Predicting Apple Tree Macronutrients Using Unmanned Aerial Vehicle-Based Hyperspectral Imagery to Manage Apple Orchard Nutrients
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Hyperspectral Image Acquisition and Processing
2.3. Leaf Macronutrients and Soil Chemical Properties
2.4. Analysis
2.4.1. Partial Least Squares Regression
2.4.2. Ridge Regression
2.4.3. k-Nearest Neighbors Regression
2.4.4. Support Vector Machine Regression
2.4.5. Gradient-Boosting Regression
2.4.6. Shapley Additive Explanation
3. Results
3.1. Reflectance Curve
3.2. Estimation Model with Full-band Ratios
3.3. Estimation Model with the Selection of Key Band Ratios
3.4. Optimized Estimation Model
3.5. Soil Chemical Properties
3.6. Estimated Macronutrients
4. Discussion
4.1. The Key Band Ratios of the Estimation Model
4.2. Fertilization Strategy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Effect of nitrogen fertilization of apple orchard on soil mineral nitrogen content, yielding of the apple trees and nutritional status of leaves and fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, A.; Kathpalia, R.; Bhatla, S.C. Plant mineral nutrition. In Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–81. [Google Scholar]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Integrative physiological, transcriptome, and metabolome analysis reveals the effects of nitrogen sufficiency and deficiency conditions in apple leaves and roots. Environ. Exp. Bot. 2021, 192, 104633. [Google Scholar] [CrossRef]
- Carranca, C.; Brunetto, G.; Tagliavini, M. Nitrogen nutrition of fruit trees to reconcile productivity and environmental concerns. Plants 2018, 7, 4. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Toivonen, P.; Herbert, L. Annual bloom-time phosphorus fertigation affects soil phosphorus, apple tree phosphorus nutrition, yield, and fruit quality. Hortscience 2008, 43, 885–890. [Google Scholar] [CrossRef]
- Taylor, B.K.; Goubran, F.H. The phosphorus nutrition of the apple tree. I. Influence of rate of application of superphosphate on the performance of young trees. Aust. J. Agric. Res. 1975, 26, 843–853. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M.; Bednarz, C.W. Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 2001, 39, 103–109. [Google Scholar] [CrossRef]
- Erner, Y.; Artzi, B.; Tagari, E.; Hamou, M. Potassium Affects Citrus Tree Performance; Volcani Center, Institute of Horticulture, Department of Fruit Trees: Tel Aviv, Israel, 2005; pp. 405–414. [Google Scholar]
- Pallas, B.; Da Silva, D.; Valsesia, P.; Yang, W.; Guillaume, O.; Lauri, P.E.; Vercambre, G.; Génard, M.; Costes, E. Simulation of carbon allocation and organ growth variability in apple tree by connecting architectural and source–sink models. Ann. Bot. 2016, 118, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Génard, M.; Dauzat, J.; Franck, N.; Lescourret, F.; Moitrier, N.; Vaast, P.; Vercambre, G. Carbon allocation in fruit trees: From theory to modelling. Trees 2008, 22, 269–282. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, F.; Ranwala, D. Nitrogen storage and its interaction with carbohydrates of young apple trees in response to nitrogen supply. Tree Physiol. 2004, 24, 91–98. [Google Scholar] [CrossRef]
- Noguera, M.; Aquino, A.; Ponce, J.M.; Cordeiro, A.; Silvestre, J.; Arias-Calderón, R.; Marcelo, M.E.; Jordão, P.; Andújar, J.M. Nutritional status assessment of olive crops by means of the analysis and modelling of multispectral images taken with UAVs. Biosyst. Eng. 2021, 211, 1–18. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Prakash, O. The Impact of Chemical Fertilizers on Our Environment and Ecosystem, Chapter 5; Chief Ed.; Uttar Pradesh: Varanasi, India, 2019; Volume 35, pp. 69–88. [Google Scholar]
- Gordon, T.R.; Swett, C.L.; Wingfield, M.J. Management of Fusarium diseases affecting conifers. Crop Prot. 2015, 73, 28–39. [Google Scholar] [CrossRef]
- Al-Busaidi, A. 5. Al-Busaidi, A. 5 fertilizer application. In Dates: Production, Processing, Food, and Medicinal Values; CRC Press: Boca Raton, FL, USA, 2012; pp. 67–80. [Google Scholar]
- López-Morales, J.A.; Martínez, J.A.; Skarmeta, A.F. Digital transformation of agriculture through the use of an interoperable platform. Sensors 2020, 20, 1153. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Martinez, P.; Ahmad, R. The digitization of agricultural industry—A systematic literature review on agriculture 4.0. Smart Agric. Technol. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Hoogenboom, G. Contribution of agrometeorology to the simulation of crop production and its applications. Agric. For. Meteorol. 2000, 103, 137–157. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Effect of nitrogen fertilization on tree growth and nutrient content in soil and cherry leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Paul, S.; Poliyapram, V.; Imamoglu, N.; Uto, K.; Nakamura, R.; Kumar, D.N. Canopy averaged chlorophyll content prediction of pear trees using convolutional autoencoder on hyperspectral data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 1426–1437. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Vescovo, L.; Wohlfahrt, G.; Balzarolo, M.; Pilloni, S.; Sottocornola, M.; Rodeghiero, M.; Gianelle, D. New spectral vegetation indices based on the near-infrared shoulder wavelengths for remote detection of grassland phytomass. Int. J. Remote Sens. 2012, 33, 2178–2195. [Google Scholar] [CrossRef] [PubMed]
- Colica, E. Geophysics and Geomatics Methods for Coastal Monitoring and Hazard Evaluation. Ph.D. Thesis, L-Università ta’ Malta, Msida, Malta, 2022. [Google Scholar]
- Wang, J.; Zhang, C.; Shi, Y.; Long, M.; Islam, F.; Yang, C.; Yang, S.; He, Y.; Zhou, W. Evaluation of quinclorac toxicity and alleviation by salicylic acid in rice seedlings using ground-based visible/near-infrared hyperspectral imaging. Plant Methods 2020, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Ryu, C.S.; Kim, S.H.; Jun, S.R.; Jang, S.H.; Park, J.W.; Song, H.Y. Yield prediction of Chinese cabbage (Brassicaceae) using broadband multispectral imagery mounted unmanned aerial system in the air and narrowband hyperspectral imagery on the ground. Korean Soc. Agric. Mach. 2018, 43, 138–147. [Google Scholar]
- Pandey, P.; Ge, Y.; Stoerger, V.; Schnable, J.C. High throughput in vivo analysis of plant leaf chemical properties using hyperspectral imaging. Front. Plant Sci. 2017, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, T.; Gao, M.; Li, Y.; Li, D.; Zhang, H. Hyperspectral inversion of nitrogen content in maize leaves based on different dimensionality reduction algorithms. Comput. Electron. Agric. 2021, 190, 106461. [Google Scholar] [CrossRef]
- Park, K.; Hong, Y.K.; Kim, G.H.; Lee, J. Classification of apple leaf conditions in hyper-spectral images for diagnosis of Marssonina blotch using mRMR and deep neural network. Comput. Electron. Agric. 2018, 148, 179–187. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Dai, H.; Xu, B.; Yang, H.; Feng, H.; Li, Z.; Yang, X. Modeling maize above-ground biomass based on machine learning approaches using UAV remote-sensing data. Plant Methods 2019, 15, 10. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Li, W.; Tang, X.; Yu, X.; Jiang, Y. Retrieval of nitrogen content in apple canopy based on unmanned aerial vehicle hyperspectral images using a modified correlation coefficient method. Sustainability 2022, 14, 1992. [Google Scholar] [CrossRef]
- Kang, Y.S.; Park, K.S.; Kim, E.R.; Jeong, J.C.; Ryu, C.S. Estimation of the total nonstructural carbohydrate concentration in apple trees using hyperspectral imaging. Horticulturae 2023, 9, 967. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, P.; Liu, H.; Fan, P.; Zeng, P.; Liu, X.; Feng, C.; Wang, W.; Yang, F. Gradient boosting estimation of the leaf area index of apple orchards in uav remote sensing. Remote Sens. 2021, 13, 3263. [Google Scholar] [CrossRef]
- Azadnia, R.; Rajabipour, A.; Jamshidi, B.; Omid, M. New approach for rapid estimation of leaf nitrogen, phosphorus, and potassium contents in apple-trees using Vis/NIR spectroscopy based on wavelength selection coupled with machine learning. Comput. Electron. Agric. 2023, 207, 107746. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, Q.; Chen, Y.; Liu, Y.; Jiang, D.; Zhang, Z. Hyperspectral estimation of chlorophyll content in apple tree leaf based on feature band selection and the CatBoost model. Agronomy 2023, 13, 2075. [Google Scholar] [CrossRef]
- Costa, L.; Kunwar, S.; Ampatzidis, Y.; Albrecht, U. Determining leaf nutrient concentrations in citrus trees using UAV imagery and machine learning. Precis. Agric. 2022, 23, 854–875. [Google Scholar] [CrossRef]
- Acosta, M.; Rodríguez-Carretero, I.; Blasco, J.; de Paz, J.M.; Quiñones, A. Nondestructive appraisal of macro- and micronutrients in persimmon leaves using Vis/NIR hyperspectral imaging. Agriculture 2023, 13, 916. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.; Cho, J.; Jung, J.; Lee, S.; Lee, D.; Kim, J. Estimation of Apple Leaf Nitrogen Concentration Using Hyperspectral Imaging-Based Wavelength Selection and Machine Learning. Horticulturae 2023, 10, 35. [Google Scholar] [CrossRef]
- Kang, Y.S.; Jang, S.H.; Park, J.W.; Song, H.Y.; Ryu, C.S.; Jun, S.R.; Kim, S.H. Yield prediction and validation of onion (Allium cepa L.) using key variables in narrowband hyperspectral imagery and effective accumulated temperature. Comput. Electron. Agric. 2020, 178, 105667. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Applications to nonorthogonal problems. Technometrics 1970, 12, 69–82. [Google Scholar] [CrossRef]
- Burba, F.; Ferraty, F.; Vieu, P. k -Nearest Neighbour method in functional nonparametric regression. J. Nonparametric Stat. 2009, 21, 453–469. [Google Scholar] [CrossRef]
- Ge, H.; Ma, F.; Li, Z.; Tan, Z.; Du, C. Improved accuracy of phenological detection in rice breeding by using ensemble models of machine learning based on UAV-RGB imagery. Remote Sens. 2021, 13, 2678. [Google Scholar] [CrossRef]
- Jakkula, V. Tutorial on support vector machine (svm). Sch. EECS Wash. State Univ. 2006, 37, 3. [Google Scholar]
- Huang, C.L.; Wang, C.J. A GA-based feature selection and parameters optimization for support vector machines. Expert Syst. Appl. 2006, 31, 231–240. [Google Scholar] [CrossRef]
- Prodhan, F.A.; Zhang, J.; Yao, F.; Shi, L.; Pangali Sharma, T.P.; Zhang, D.; Cao, D.; Zheng, M.; Ahmed, N.; Mohana, H.P. Deep learning for monitoring agricultural drought in South Asia using remote sensing data. Remote Sens. 2021, 13, 1715. [Google Scholar] [CrossRef]
- Arumugam, P.; Chemura, A.; Schauberger, B.; Gornott, C. Remote sensing based yield estimation of Rice (Oryza sativa L.) using gradient boosted regression in India. Remote Sens. 2021, 13, 2379. [Google Scholar] [CrossRef]
- Kannangara, K.K.P.M.; Zhou, W.; Ding, Z.; Hong, Z. Investigation of feature contribution to shield tunneling-induced settlement using Shapley additive explanations method. J. Rock Mech. Geotech. Eng. 2022, 14, 1052–1063. [Google Scholar] [CrossRef]
- Marcílio, W.E.; Eler, D.M. From explanations to feature selection: Assessing SHAP values as feature selection mechanism. In Proceedings of the 33rd SIBGRAPI Conference on Graphics, Patterns and Images (SIBGRAPI), Porto de Galinhas, Brazil, 7−10 November 2020. [Google Scholar]
- Zhang, Y.; Gao, J.; Cen, H.; Lu, Y.; Yu, X.; He, Y.; Pieters, J.G. Automated spectral feature extraction from hyperspectral images to differentiate weedy rice and barnyard grass from a rice crop. Comput. Electron. Agric. 2019, 159, 42–49. [Google Scholar] [CrossRef]
- Sánchez, E.E.; Giayetto, A.; Cichón, L.; Fernández, D.; Aruani, M.C.; Curetti, M. Cover crops influence soil properties and tree performance in an organic apple (Malus domestica Borkh) orchard in northern Patagonia. Plant Soil 2007, 292, 193–203. [Google Scholar] [CrossRef]
- Laurén, A.; Lappalainen, M.; Kieloaho, A.J.; Karhu, K.; Palviainen, M. Temperature sensitivity patterns of carbon and nitrogen processes in decomposition of boreal organic soils—Quantification in different compounds and molecule sizes based on a multifactorial experiment. PLoS ONE 2019, 14, e0223446. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A., Jr.; Genter, C.F.; Hawkins, G.W. Effect of soil pH on element concentration and uptake by maize: I. P, K, Mg, and Na 1. Agron. J. 1972, 64, 581–583. [Google Scholar] [CrossRef]
- Agboola, A.A.; Corey, R.B. The relationship between soil pH, organic matter, available phosphorus, exchangeable potassium, calcium, magnesium, and nine elements in the maize tissue. Soil Sci. 1973, 115, 367–375. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Acta Ecol. Sin. 2001, 31, 212–216. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium regulates growth and nutrient absorption in poplar seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef]
- Haboudane, D.; Tremblay, N.; Miller, J.R.; Vigneault, P. Remote estimation of crop chlorophyll content using spectral indices derived from hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2008, 46, 423–437. [Google Scholar] [CrossRef]
- Amirruddin, A.D.; Muharam, F.M.; Ismail, M.H.; Tan, N.P.; Ismail, M.F. Hyperspectral spectroscopy and imbalance data approaches for classification of oil palm’s macronutrients observed from frond 9 and 17. Comput. Electron. Agric. 2020, 178, 105768. [Google Scholar] [CrossRef]
- Mohd Asaari, M.S.M.; Mishra, P.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. Close-range hyperspectral image analysis for the early detection of stress responses in individual plants in a high-throughput phenotyping platform. ISPRS J. Photogramm. Remote Sens. 2018, 138, 121–138. [Google Scholar] [CrossRef]
- Khoi, C.M. Management of Chaetoceros Calcitrans Growth in Hypersaline Artemia Franciscana Ponds by Optimizing Nitrogen and Phosphorus Availability. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2006. [Google Scholar]
- Chang-Hua, J.U.; Yong-Chao, T.I.A.N.; Xia, Y.A.O.; Wei-Xing, C.A.O.; Yan, Z.H.U.; Hannaway, D. Estimating leaf chlorophyll content using red edge parameters. Pedosphere 2010, 20, 633–644. [Google Scholar]
- Ennab, H. Effect of organic manures, biofertilizers and NPK on vegetative growth, yield, fruit quality and soil fertility of eureka lemon trees (Citrus limon (L.) Burm). J. Soil Sci. Agric. Eng. 2016, 7, 767–774. [Google Scholar] [CrossRef]
- Ullah, H.; Santiago-Arenas, R.; Ferdous, Z.; Attia, A.; Datta, A. Improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: A review. Adv. Agron. 2019, 156, 109–157. [Google Scholar]
- Aminifard, M.H.; Aroiee, H.; Nemati, H.; Azizi, M.; Khayyat, M. Effect of nitrogen fertilizer on vegetative and reproductive growth of pepper plants under field conditions. J. Plant Nutr. 2012, 35, 235–242. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2021; pp. 135–189. [Google Scholar]
- Vashisth, T.; Kadyampakeni, D. Diagnosis and management of nutrient constraints in citrus. In Fruit Crops; Elsevier: New Dehli, India, 2020; pp. 723–737. [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, A. Mechanisms of nitrogen use in maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]


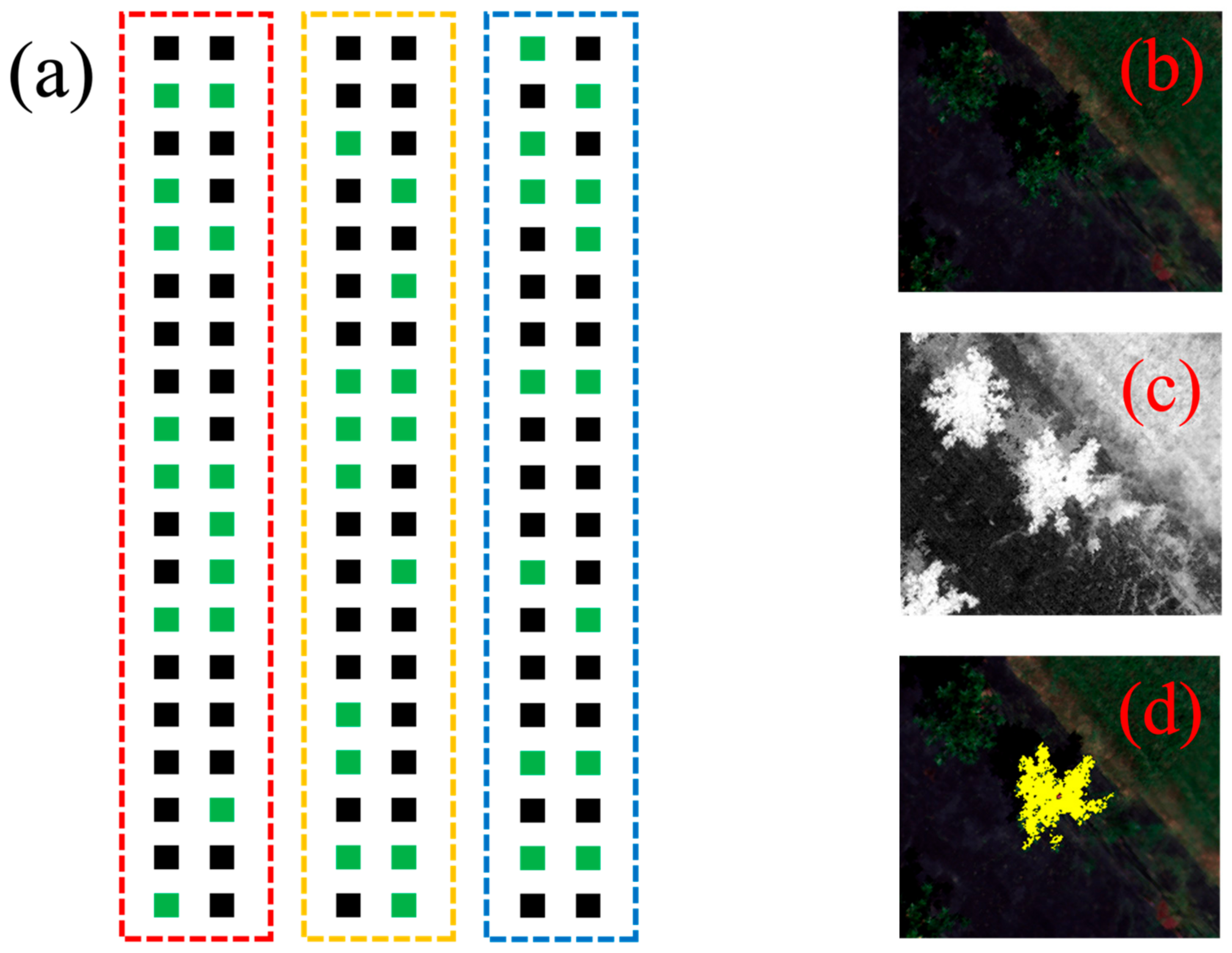


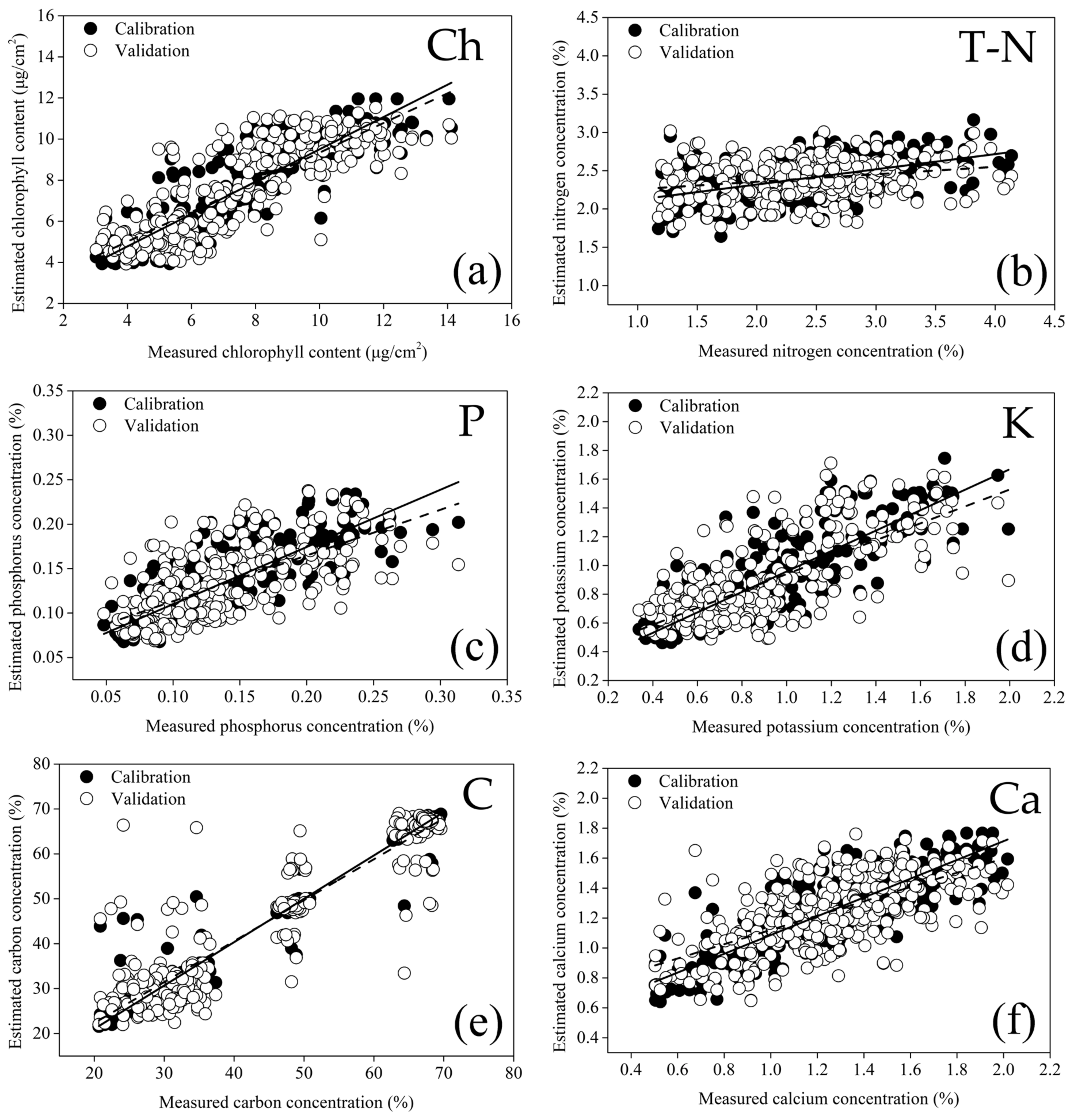
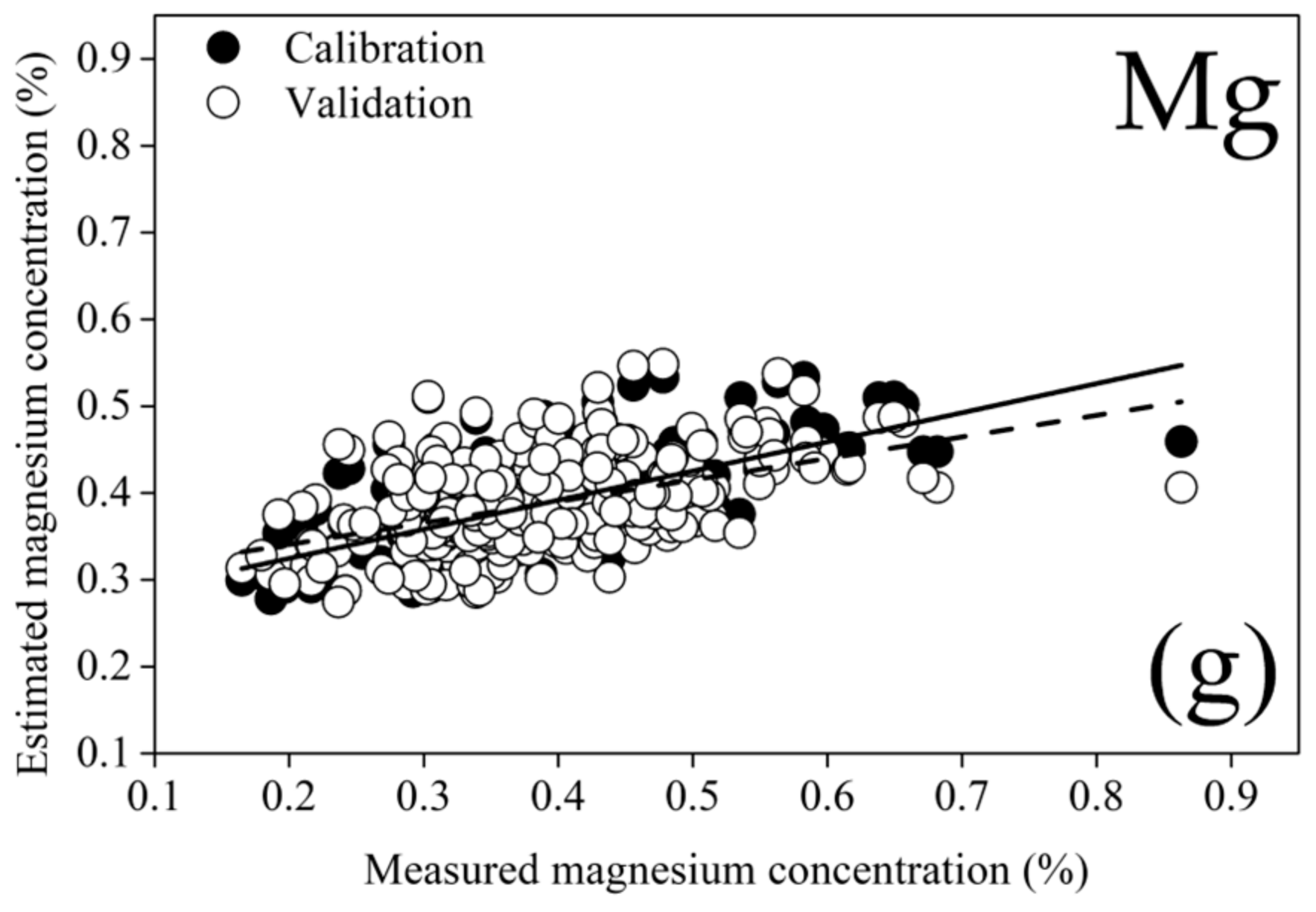
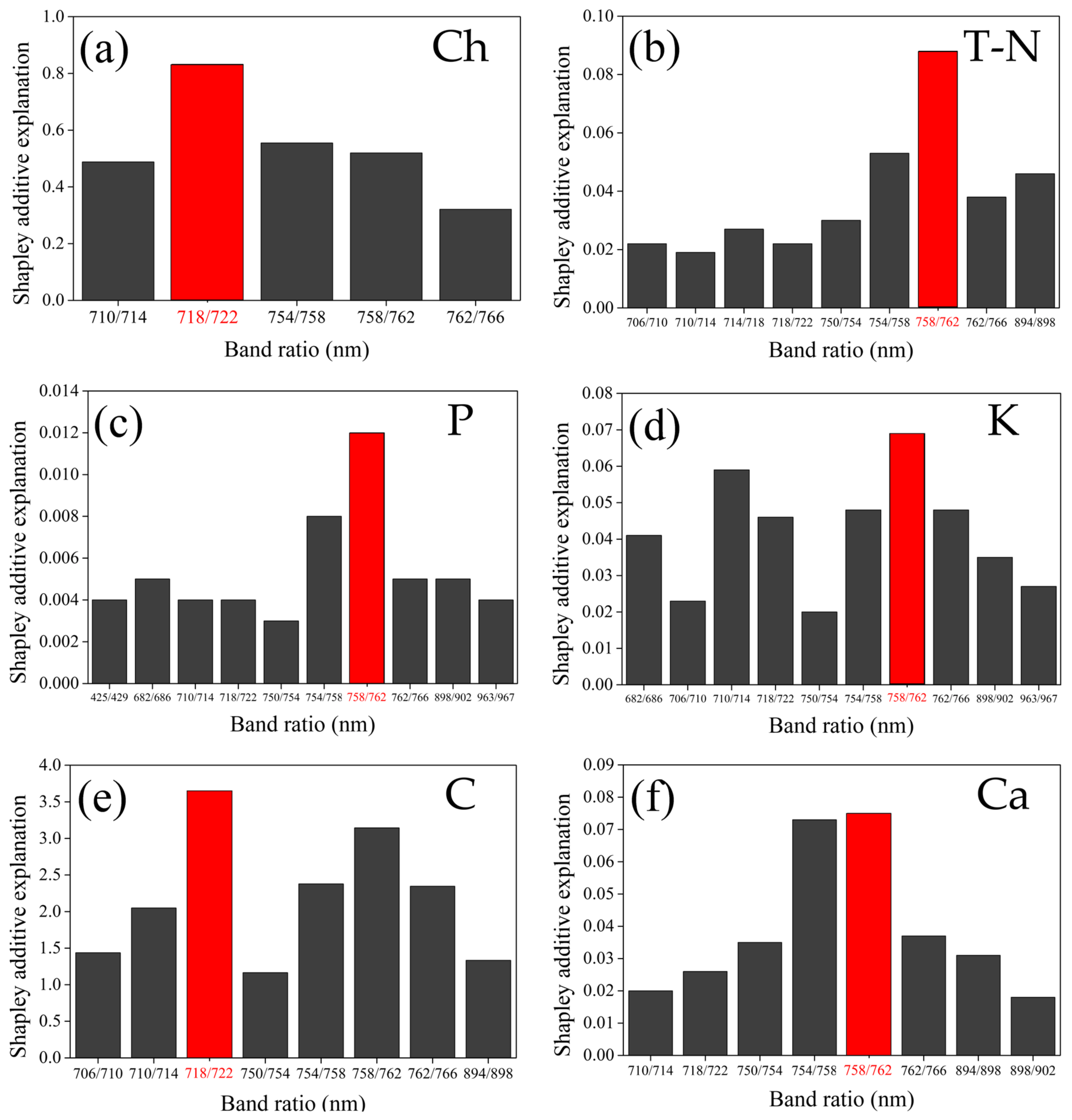

| 2021 | 2022 | All Years | ||||
|---|---|---|---|---|---|---|
| n 1 | Mean ± S.D. 2 | n | Mean ± S.D. | n | Mean ± S.D. | |
| Ch (μg/cm2) | 147 | 5.03 ± 1.12 | 182 | 9.22 ± 1.85 | 329 | 7.35 ± 2.61 |
| T-N (%) | 175 | 2.30 ± 1.74 | 182 | 2.49 ± 0.46 | 357 | 2.40 ± 0.62 |
| P (%) | 0.12 ± 0.04 | 0.14 ± 0.05 | 0.13 ± 0.05 | |||
| K (%) | 0.67 ± 0.16 | 1.02 ± 0.37 | 0.85 ± 0.34 | |||
| C (%) | 29.8 ± 4.06 | 58.8 ± 9.02 | 44.6 ± 16.1 | |||
| Ca (%) | 1.17 ± 0.34 | 1.32 ± 0.30 | 1.25 ± 0.33 | |||
| Mg (%) | 0.36 ± 0.08 | 0.32 ± 0.10 | 0.39 ± 0.09 | |||
| 2021 | 2022 | All Years | |||||
|---|---|---|---|---|---|---|---|
| Calibration | Validation | Calibration | Validation | Calibration | Validation | ||
| Ch | PLSR | 0.51 | 0.20 | 0.36 | 0.11 | 0.72 | 0.46 |
| RR | 0.44 | 0.13 | 0.45 | 0.13 | 0.78 | 0.46 | |
| KNR | 0.39 | 0.06 | 0.63 | 0.35 | 0.82 | 0.72 | |
| SVR | 0.40 | 0.32 | 0.42 | 0.35 | 0.75 | 0.73 | |
| GBR | 0.45 | 0.12 | 0.99 | 0.21 | 0.99 | 0.52 | |
| T-N | PLSR | 0.10 | N/A | 0.39 | 0.12 | 0.17 | 0.02 |
| RR | 0.09 | N/A | 0.42 | 0.18 | 0.30 | 0.06 | |
| KNR | 0.09 | N/A | 0.37 | 0.21 | 0.18 | N/A | |
| SVR | 0.23 | 0.10 | 0.47 | 0.33 | 0.28 | 0.18 | |
| GBR | 0.01 | N/A | 0.96 | 0.16 | 0.05 | N/A | |
| P | PLSR | 0.47 | 0.04 | 0.78 | N/A | 0.16 | N/A |
| RR | 0.67 | 0.04 | 0.87 | N/A | 0.24 | N/A | |
| KNR | 0.47 | 0.30 | 0.75 | 0.64 | 0.65 | 0.48 | |
| SVR | N/A | N/A | N/A | N/A | N/A | N/A | |
| GBR | 0.58 | 0.03 | 0.99 | N/A | 0.56 | N/A | |
| K | PLSR | 0.44 | N/A | 0.81 | 0.30 | 0.67 | 0.16 |
| RR | 0.40 | 0.02 | 0.86 | 0.01 | 0.61 | 0.12 | |
| KNR | 0.23 | 0.02 | 0.72 | 0.61 | 0.70 | 0.58 | |
| SVR | 0.37 | 0.22 | 0.70 | 0.62 | 0.70 | 0.62 | |
| GBR | N/A | N/A | 0.12 | N/A | 0.98 | 0.05 | |
| C | PLSR | 0.80 | N/A | 0.96 | 0.18 | 0.76 | 0.39 |
| RR | 0.17 | N/A | 0.98 | 0.06 | 0.80 | 0.27 | |
| KNR | 0.49 | 0.23 | 0.99 | 0.95 | 0.99 | 0.90 | |
| SVR | 0.23 | 0.15 | 0.42 | 0.39 | 0.63 | 0.61 | |
| GBR | 0.09 | N/A | 0.99 | N/A | 0.99 | 0.39 | |
| Ca | PLSR | 0.52 | N/A | 0.56 | N/A | 0.59 | N/A |
| RR | 0.75 | N/A | 0.61 | N/A | 0.67 | N/A | |
| KNR | 0.77 | 0.54 | 0.57 | 0.43 | 0.77 | 0.50 | |
| SVR | 0.66 | 0.46 | 0.54 | 0.43 | 0.60 | 0.49 | |
| GBR | 0.99 | N/A | 0.11 | N/A | 0.28 | N/A | |
| Mg | PLSR | 0.43 | N/A | 0.15 | N/A | 0.49 | N/A |
| RR | 0.42 | N/A | 0.52 | N/A | 0.57 | 0.06 | |
| KNR | 0.48 | 0.27 | 0.34 | 0.15 | 0.47 | 0.24 | |
| SVR | 0.39 | 0.24 | 0.38 | 0.26 | 0.41 | 0.27 | |
| GBR | 0.99 | N/A | 0.09 | N/A | 0.82 | N/A | |
| n 1 | Ch | N | P | K | C | Ca | Mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal | Val | Cal | Val | Cal | Val | Cal | Val | Cal | Val | Cal | Val | Cal | Val | ||
| KNR | 10 | 0.82 | 0.73 | 0.19 | 0.01 | 0.66 | 0.44 | 0.73 | 0.53 | 0.97 | 0.88 | 0.63 | 0.43 | 0.37 | 0.21 |
| 9 | 0.82 | 0.73 | 0.22 | 0.04 | 0.65 | 0.39 | 0.75 | 0.48 | 0.96 | 0.87 | 0.66 | 0.41 | 0.43 | 0.19 | |
| 8 | 0.82 | 0.73 | 0.20 | 0.03 | 0.57 | 0.36 | 0.75 | 0.45 | 0.96 | 0.85 | 0.66 | 0.42 | 0.43 | 0.18 | |
| 7 | 0.82 | 0.72 | 0.24 | 0.03 | 0.55 | 0.32 | 0.75 | 0.44 | 0.89 | 0.83 | 0.60 | 0.39 | 0.43 | 0.19 | |
| 6 | 0.81 | 0.71 | 0.20 | 0.01 | 0.49 | 0.28 | 0.75 | 0.45 | 0.95 | 0.84 | 0.58 | 0.36 | 0.32 | 0.17 | |
| 5 | 0.802 | 0.70 | 0.18 | N/A | 0.40 | 0.26 | 0.84 | 0.45 | 0.92 | 0.83 | 0.56 | 0.36 | 0.41 | 0.16 | |
| 4 | 0.80 | 0.67 | 0.17 | N/A | 0.43 | 0.23 | 0.75 | 0.45 | 0.91 | 0.82 | 0.52 | 0.34 | 0.37 | 0.15 | |
| 3 | 0.79 | 0.65 | 0.17 | N/A | 0.47 | 0.19 | 0.72 | 0.39 | 0.81 | 0.66 | 0.45 | 0.29 | 0.31 | 0.11 | |
| 2 | 0.53 | 0.33 | 0.21 | 0.01 | 0.34 | 0.19 | 0.38 | 0.22 | 0.82 | 0.60 | 0.39 | 0.24 | 0.26 | 0.08 | |
| SVR | 10 | 0.71 | 0.70 | 0.17 | 0.14 | N/A | N/A | 0.42 | 0.41 | 0.53 | 0.52 | 0.31 | 0.29 | 0.23 | 0.19 |
| 9 | 0.70 | 0.70 | 0.17 | 0.13 | N/A | N/A | 0.40 | 0.38 | 0.49 | 0.48 | 0.30 | 0.27 | 0.23 | 0.20 | |
| 8 | 0.69 | 0.68 | 0.13 | 0.11 | N/A | N/A | 0.39 | 0.37 | 0.47 | 0.47 | 0.26 | 0.24 | 0.24 | 0.19 | |
| 7 | 0.69 | 0.68 | 0.14 | 0.12 | N/A | N/A | 0.39 | 0.38 | 0.46 | 0.45 | 0.24 | 0.22 | 0.25 | 0.23 | |
| 6 | 0.68 | 0.67 | 0.13 | 0.11 | N/A | N/A | 0.29 | 0.28 | 0.43 | 0.41 | 0.14 | 0.13 | 0.24 | 0.21 | |
| 5 | 0.67 | 0.66 | 0.11 | 0.10 | N/A | N/A | 0.28 | 0.27 | 0.41 | 0.40 | 0.13 | 0.12 | 0.06 | 0.05 | |
| 4 | 0.63 | 0.62 | 0.10 | 0.09 | N/A | N/A | 0.24 | 0.23 | 0.40 | 0.40 | 0.08 | 0.07 | 0.05 | 0.04 | |
| 3 | 0.45 | 0.44 | 0.10 | 0.09 | N/A | N/A | 0.23 | 0.22 | 0.37 | 0.36 | 0.07 | 0.07 | 0.04 | 0.03 | |
| 2 | 0.44 | 0.43 | 0.05 | 0.04 | N/A | N/A | 0.02 | 0.01 | 0.32 | 0.32 | 0.06 | 0.06 | 0.01 | 0.01 | |
| n 1 | Ch | N | P | K | C | Ca | Mg | |
|---|---|---|---|---|---|---|---|---|
| KNR | 1 | 710/714 | 706/710 | 425/429 | 682/686 | 706/710 | 710/714 | 710/714 |
| 2 | 718/722 | 710/714 | 682/686 | 706/710 | 710/714 | 718/722 | 714/718 | |
| 3 | 754/758 | 714/718 | 710/714 | 710/714 | 718/722 | 750/754 | 718/722 | |
| 4 | 758/762 | 718/722 | 718/722 | 718/722 | 750/754 | 754/758 | 750/754 | |
| 5 | 762/766 | 750/754 | 750/754 | 750/754 | 754/758 | 758/762 | 754/758 | |
| 6 | 754/758 | 754/758 | 754/758 | 758/762 | 762/766 | 758/762 | ||
| 7 | 758/762 | 758/762 | 758/762 | 762/766 | 894/898 | 762/766 | ||
| 8 | 762/766 | 762/766 | 762/766 | 894/898 | 898/902 | 894/898 | ||
| 9 | 894/898 | 898/902 | 898/902 | 898/902 | ||||
| 10 | 963/967 | 963/967 | 906/911 | |||||
| n_neighbors | 5 | 9 | 4 | 4 | 2 | 4 | 9 |
| Nutrient | Variables | Algorithm | Performance |
|---|---|---|---|
| Ch | Calibration | R2 | 0.81 |
| RMSE (μg/cm2) | 1.15 | ||
| RE (%) | 15.6 | ||
| Validation | R2 | 0.70 | |
| RMSE (μg/cm2) | 1.43 | ||
| RE (%) | 19.5 | ||
| N | Calibration | R2 | 0.22 |
| RMSE (%) | 0.55 | ||
| RE (%) | 22.8 | ||
| Validation | R2 | 0.04 | |
| RMSE (%) | 0.61 | ||
| RE (%) | 25.3 | ||
| P | Calibration | R2 | 0.66 |
| RMSE (%) | 0.03 | ||
| RE (%) | 21.8 | ||
| Validation | R2 | 0.44 | |
| RMSE (%) | 0.04 | ||
| RE (%) | 28.2 | ||
| K | Calibration | R2 | 0.73 |
| RMSE (%) | 0.18 | ||
| RE (%) | 20.9 | ||
| Validation | R2 | 0.53 | |
| RMSE (%) | 0.23 | ||
| RE (%) | 27.6 | ||
| C | Calibration | R2 | 0.96 |
| RMSE (%) | 3.31 | ||
| RE (%) | 7.42 | ||
| Validation | R2 | 0.85 | |
| RMSE (%) | 6.18 | ||
| RE (%) | 13.9 | ||
| Ca | Calibration | R2 | 0.66 |
| RMSE (%) | 0.19 | ||
| RE (%) | 15.2 | ||
| Validation | R2 | 0.42 | |
| RMSE (%) | 0.25 | ||
| RE (%) | 20.0 | ||
| Mg | Calibration | R2 | 0.37 |
| RMSE (%) | 0.07 | ||
| RE (%) | 18.8 | ||
| Validation | R2 | 0.21 | |
| RMSE (%) | 0.08 | ||
| RE (%) | 21.0 |
| 2021 | 9 June | 22 June | 13 July | 28 July | 11 August | 31 August | 15 September | 30 September | 15 October | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH (1:5) | E 1 | a 0.15 E 2 | a 0.13 D | a 0.16 B | a 0.17 A | a 0.31 C | a 0.25 AB | a 0.15 AB | a 0.14 C | a 0.18 B |
| M | b 0.11 D | b 0.11 D | b 0.31 BC | b 0.09 B | b 0.42 AB | b 0.67 BCD | b 0.14 A | b 0.08 C | b 0.12 BC | |
| U | c 0.10 BC | c 0.13 BC | c 0.10 BC | c 0.13 CD | c 0.47 AB | b 0.34 A | c 0.06 D | c 0.15 E | c 0.17 BCDE | |
| All | 0.37 D | 0.52 CD | 0.87 BD | 1.00 AB | 0.68 AB | 0.99 AB | 0.95 AB | 0.88 BD | 0.93 BC | |
| OM (%) | E | a 0.21 AB | a2.66 0.21 A | b2.85 0.52 ABC | b3.19 0.13 C | c2.92 0.17 B | b2.92 0.15 B | a2.69 0.30 AB | a2.84 0.38 AB | a3.05 0.78 ABC |
| M | a 0.14 C | a2.61 0.18 B | a2.38 0.22 A | a2.78 0.26 BC | b2.69 0.12 BC | a2.72 0.17 BC | a2.81 0.17 C | a2.73 0.16 BC | a2.86 0.20 C | |
| U | a2.90 0.37 C | a2.71 0.23 BC | ab2.58 0.14 B | ab3.04 0.30 CD | a2.39 0.21 A | ab2.82 0.30 CD | a2.97 0.62 BC | a2.86 0.21 CD | a3.05 0.25 CD | |
| All | 0.25 DE | 2.66 0.21 AB | 2.60 0.38 A | 3.00 0.29 E | 2.67 0.28 ABC | 2.82 0.22 D | 2.82 0.41 BDE | 2.81 0.27 CD | 2.99 0.48 DE | |
| T-N (%) | E | a 0.01 C | b0.12 0.01 D | b0.05 0.01 A | b0.06 0.01 AB | b0.05 0.03 A | b0.16 0.02 F | c0.13 0.01 E | b0.07 0.04 ABC | b0.08 0.04 BC |
| M | a 0.01 E | a0.08 0.01 E | a0.02 0.01 A | a0.03 0.02 AB | a0.02 0.02 A | a0.04 0.01 BC | b0.06 0.01 D | b0.07 0.03 CDE | b0.07 0.03 CDE | |
| U | a 0.02 C | a0.08 0.02 C | a0.01 0.01 A | a0.02 0.02 A | a0.01 0.01 A | a0.04 0.01 B | a0.04 0.01 B | a0.04 0.01 B | a0.04 0.01 B | |
| All | 0.01 C | 0.09 0.02 D | 0.03 0.02 A | 0.04 0.02 A | 0.03 0.03 A | 0.08 0.06 CD | 0.08 0.04 CD | 0.06 0.03 B | 0.06 0.03 B | |
| P2O5 (mg/kg) | E | a 4.06 B | b25.5 0.64 A | b88.3 5.43 E | c76.9 2.98 D | b80.1 5.41 D | c35.1 5.46 B | c39.9 10.1 BC | c45.0 7.39 C | c45.5 4.93 C |
| M | b 4.72 E | b28.6 5.08 D | a71.7 4.38 H | a61.8 2.72 G | a58.5 3.26 F | a7.97 1.36 A | b13.8 5.15 BC | b15.8 1.32 C | b11.7 2.32 B | |
| U | a 3.72 C | a19.8 7.78 A | a71.8 6.70 E | b66.9 5.78 E | a59.7 6.84 D | b13.8 1.04 B | a9.09 1.59 B | a13.0 3.08 B | a5.31 0.76 AB | |
| All | 4.58 C | 24.6 6.37 B | 77.3 9.58 E | 68.5 7.52 D | 66.1 11.3 D | 19.0 12.3 A | 20.9 15.2 AB | 24.6 15.4 AB | 20.8 18.2 AB | |
| K (cmol/kg) | E | a 0.03 G | a0.19 0.02 F | b0.24 0.03 G | a0.16 0.01 DE | a0.17 0.01 E | a0.13 0.03 BC | a0.09 0.02 A | a0.10 0.03 AB | a0.15 0.02 CD |
| M | a 0.03 E | a0.19 0.03 DE | a0.21 0.03 E | ab0.18 0.03 DE | a0.19 0.07 CDE | a0.12 0.02 A | b0.14 0.03 AB | b0.15 0.01 BC | b0.18 0.03 D | |
| U | a 0.02 C | a0.20 0.04 BC | a0.20 0.03 BC | b0.20 0.04 BC | b0.25 0.03 D | b0.17 0.02 B | b0.14 0.02 A | b0.15 0.03 A | a0.14 0.01 A | |
| All | 0.03 E | 0.20 0.03 DE | 0.21 0.03 E | 0.18 0.03 D | 0.20 0.05 DE | 0.14 0.03 BC | 0.12 0.03 A | 0.13 0.03 AB | 0.16 0.02 C | |
| Ca (cmol/kg) | E | a 0.26 D | a6.59 0.49 E | a5.66 0.49 CD | a5.23 0.56 C | a4.71 1.54 BC | a4.00 0.13 B | a4.84 1.48 BC | a6.01 0.35 D | a3.50 0.22 A |
| M | b 0.32 C | b7.19 0.13 D | b7.86 0.64 E | b6.80 0.43 C | ab5.84 1.94 BCD | b7.79 0.92 DE | a3.98 0.66 A | a5.04 1.42 AB | b6.39 0.34 C | |
| U | b 0.47 BC | c8.46 1.11 DE | b8.34 0.76 E | c8.18 0.56 E | b6.36 0.67 A | b7.63 0.47 CD | b7.90 1.12 CDE | b7.27 0.35 BC | c7.13 0.54 AB | |
| All | 0.61 C | 7.41 1.04 E | 7.29 1.34 DE | 6.74 1.33 BCD | 5.64 1.59 A | 6.47 1.88 AC | 5.58 2.03 A | 6.10 1.25 AB | 5.67 1.64 A | |
| Mg (cmol/kg) | E | a 0.12 G | a1.85 0.09 F | a1.28 0.13 DE | a1.34 0.10 E | a0.97 0.42 C | a0.55 0.03 A | a0.60 0.05 B | a0.66 0.12 BC | a0.61 0.09 AB |
| M | b 0.13 F | b2.45 0.14 E | b2.27 0.16 D | b2.02 0.09 BC | a1.39 0.91 AB | b1.99 0.48 BCD | b1.53 0.24 A | b1.80 0.23 B | b1.79 0.20 AB | |
| U | b 0.12 B | c2.69 0.24 BC | c2.70 0.07 B | c2.88 0.21 C | b2.35 0.63 AB | b2.05 0.24 A | c2.23 0.11 A | c2.61 0.13 B | c2.19 0.31 A | |
| All | 0.12 C | 2.33 0.40 B | 2.08 0.61 B | 2.08 0.66 B | 1.57 0.88 A | 1.53 0.77 A | 1.45 0.69 A | 1.69 0.83 A | 1.53 0.71 A | |
| 2022 | 23 May | 3 June | 17 June | 4 July | 19 July | 28 July | 16 August | 7 September | 21 September | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH (1:5) | E 1 | a 0.23 E 2 | a 0.08 B | a 0.05 E | a 0.12 A | a 0.21 AB | a 0.15 A | a 0.10 C | a 0.09 D | a 0.18 F |
| M | b 0.24 CD | b 0.30 C | b 0.53 BC | b 0.13 A | b 0.10 A | b 0.28 A | b 0.15 B | b 0.35 B | a 0.10 D | |
| U | b 0.18 A | b 0.53 ABC | c 0.11 C | c 0.09 C | c 0.22 AB | c 0.04 BC | c 0.35 AB | c 0.16 C | b 0.45 D | |
| All | 0.49 B | 0.83 AB | 0.70 B | 0.90 A | 0.68 A | 0.85 A | 0.56 A | 0.63 AB | 0.38 C | |
| OM (%) | E | b 0.23 C | a2.72 0.16 A | c2.92 0.16 B | b2.72 0.12 A | b3.03 0.21 ABC | a3.14 0.15 C | b3.18 0.10 C | a2.81 0.09 AB | b2.82 0.18 AB |
| M | a2.67 0.09 B | a2.74 0.09 BC | a2.51 0.18 AB | a2.54 0.13 A | b2.71 0.10 BCD | a2.94 0.28 CD | ab2.94 0.15 ABCD | a2.84 0.35 D | b2.88 0.10 BCD | |
| U | a2.62 0.35 ABC | a2.64 0.15 BC | b2.75 0.11 C | ab2.60 0.09 B | a2.39 0.22 AB | a3.15 0.04 D | a2.93 0.35 CD | a2.90 0.16 BCD | a2.42 0.45 A | |
| All | 2.84 0.37 BDE | 2.70 0.14 AB | 2.73 0.22 AB | 2.62 0.16 A | 2.71 0.39 ABC | 3.07 0.38 F | 3.02 0.34 DF | 2.85 0.11 CE | 2.70 0.36 AB | |
| T-N (%) | E | c0.10 0.01 C | c0.12 0.04 CD | c0.10 0.02 BC | c0.18 0.02 E | b0.15 0.02 D | b0.47 0.11 F | b0.09 0.01 B | ab0.07 0.01 A | b0.43 0.21 F |
| M | a 0.01 A | b0.05 0.01 AB | b0.04 0.01 A | b0.08 0.01 C | a0.12 0.03 D | ab0.28 0.27 D | ab0.07 0.03 AC | a0.06 0.01 BC | ab0.24 0.51 ABCD | |
| U | b 0.01 B | a0.03 0.01 A | a0.02 0.01 A | a0.06 0.01 B | a0.09 0.01 D | a0.14 0.03 D | a0.06 0.02 B | b0.08 0.01 C | a0.13 0.08 CD | |
| All | 0.03 AB | 0.07 0.04 AB | 0.05 0.04 A | 0.10 0.06 C | 0.12 0.03 C | 0.30 0.21 D | 0.07 0.02 B | 0.07 0.01 B | 0.27 0.34 D | |
| P2O5 (mg/kg) | E | b 42.9 E | b92.6 36.7 D | b16.4 1.55 A | c39.2 11.5 C | c110 36.0 DE | a77.7 30.4 D | b34.7 6.15 C | b25.0 7.01 B | c477 328 F |
| M | a 17.8 E | a38.9 28.8 BE | b16.1 6.11 BC | b18.6 2.17 B | b49.7 9.61 DE | a71.7 7.53 E | ab27.4 14.9 BD | a14.4 0.33 AB | a9.40 10.4 AC | |
| U | a 30.6 D | a34.8 7.26 C | a8.99 0.71 A | a12.3 2.27 ABC | a69.1 8.93 D | b231 108 E | a19.5 2.14 B | a14.6 1.00 B | b128 139 DE | |
| All | 44.4 E | 56.6 38.8 D | 13.8 4.96 A | 23.4 14.5 BC | 76.3 33.3 D | 127 97.6 E | 27.2 11.0 C | 18.0 6.41 B | 205 282 E | |
| K (cmol/kg) | E | b 0.18 D | a0.17 0.02 B | a0.07 0.04 A | b0.18 0.04 BC | a0.19 0.02 C | a0.17 0.02 B | a0.18 0.02 BC | a0.11 0.04 A | c0.82 0.55 D |
| M | a 0.12 C | a0.17 0.04 BC | a0.06 0.02 A | a0.14 0.03 B | a0.19 0.03 C | b0.23 0.04 C | a0.19 0.04 C | ab0.13 0.02 B | a0.15 0.04 B | |
| U | a 0.12 BC | a0.15 0.07 B | a0.07 0.02 A | a0.15 0.02 B | b0.23 0.02 C | b0.28 0.08 C | a0.22 0.06 C | b0.15 0.02 B | b0.28 0.18 C | |
| All | 0.16 FG | 0.16 0.05 C | 0.07 0.03 A | 0.15 0.03 C | 0.21 0.03 DE | 0.23 0.07 EF | 0.19 0.05 D | 0.13 0.03 B | 0.42 0.43 G | |
| Ca (cmol/kg) | E | a 0.38 C | a5.32 1.64 CD | a4.71 1.19 AC | a4.16 0.62 AB | a4.00 0.10 A | a4.39 0.42 BC | a4.51 0.58 C | a4.56 0.53 BC | a6.13 0.53 D |
| M | b 0.28 C | a6.18 0.98 CD | a5.72 1.15 AC | b5.87 0.49 AB | b6.26 0.25 C | b6.69 0.70 C | b5.85 0.27 BC | b6.96 0.41 D | b7.37 0.28 F | |
| U | ab 3.47 ACD | a6.34 0.67 A | b7.34 0.79 C | c7.27 0.17B C | b6.54 0.31 A | c8.50 1.48 CE | b6.02 0.46 AB | c8.60 0.44 DE | a6.63 0.51 A | |
| All | 2.19 ABC | 5.95 1.21 A | 5.93 1.50 A | 5.77 1.37 A | 5.60 1.18 A | 6.53 1.96 B | 5.46 0.81 A | 6.70 1.75 C | 6.71 0.68 C | |
| Mg (cmol/kg) | E | a 0.19 D | a1.23 0.40 CD | a0.77 0.19 A | a0.82 0.12 A | a0.92 0.05 B | a1.08 0.08 C | a1.07 0.11 C | a1.06 0.11 C | a1.44 0.08 D |
| M | b 0.24 C | b1.91 0.18 C | b1.36 0.31 AB | b1.41 0.10 B | b1.64 0.06 A | b1.48 0.16 B | b1.24 0.06 A | b1.60 0.16 B | b1.56 0.07 A | |
| U | c 1.43 D | b1.76 0.15 B | c1.87 0.09 C | c1.92 0.06 C | c2.21 0.16 D | c2.44 0.30 D | c1.61 0.33 B | b1.82 0.30 BC | a1.45 0.09 A | |
| All | 1.08 F | 1.63 0.39 D | 1.33 0.50 AB | 1.38 0.47 BE | 1.59 0.54 BD | 1.66 0.61 DEF | 1.31 0.30 AC | 1.49 0.38 BCD | 1.49 0.09 BD | |
| 2021 | 9 June | 22 June | 13 July | 28 July | 11 August | 31 August | 15 September | 30 September | 15 October | |
|---|---|---|---|---|---|---|---|---|---|---|
| ) | E 1 | a 0.68 B 2 | a 0.57 A | a 0.59 A | a 0.17 A | a 0.88 AB | a 1.20 AB | a 0.86 A | ||
| M | a 0.44 B | a 0.46 A | a 0.65 A | b 0.36 A | a 0.69 A | a 2.31 AB | a 0.62 A | |||
| U | a 0.36 C | a 0.69 A | a 0.29 AB | ab 0.84 ABC | a 0.89 BC | a 1.61 AC | a 0.59 AB | |||
| All | 0.52 C | 0.56 A | 0.51 A | 0.64 AB | 0.82 A | 1.81 BC | 0.63 A | |||
| P (%) | E | a 0.03 AB | a0.10 0.02 A | a0.10 0.02 A | a0.11 0.01 A | a0.13 0.02 C | a0.12 0.01 AC | a0.14 0.02 BC | a0.15 0.02 C | a0.14 0.02 BC |
| M | a 0.03 B | a0.11 0.03 B | a0.10 0.02 A | a0.11 0.01 AB | a0.12 0.01 AB | a0.11 0.01 A | a0.14 0.02 C | a0.14 0.02 C | a0.13 0.02 BC | |
| U | a 0.02B C | a0.10 0.02 B | a0.10 0.01 B | a0.11 0.02 BC | a0.12 0.03 BC | a0.13 0.04 BCD | a0.14 0.03 CD | a0.15 0.02 D | a0.14 0.03 CD | |
| All | 0.02 AB | 0.10 0.02 A | 0.10 0.02 A | 0.11 0.01 B | 0.12 0.02 BC | 0.12 0.03 B | 0.14 0.02 CD | 0.15 0.02 D | 0.14 0.03 CD | |
| K (%) | E | a 0.09 AB | a0.73 0.12 AB | a0.74 0.12 AB | a0.73 0.06 B | a0.67 0.06 AB | a0.57 0.04 A | a0.72 0.08 AB | a0.68 0.06 AB | b0.71 0.09 B |
| M | a 0.08 BCE | a0.68 0.10 ABD | a0.77 0.15 CDE | a0.74 0.06 DE | a0.69 0.11 AE | a0.65 0.06 ACE | a0.63 0.06 AB | a0.69 0.06 BE | a0.60 0.07 A | |
| U | a 0.08 AB | a0.70 0.14 AB | a0.74 0.07 B | a0.69 0.05 AB | a0.65 0.08 A | a0.73 0.26 AB | a0.70 0.14 AB | a0.67 0.09 AB | ab0.67 0.18 AB | |
| All | 0.08 AB | 0.70 0.12 BC | 0.75 0.11 C | 0.72 0.06 BC | 0.67 0.08 A | 0.67 0.18 AB | 0.68 0.11 AB | 0.68 0.07 AB | 0.65 0.13 A | |
| C (%) | E | a 7.43 ABC | a30.2 3.00 B | a34.9 3.69 CD | a30.2 1.83 B | a30.8 2.83 B | a32.2 2.16 CD | a34.3 7.37 BC | a28.1 2.56 B | a27.4 2.69 A |
| M | a 3.41 AB | a35.4 13.7 BCF | a35.3 3.85 CD | a31.0 1.66 CE | a30.9 2.17 CE | a31.8 1.59 C | a30.4 1.94 BE | a32.7 8.40 BCF | a27.4 2.97 ADF | |
| U | a 2.24 A | a29.5 4.10 ABC | a33.7 4.53 BC | a31.0 2.09 BC | a28.8 2.17 A | a33.9 6.28 C | a32.1 7.80 A | a29.7 8.10 ABC | a27.1 4.62 AB | |
| All | 4.69 AC | 31.7 8.46 CE | 34.6 3.90 E | 30.8 1.82 CD | 30.2 2.29 C | 32.8 4.23 DE | 31.9 6.55 CE | 30.5 7.22 BCD | 27.3 3.41 AB | |
| Ca (%) | E | a 0.16 A | a0.95 0.18 A | a0.98 0.10 A | a1.21 0.08 B | a1.21 0.11 B | a1.31 0.05 BC | b1.51 0.13 D | a1.38 0.19 BD | b1.46 0.15 CD |
| M | a 0.28 AB | a1.03 0.31 BC | a0.96 0.13 A | a1.12 0.14 BC | a1.14 0.08 BC | a1.23 0.09 C | a1.36 0.08 D | a1.44 0.13 E | a1.26 0.08 CD | |
| U | a 0.23 ABC | a0.84 0.10 A | a1.01 0.09 C | a1.19 0.15 BE | a1.10 0.20 BCD | a1.17 0.14 BE | ab1.30 0.23 E | a1.33 0.18 DE | ab1.29 0.21 DE | |
| All | 0.22 A | 0.93 0.22 A | 0.98 0.10 A | 1.17 0.13 B | 1.15 0.14 B | 1.22 0.12 B | 1.37 0.17 C | 1.39 0.18 C | 1.32 0.16 C | |
| 2022 | May 23 | June 03 | June 17 | July 04 | July 19 | July 28 | August 16 | September 07 | September 21 | |
|---|---|---|---|---|---|---|---|---|---|---|
| ) | E 1 | a 0.51 A 2 | a 0.72 B | a 1.62 D | a 2.49 D | a 1.44 CD | a 0.65 D | a 1.00 D | ab 1.25 D | b 0.37 BC |
| M | a 0.80 A | a 1.08 B | a 0.48 BC | a 0.72 C | a 0.54 CD | b 0.40 D | a 0.78 C | b 0.27 D | b 0.76 B | |
| U | a 0.86 A | a 0.97 B | a 0.38 C | a 0.86 CD | a 0.57 D | ab 0.74 D | a 1.30 CD | a 0.49 D | a 0.41 AB | |
| All | 0.71 A | 0.90 B | 0.52C | 0.67 D | 1.01 DE | 0.67 D | 1.00 CD | 0.77 E | 0.66 B | |
| P (%) | E | a 0.03C D | ab0.17 0.01 C | b0.21 0.01 E | a0.19 0.02 D | b0.13 0.01 B | a0.14 0.03 B | a0.10 0.01 A | c0.12 0.02 B | a0.09 0.02 A |
| M | a 0.04 E | b0.18 0.02 EF | b0.20 0.03 F | a0.19 0.02 EF | ab0.13 0.02 DE | a0.11 0.01 CD | a0.11 0.01 C | b0.10 0.01 B | a0.07 0.01 A | |
| U | a 0.03 DF | a0.16 0.01 E | a0.17 0.01 F | a0.19 0.02 F | a0.11 0.01 D | a0.13 0.01 C | a0.11 0.01 D | a0.09 0.01 B | a0.08 0.01 A | |
| All | 0.04 DE | 0.17 0.04 D | 0.19 0.02 F | 0.19 0.02 EF | 0.12 0.02 C | 0.13 0.02 C | 0.11 0.01 B | 0.10 0.02 B | 0.08 0.01 A | |
| K (%) | E | a 0.23C D | a1.29 0.13 D | a1.36 0.09 D | a1.13 0.12 C | a0.88 0.21 B | a0.94 0.30 BC | a0.67 0.08 A | a0.61 0.05 A | a0.80 0.10 B |
| M | a 0.27 DE | a1.32 0.09 E | a1.44 0.08 F | a1.10 0.19 D | a0.92 0.17 CD | a0.86 0.09 C | a0.70 0.10 A | b0.78 0.03 AB | a0.81 0.07 BC | |
| U | a 0.20 C | a1.21 0.14 C | b1.58 0.10 D | a1.12 0.18 C | a0.79 0.12 B | a0.88 0.17 B | a0.63 0.12 A | c0.89 0.10 B | a0.86 0.10 B | |
| All | 0.23 E | 1.27 0.12 E | 1.46 0.13 F | 1.11 0.16 D | 0.86 0.17 C | 0.89 0.20 C | 0.67 0.10 A | 0.76 0.13 B | 0.83 0.09 BC | |
| C (%) | E | a 4.18 AB | a48.2 6.35 AC | b49.2 0.31 C | a52.3 6.77 BCD | a63.4 7.11 E | a63.5 8.06 E | a58.3 10.3 DE | ab63.3 7.54 E | a65.3 7.24 E |
| M | a 2.82 A | a48.4 0.63 A | b49.0 0.39 B | a53.8 4.66 C | a65.3 4.06 DEF | a67.0 0.84 EF | a65.1 1.57 D | b66.8 0.13 E | a67.4 0.52 F | |
| U | a 3.52 A | a50.1 3.57 B | a48.3 0.53 B | a51.8 4.28 B | a64.3 5.04 CDE | a67.9 0.52 E | a62.9 2.97 C | a66.0 0.59 D | a67.6 0.36 E | |
| All | 3.34 A | 48.9 4.10 B | 48.8 0.57 B | 52.6 5.14 C | 64.3 5.32 DE | 66.1 5.19 E | 62.1 6.61 D | 65.4 4.41 E | 66.8 4.11 E | |
| Ca (%) | E | a 0.10 AB | a1.08 0.09 A | b1.43 0.05 CD | a1.43 0.09 DE | a1.24 0.17 CD | a1.19 0.08 BC | a1.46 0.08 DF | a1.47 0.13 DE | ab1.57 0.15 EF |
| M | a 0.13 A | a1.08 0.11 B | b1.31 0.14 BCD | a1.39 0.17 C | a1.33 0.12 C | a1.20 0.09 D | a1.39 0.10 C | a1.52 0.04 E | b1.64 0.10 F | |
| U | a 0.12 A | a1.08 0.09 AB | a1.13 0.07 B | a1.39 0.17 D | a1.34 0.11 CD | a1.24 0.08 C | a1.41 0.11 D | a1.56 0.03 E | a1.55 0.04 E | |
| All | 0.11 A | 1.08 0.09 B | 1.29 0.15 D | 1.41 0.14 E | 1.31 0.14 D | 1.21 0.10 C | 1.42 0.10 E | 1.52 0.08 F | 1.58 0.11 G | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.S.; Ryu, C.S.; Cho, J.G.; Park, K.S. Predicting Apple Tree Macronutrients Using Unmanned Aerial Vehicle-Based Hyperspectral Imagery to Manage Apple Orchard Nutrients. Drones 2024, 8, 369. https://doi.org/10.3390/drones8080369
Kang YS, Ryu CS, Cho JG, Park KS. Predicting Apple Tree Macronutrients Using Unmanned Aerial Vehicle-Based Hyperspectral Imagery to Manage Apple Orchard Nutrients. Drones. 2024; 8(8):369. https://doi.org/10.3390/drones8080369
Chicago/Turabian StyleKang, Ye Seong, Chan Seok Ryu, Jung Gun Cho, and Ki Su Park. 2024. "Predicting Apple Tree Macronutrients Using Unmanned Aerial Vehicle-Based Hyperspectral Imagery to Manage Apple Orchard Nutrients" Drones 8, no. 8: 369. https://doi.org/10.3390/drones8080369
APA StyleKang, Y. S., Ryu, C. S., Cho, J. G., & Park, K. S. (2024). Predicting Apple Tree Macronutrients Using Unmanned Aerial Vehicle-Based Hyperspectral Imagery to Manage Apple Orchard Nutrients. Drones, 8(8), 369. https://doi.org/10.3390/drones8080369







