. Available online: https://www.statista.com/statistics/687526/plastic-materials-applications-european-union-eu/ (accessed on 18 January 2023).
- Asociación Ecuatoriana de Plásticos. Estadísticas del Sector Plástico. Integra 2022, 67, 35. [Google Scholar]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. On the Determination of the Enthalpy of Fusion of A-Crystalline Isotactic Polypropylene Using Differential Scanning Calorimetry, X-Ray Diffraction, and Fourier-Transform Infrared Spectroscopy: An Old Story Revisited. Adv. Eng. Mater. 2020, 22, 1900796. [Google Scholar] [CrossRef]
- Won, J.S.; Lee, J.M.; Lee, P.G.; Choi, H.Y.; Kwak, T.J.; Lee, S.G. Effects of Nanocrystallization on Surface Migration of Polypropylene/Slip Agent Composites in Accelerated Aging. J. Mater. Sci. 2022, 57, 1489–1505. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of Shear Rate and Viscosity from Batch Mixer Data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Alzamel, N.O.; Alakhras, F.; Al-Arfaj, A.A.; Al-Khaldi, M.A.; Al-Omair, N.A.; Al-Abbad, E.; Wassel, A.A.; Ouerfelli, N. On the Homographic Dependence of Activation Energy and Viscosity Arrhenius’ Temperature for Some Pure Fluids. Asian J. Chem. 2018, 30, 1937–1943. [Google Scholar] [CrossRef]
- Lou, Y.; Lei, Q.; Wu, G. Research on Polymer Viscous Flow Activation Energy and Non-Newtonian Index Model Based on Feature Size. Adv. Polym. Technol. 2019, 2019, 1070427. [Google Scholar] [CrossRef]
- Kealy, T. Explanation and Evaluation of Processability; Rheology Solutions Pty Ltd.: Bacchus Marsh, VIC, Australia, 2006. [Google Scholar]
- Kalinchev, E.L. Controlling the Processability of Polymeric Materials. Int. Polym. Sci. Technol. 2002, 29, 55–62. [Google Scholar] [CrossRef]
- Hoja de Datos del Producto Ultra Plast Po-E; Kalay do Brasil Ltda: Pinhais, Brazil, 2016; pp. 1–3.
- INEOS Olefins & Polymers USA. Polypropylene Processing Guide. Available online: https://www.ineos.com/globalassets/ineos-group/businesses/ineos-olefins-and-polymers-usa/products/technical-information--patents/ineos_polypropylene_processing_guide.pdf (accessed on 17 January 2023).
- Elduque, A.; Elduque, D.; Pina, C.; Clavería, I.; Javierre, C. Electricity Consumption Estimation of the Polymer Material Injection-Molding Manufacturing Process: Empirical Model and Application. Materials 2018, 11, 1740. [Google Scholar] [CrossRef]
- Callister, W.; Rethwisch, D. Characteristics, Applications, and Processing of Polymers. In Materials Science and Engineering: An Introduction; Wiley: Hoboken, NJ, USA, 2018; pp. 511–563. ISBN 9781119405436. [Google Scholar]
- Nitzsche, N. Composition and Method for Purging Polymer Processing Equipment. U.S. Patent US20020193267A1, 19 December 2002. [Google Scholar]
- Nitzsche, N. Composition and Method for Purging Polymer Processing Equipment. U.S. Patent US6384002B1, 2001. [Google Scholar]
- Harper, C.A. Modern Plastics Handbook; McGraw-Hill Education: New York, NY, USA, 2000; ISBN 0070267146. [Google Scholar]
- Rueda, M.M.; Auscher, M.C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and Applications of Highly Filled Polymers: A Review of Current Understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Coleman, E.A. Plastics Additives. In Applied Plastics Engineering Handbook: Processing and Materials; William Andrew: New York, NY, USA, 2011; pp. 419–428. ISBN 9781437735147. [Google Scholar]
- Patti, A.; Lecocq, H.; Serghei, A.; Acierno, D.; Cassagnau, P. The Universal Usefulness of Stearic Acid as Surface Modifier: Applications to the Polymer Formulations and Composite Processing. J. Ind. Eng. Chem. 2021, 96, 1–33. [Google Scholar] [CrossRef]
- Leung, P.; Ramdatt, P.; King, R. Purging Composition for Cleaning Thermoplastic Processing Equipment. U.S. Patent US005236514A, 17 August 1993. [Google Scholar]
- Wang, K.; Addiego, F.; Bahlouli, N.; Ahzi, S.; Rémond, Y.; Toniazzo, V.; Muller, R. Analysis of Thermomechanical Reprocessing Effects on Polypropylene/Ethylene Octene Copolymer Blends. Polym. Degrad. Stab. 2012, 97, 1475–1484. [Google Scholar] [CrossRef]
- Garzón, E.; Pérez-Villarejo, L.; Eliche-Quesada, D.; Martínez-Martínez, S.; Sánchez-Soto, P.J. Vitrification Rate and Estimation of the Optimum Firing Conditions of Ceramic Materials from Raw Clays: A Review. Ceram. Int. 2022, 48, 15889–15898. [Google Scholar] [CrossRef]
- Sadik, T.; Pillon, C.; Carrot, C.; Ruiz, J.A.R. Dsc Studies on the Decomposition of Chemical Blowing Agents Based on Citric Acid and Sodium Bicarbonate. Thermochim. Acta 2018, 659, 74–81. [Google Scholar] [CrossRef]
- Şirin, K.; Doğan, F.; Şirin, M.; Beşergil, B. Mechanical and Thermal Properties of Polypropylene (IPP)-High Density Polyethylene (HDPE) Binary Blends. Celal Bayar Üniv. Bilim. Derg. 2017, 13, 15–23. [Google Scholar] [CrossRef]
- Dashora, P.; Bafna, M.; Pratap, A.; Saxena, N.S. A Study of Temperature Dependence of Heat Capacity of Amorphous and Semi-Crystalline Polymers. AIP Conf. Proc. 2010, 1249, 47–50. [Google Scholar]
- Feng, C.; Li, Z.; Wang, Z.; Wang, B.; Wang, Z. Optimizing Torque Rheometry Parameters for Assessing the Rheological Characteristics and Extrusion Processability of Wood Plastic Composites. J. Thermoplast. Compos. Mater. 2019, 32, 123–140. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A.; Schweizer, T.; Öttinger, H.C. Particle—Particle and Particle-Matrix Interactions in Calcite Filled High-Density Polyethylene—Steady Shear. J. Rheol. 2004, 48, 1167–1184. [Google Scholar] [CrossRef]
- Elleithy, R.H.; Ali, I.; Ali, M.A.; Al-Zahrani, S.M. High Density Polyethylene/Micro Calcium Carbonate Composites: A Study of the Morphological, Thermal and Viscoelastic Properties. Appl. Polym. 2009, 117, 2413–2421. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.J. Shear Yield Behavior of Calcium Carbonate-Filled Polypropylene. Polym. Eng. Sci. 1999, 39, 190–198. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D.; Latteri, A.; Tosto, C.; Pergolizzi, E.; Recca, G.; Cristaudo, M.; Cicala, G. Influence of the Processing Conditions on the Mechanical Performance of Sustainable Bio-Based PLA Compounds. Polymers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Drabek, J.; Zatloukal, M.; Martyn, M. Effect of Molecular Weight, Branching and Temperature on Dynamics of Polypropylene Melts at Very High Shear Rates. Polymer 2018, 144, 179–183. [Google Scholar] [CrossRef]
- Toth, G.; Nagy, D.; Bata, A.; Belina, K. Determination of Polymer Melts Flow-Activation Energy a Function of Wide Range Shear Rate. J. Phys. Conf. Ser. 2018, 1045, 012040. [Google Scholar] [CrossRef]
- Collins, E.A.; Metzger, A.P. Polyvinylchloride Melt Rheology II—The Influence of Molecular Weight on Flow Activation Energy. Polym. Eng. Sci. 1970, 10, 57–65. [Google Scholar] [CrossRef]
- Saini, D.R.; Shenoy, A.V. A New Method for the Determination of Flow Activation Energy of Polymer Melts. J. Macromol. Sci. Part B 1983, 22, 437–449. [Google Scholar] [CrossRef]
- Singh, V.P.; Kumar, R.; Ashwith; Singh, P.; Samanta, S.; Banerjee, S. Melt Rheological Behaviour of High-Density Polyethylene/Montmorillonite Nanocomposites. Polym. Polym. Compos. 2021, 29, S511–S520. [Google Scholar] [CrossRef]



| Material | Density [g·cm−3] | Melt Flow Rate (MFR) [g/10 min] | Recommended Process Temperatures [°C] |
|---|---|---|---|
| PP-MP | 1.09 (23 °C) | N/A | 170–300 |
| PE-CP | 0.70 (25 °C) | N/A | 140–300 |
| PP | 0.905 (23 °C) | 23 a | N/A |
| Section | Size [cm] |
|---|---|
| Internal Radius | 1.65 |
| External Radius | 1.85 |
| β | 1.12 |
| Cylinder Length | 4.60 |
| Purging Compound | Injection Process | Temperature Profile | ||
|---|---|---|---|---|
| 220 °C | 240 °C | 260 °C | ||
| PE-CP | Injection time | 3.68 | 3.97 | 3.73 |
| Cycle | 33.15 | 29.65 | 28.10 | |
| PP-MP | Injection time | 3.53 | 3.57 | 3.62 |
| Cycle | 24.86 | 24.93 | 25.08 | |
| Zone | Purging Temperature Profiles [°C] | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | 220 | 240 | 260 |
| 2 | 220 | 240 | 260 |
| 3 | 200 | 200 | 200 |
| 4 | 190 | 190 | 190 |
| IR Peak [cm−1] | Functional Group |
|---|---|
| 3696/3621 | –NH2 stretching |
| 3434 | –OH stretching |
| 2919 | –CH3 asymmetric vibration |
| 1727 | C=O aliphatic carbonyl stretch |
| 1633 | –NH bending |
| 1413 | –OH bending |
| Material | Tm [°C] | Tc [°C] | ΔHm [J·g−1] | Xc [%] |
|---|---|---|---|---|
| PP-MP | 165.43 (131.02) | 125.33 | 45.26 (5.28) | 21.86 |
| PE-CP | 136.47 | 112.79 | 148.30 | 50.61 |
| Virgin PP | 164.68 | 134.17 | 114.10 | 55.12 |
| Temperature [°C] | PE-CP | PP-MP |
|---|---|---|
| 220 | 3.682 | 1.824 |
| 240 | 4.119 | 2.448 |
| 260 | 4.717 | 2.511 |
| Rotor Speed [rpm] | 220 °C | 240 °C | 260 °C | |||
|---|---|---|---|---|---|---|
| STME [kWh·kg−1] | Stabilized Torque [N·m] | STME [kWh·kg−1] | Stabilized Torque [N·m] | STME [kWh·kg−1] | Stabilized Torque [N·m] | |
| 10 | 0.03 | 4.81 | 0.03 | 3.57 | 0.02 | 3.18 |
| 30 | 0.11 | 4.67 | 0.09 | 4.26 | 0.08 | 3.89 |
| 50 | 0.19 | 4.75 | 0.16 | 4.41 | 0.15 | 3.94 |
| 70 | 0.25 | 4.50 | 0.22 | 4.26 | 0.20 | 3.78 |
| 90 | 0.31 | 4.20 | 0.27 | 3.16 | 0.26 | 2.94 |
| Rotor Speed [rpm] | 220 °C | 240 °C | 260 °C | |||
|---|---|---|---|---|---|---|
| STME [kWh·kg−1] | Stabilized Torque [N·m] | STME [kWh·kg−1] | Stabilized Torque [N·m] | STME [kWh·kg−1] | Stabilized Torque [N·m] | |
| 10 | 0.01 | 1.86 | 0.01 | 1.65 | 0.01 | 1.27 |
| 30 | 0.06 | 3.84 | 0.05 | 3.50 | 0.04 | 2.82 |
| 50 | 0.12 | 4.40 | 0.11 | 4.05 | 0.07 | 2.75 |
| 70 | 0.20 | 5.23 | 0.17 | 5.22 | 0.12 | 3.51 |
| 90 | 0.29 | 5.52 | 0.22 | 4.31 | 0.19 | 4.37 |
| Speed [rpm] | PP-MP | PE-CP |
|---|---|---|
| 10 | 18.35 | 33.13 |
| 30 | 12.17 | 19.23 |
| 50 | 9.16 | 25.22 |
| 70 | 6.56 | 26.94 |
| 90 | 10.35 | 19.83 |
| Purge | Injection Temperatures [°C] | ||
|---|---|---|---|
| 220 | 240 | 260 | |
| PE-CP | 18.48 | 19.20 | 14.74 |
| PP-MP | 13.26 | 13.30 | 13.38 |
| Purge | Control Factor | Degrees of Freedom | Contribution [%] | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|---|---|---|
| PE-CP | Temperature | 2 | 76.79 | 36.61 | 17.80 | 19.85 | 0.0002 |
| Error | 12 | 23.21 | 10.76 | 0.90 | |||
| Total | 14 | 100.00 | 46.37 | ||||
| PP-MP | Temperature | 2 | 14.44 | 0.037 | 0.018 | 1.01 | 0.3922 |
| Error | 12 | 85.56 | 0.22 | 0.018 | |||
| Total | 14 | 100.00 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, M.; Guerrero, J.; Lazo, M.; Adrián, E.; Medina-Perilla, J.A.; Rigail-Cedeño, A. Evaluation of Processing Conditions in the Performance of Purging Compounds for Polypropylene Injection Molding. J. Manuf. Mater. Process. 2023, 7, 31. https://doi.org/10.3390/jmmp7010031
Carrasco M, Guerrero J, Lazo M, Adrián E, Medina-Perilla JA, Rigail-Cedeño A. Evaluation of Processing Conditions in the Performance of Purging Compounds for Polypropylene Injection Molding. Journal of Manufacturing and Materials Processing. 2023; 7(1):31. https://doi.org/10.3390/jmmp7010031
Chicago/Turabian StyleCarrasco, Miguel, Jorge Guerrero, Miriam Lazo, Estephany Adrián, Jorge Alberto Medina-Perilla, and Andrés Rigail-Cedeño. 2023. "Evaluation of Processing Conditions in the Performance of Purging Compounds for Polypropylene Injection Molding" Journal of Manufacturing and Materials Processing 7, no. 1: 31. https://doi.org/10.3390/jmmp7010031
APA StyleCarrasco, M., Guerrero, J., Lazo, M., Adrián, E., Medina-Perilla, J. A., & Rigail-Cedeño, A. (2023). Evaluation of Processing Conditions in the Performance of Purging Compounds for Polypropylene Injection Molding. Journal of Manufacturing and Materials Processing, 7(1), 31. https://doi.org/10.3390/jmmp7010031









