Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area and Water Quality Parameters
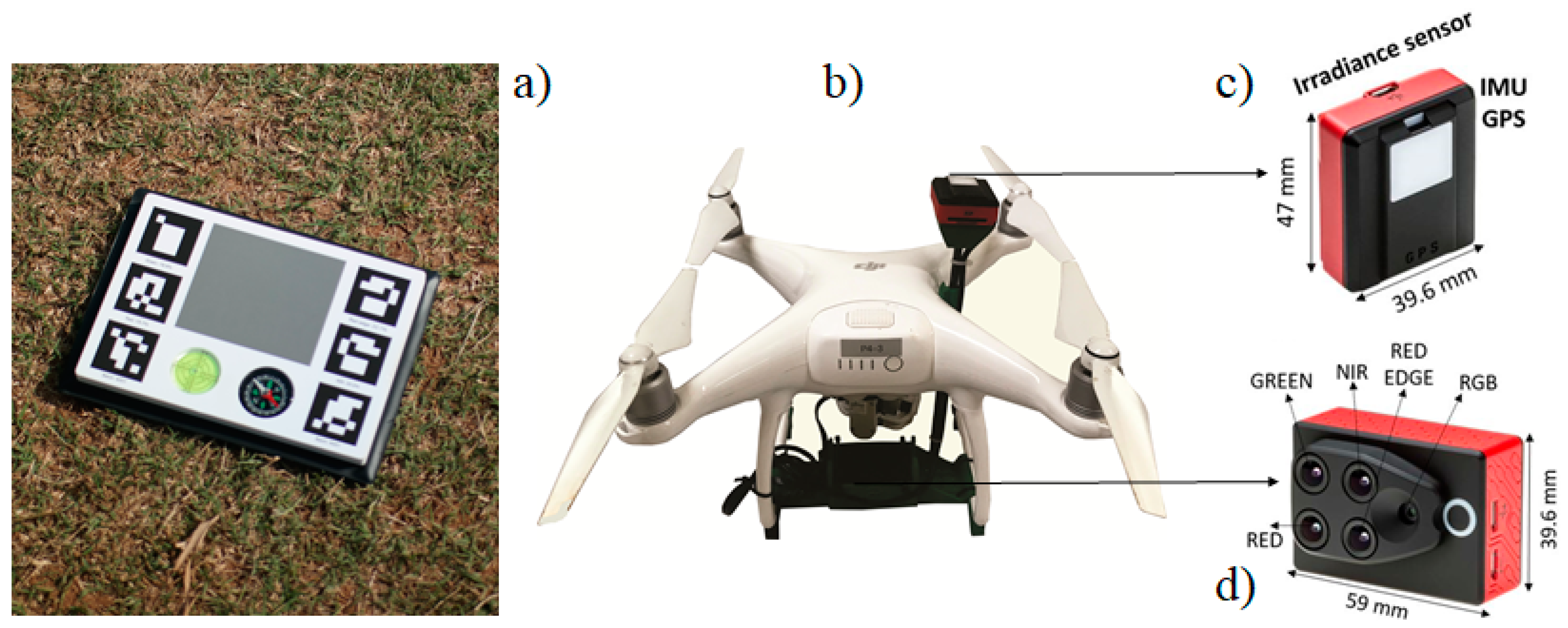
2.2. Multispectral Platform: Data Collection and Processing
2.3. Hyperspectral Platform: Data Collection and Processing
2.4. Chl-a and HABs Algorithms and Analysis of Performances
3. Results
3.1. Exploratory In Situ Analysis
3.2. Spectral Analysis from Multi- and Hyper-Spectral UAV Platforms
3.3. Chl-a Bio-Optical Models from Multi- and Hyper-Spectral UAV Platforms
3.4. Cyanobacteria Bio-Optical Models from Multi- and Hyper-Spectral UAV Platforms
4. Discussion
4.1. Retrieval of Water Quality in the Artificial Ponds
4.2. Chl-a Retrieved from Multi- and Hyper-Spectral UAV Platforms
4.3. Cyanobacteria Retrieved from Multi- and Hyper-Spectral UAV Platforms
4.4. Benefits and Costs of Multi- and Hyper-Spectral UAV Platforms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Model Algorithm | Nano-Hyperspec | Parrot Sequoia * | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est. | Std. e. | Min. | Max. | Est. | Std. e. | Min. | Max. | |||
| Two-band NIR and Red models | ||||||||||
| 1 | SR716/SR676 | Not applicable | ||||||||
| Intercept | −68.9 | 15.2 | −110.1 | −32.3 | ||||||
| coefficient | 98.8 | 11.1 | 72.5 | 128.1 | ||||||
| 2 | SR709/SR665 | Not applicable | [20] | |||||||
| Intercept | −124.9 | 21.2 | −183.5 | −68.1 | ||||||
| coefficient | 147.6 | 15.7 | 106.7 | 187.7 | ||||||
| 3 | SR705/SR665 | Not applicable | [46] | |||||||
| Intercept | −175.6 | 29.5 | −252.4 | −99.2 | ||||||
| coefficient | 189.1 | 22.0 | 129.1 | 244.6 | ||||||
| 4 | SR740/SR665 | [47] | ||||||||
| Intercept | −41.6 | 19.2 | −86.4 | −12.5 | −35.3 | 35.9 | −131.8 | 37.4 | ||
| coefficient | 195.4 | 28.7 | 126.9 | 262.6 | 125 | 34.5 | 68.4 | 221.5 | ||
| 5 | SR666−1 × SR704 | Not applicable | [48] | |||||||
| Intercept | −212.4 | 38.9 | −325.6 | −123 | ||||||
| coefficient | 221.3 | 29.4 | 149.2 | 302.6 | ||||||
| 6 | SR665−1 × SR783 | [38] | ||||||||
| Intercept | −43.4 | 21.7 | −98.6 | 8.7 | −18.1 | 45.4 | −122.0 | 64.6 | ||
| coefficient | 191.8 | 30.6 | 122.2 | 267.1 | 110.8 | 42.5 | 47.4 | 207.9 | ||
| Three-band NIR and Red models | ||||||||||
| 7 | (SR666−1 − SR704−1) × SR723 | Not applicable | [38] | |||||||
| Intercept | 25.3 | 7.1 | 8.4 | 44.8 | ||||||
| coefficient | 174.3 | 19.8 | 123.9 | 224.2 | ||||||
| 8 | (SR665−1 − SR705−1) × SR740 | Not applicable | [49] | |||||||
| Intercept | 29.6 | 8.0 | 11.6 | 51.4 | ||||||
| coefficient | 281.2 | 36.7 | 195.7 | 368.9 | ||||||
| 9 | (SR665−1 − SR705−1) × SR783 | [47] | ||||||||
| Intercept | 29.3 | 8.5 | 9.11 | 53.2 | 91.7 | 9.1 | 69.8 | 123.5 | ||
| coefficient | 274.8 | 37.2 | 189.7 | 366.2 | 115.6 | 39.6 | 54.7 | 215.9 | ||
| 10 | (1/SR670 − 1/SR710) × SR750 | Not applicable | [50] | |||||||
| Intercept | 29.9 | 7.4 | 11.6 | 55.8 | ||||||
| coefficient | 210.1 | 26.3 | 142.4 | 281.1 | ||||||
| 11 | (SR665−1 − SR708−1) × SR753 | Not applicable | [51] | |||||||
| Intercept | 31.2 | 8.6 | 12.1 | 55.5 | ||||||
| coefficient | 257.2 | 35.3 | 178.4 | 338.1 | ||||||
| 12 | (1/SR660−1/SR708) × SR755 | Not applicable | [52] | |||||||
| Intercept | 38.1 | 7.6 | 20.9 | 59.7 | ||||||
| coefficient | 281.3 | 37.7 | 190.9 | 369.2 | ||||||
| Index models | ||||||||||
| 13 | FLH: SR680 − [SR665 + (SR708/SR665) × ((λ680 − λ665)/(λ708 − λ665))] | Not applicable | [53] | |||||||
| Intercept | −147.7 | 25.3 | −220 | −86.2 | ||||||
| coefficient | −474.6 | 53.9 | −607.5 | −341.3 | ||||||
| 14 | NDCI: (SR708 − SR665)/(SR708 + SR665) | [54] | ||||||||
| Intercept | 19.3 | 11 | −17.7 | 39.5 | 99.1 | 8.7 | 79.1 | 121.5 | ||
| coefficient | 379.2 | 49.6 | 266.5 | 517.2 | 329.3 | 56.5 | 205.8 | 502.3 | ||
| 15 | BNDVI: (N − B)/(N + B) | Not applicable | [55] | |||||||
| Intercept | 84.8 | 12.6 | 50.2 | 117.9 | ||||||
| coefficient | 513.6 | 97.3 | 265.7 | 797.7 | ||||||
| 16 | INDEX: (SR665−1 − SR708−1)/(SR753−1 + SR708−1) | Not applicable | [56] | |||||||
| Intercept | 51.9 | 12.1 | 22.1 | 84.1 | ||||||
| coefficient | 79.4 | 20.2 | 44.5 | 139.2 | ||||||
| 17 | SABI: (N − R)/(B + G) | Not applicable | [57] | |||||||
| Intercept | 122.9 | 13.1 | 90.1 | 156.5 | ||||||
| coefficient | 435.3 | 82.6 | 248.1 | 659.6 | ||||||
| 18 | NDVI: (N − R)/(N + R) | [58] | ||||||||
| Intercept | 125 | 13.7 | 93.2 | 159.6 | ||||||
| coefficient | 314.9 | 64.2 | 170.4 | 506.8 | ||||||
| 19 | AI: ((SR850 − SR660)/(SR850 + SR660)) + ((SR850 − SR625)/(SR850 + SR625)) | Not applicable | [59] | |||||||
| Intercept | 180.1 | 22.3 | 120.6 | 231.9 | ||||||
| coefficient | 169.9 | 38.6 | 75.9 | 273.7 | ||||||
| 20 | GNDVI: (N − G)/(N + G) | [14] | ||||||||
| Intercept | 236.8 | 44.4 | 126.7 | 365.2 | 195.1 | 29.0 | 115.6 | 259.7 | ||
| coefficient | 375.4 | 124.3 | 105 | 792.9 | 336.2 | 95.8 | 84.0 | 584.4 | ||
| 21 | NGRDVI: (G − R)/(G + R) | [60] | ||||||||
| Intercept | −18.2 | 39.5 | −124.2 | 149.3 | −45.6 | 50.8 | −201.5 | 92.7 | ||
| coefficient | 448.5 | 126.2 | −17.5 | 780 | 562.0 | 180.1 | 182.0 | 1128.5 | ||
| 22 | KIVU: (B − R)/G | Not applicable | [61] | |||||||
| Intercept | 144.6 | 24.7 | 87.7 | 227.3 | ||||||
| coefficient | 290.5 | 140.2 | −99.7 | 805.7 | ||||||
| 23 | GLI: (2 × G − R − B)/(2 × G + R + B) | Not applicable | [62] | |||||||
| Intercept | 19.1 | 50.5 | −100.4 | 177 | ||||||
| coefficient | 177.8 | 81.7 | −51.1 | 451 | ||||||
| 24 | NGDBI: (G − B)/(G + B) | Not applicable | [63] | |||||||
| Intercept | 37.2 | 91.2 | −226.6 | 266.1 | ||||||
| coefficient | 206.1 | 220.5 | −386.8 | 826.5 | ||||||
| 25 | EXG: 2 × G − R − B | Not applicable | [64] | |||||||
| Intercept | 96.3 | 51.9 | −22.6 | 249.7 | ||||||
| coefficient | 5915.4 | 14,524 | −43.145 | 411,666 | ||||||
| Semi-analytical models | ||||||||||
| 26 | [35.7 × (SR708/SR665) − 19.3]1.124 | Not applicable | [62] | |||||||
| Intercept | −42.7 | 13.2 | −80.7 | −9.1 | ||||||
| coefficient | 2.7 | 0.3 | 1.9 | 3.4 | ||||||
| 27 | (SR662 − SR693)/(SR740 + SR705) | Not applicable | [63] | |||||||
| Intercept | 93.8 | 19.4 | 48.6 | 151.3 | ||||||
| coefficient | 212.6 | 109.5 | 41.4 | 491.5 | ||||||
| Code | Model Algorithm | Nano-Hyperspec | Parrot Sequoia | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Est. | Std. e. | Min. | Max. | Est. | Std. e. | Min. | Max. | |||
| Two-band models | ||||||||||
| 28 | SR708/SR622 | Not applicable | [52] | |||||||
| a | 117.5 | 28.0 | 32.3 | 206.0 | ||||||
| b | −238 | 71.9 | −475.2 | −41.5 | ||||||
| c | 121.3 | 43.2 | 6.8 | 264.6 | ||||||
| 29 | SR710/SR620 | Not applicable | [20] | |||||||
| a | 94.4 | 24.8 | 15.0 | 183.4 | ||||||
| b | −196.4 | 65.5 | −424.4 | −10.0 | ||||||
| c | 102.0 | 40.0 | −3.0 | 250.0 | ||||||
| 30 | SR709/SR600 | Not applicable | [11] | |||||||
| a | 174.1 | 44.9 | 30.2 | 306.8 | ||||||
| b | −305.8 | 98.3 | −628.6 | −20.5 | ||||||
| c | 134.0 | 50.9 | 0.98 | 310.7 | ||||||
| 31 | SR700/SR600 | Not applicable | [66] | |||||||
| a | 898.5 | 343.4 | 336.9 | 1959.2 | ||||||
| b | −1417.3 | 600.1 | −3316.4 | −404.0 | ||||||
| c | 558.8 | 259.5 | 99.4 | 1405.0 | ||||||
| 32 | SR724/SR600 | Not applicable | [12] | |||||||
| a | 116.2 | 54.8 | −41.3 | 279.1 | ||||||
| b | −149.3 | 100.7 | −472.5 | 121.9 | ||||||
| c | 49.3 | 40.9 | −59.9 | 186.5 | ||||||
| 33 | SR650/SR625 | Not applicable | [67] | |||||||
| a | 2074.7 | 1613.7 | −7440.6 | 7712.1 | ||||||
| b | −3691.7 | 3043.5 | −14,563 | 13,896 | ||||||
| c | 1644.3 | 1434.0 | −6468.2 | 6877.4 | ||||||
| 34 | SR724/SR660 | [68] | ||||||||
| Intercept | −16.6 | 52.5 | −168.3 | 150.1 | −23.8 | 17.6 | −72.9 | 7.6 | ||
| coefficient * | 18.2 | 45.5 | −78.8 | 188.3 | 38.0 | 18.0 | 10.1 | 88.6 | ||
| coefficient | 9.0 | 103.3 | −341.4 | 267.7 | ||||||
| Three-band NIR and Red models | ||||||||||
| 35 | (SR624−1 − SR600−1) × SR725 | Not applicable | [69] | |||||||
| a | 1007.3 | 248.8 | 67.8 | 1480.0 | ||||||
| b | −199.3 | 97.7 | −424.7 | 94.5 | ||||||
| c | 11.1 | 6.4 | −5.8 | −31.9 | ||||||
| 36 | (1/SR622 − 1/SR708) × SR755 | [52] | ||||||||
| a | 316.3 | 115.7 | −64.7 | 640.0 | 34.9 | 19.4 | 8.6 | 90.3 | ||
| b | −15.3 | 48.8 | −176.6 | 95.2 | ||||||
| c | 1.6 | 1.9 | −3.9 | 6.4 | 15.0 | 5.7 | 3.7 | 31.1 | ||
| 37 | (SR615−1 − SR600−1) × SR725 | Not applicable | [70] | |||||||
| a | 4108.8 | 1122.7 | 5.6 | 6832.1 | ||||||
| b | −705.9 | 283.2 | −1540.5 | 171.9 | ||||||
| c | 29.8 | 14.5 | −6.5 | 78.0 | ||||||
| Index models | ||||||||||
| 38 | PCI: SR555 − (SR555 − SR665)/(λ655 − λ555) × (λ630 − λ555) − SR630 | Not applicable | [71] | |||||||
| a | 2.2 × 1016 | 1.1 × 1016 | −1.5 × 1016 | 5.4 × 1015 | ||||||
| b | 90.5 | 27.2 | 36.6 | 159.9 | ||||||
| c | 10.4 | 4.2 | 1.7 | 22.9 | ||||||
| 39 | SLH: SR714 − [SR654 + ((SR754 − SR654)/(λ754 − λ654)) × (λ714 − λ654)] | Not applicable | [72] | |||||||
| a | 1.4 × 1016 | 1.9 × 1016 | −1.9 × 1016 | 9.6 × 1015 | ||||||
| b | −4984.5 | 46.9 | −194.1 | 98.6 | ||||||
| c | 0.16 | 24.1 | −65.9 | 99.2 | ||||||
| 40 | CI: SR681 − SR665 − (SR709 − SR665) × ((λ681 − λ665))/(λ709 − λ665)) | Not applicable | [72] | |||||||
| a | 2.77 × 1015 | 4.73 × 1015 | −6.87 × 1015 | 2.17 × 1016 | ||||||
| b | −13,755.4 | 59,481 | −161,755 | 179,961 | ||||||
| c | −1.1 | 12.5 | −39.1 | 33.5 | ||||||
| 41 | (SR556 − SR510)/(λ556 − λ510) | Not applicable | [73] | |||||||
| a | 239.4 | 1850.6 | −7550.6 | 4976.9 | ||||||
| b | 531.7 | 2266.3 | −8860.5 | 6515.2 | ||||||
| c | 253.2 | 689.0 | −2551.5 | 2139.5 | ||||||
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Matthews, M.W. Bio-Optical Modeling of Phytoplanton Chla. In Bio-optical Modeling and Remote Sensing of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 97801280465482017. [Google Scholar]
- Codd, G.A. Cyanobacterial Toxins, the Perception of Water Quality, and the Prioritisation of Eutrophication Control. Ecol. Eng. 2000, 16, 51–60. [Google Scholar] [CrossRef]
- Matthews, M.W. A Current Review of Empirical Procedures of Remote Sensing in Inland and Near-Coastal Transitional Waters. Int. J. Remote Sens. 2011, 32, 6855–6899. [Google Scholar] [CrossRef]
- Odermatt, D.; Gitelson, A.; Brando, V.E.; Schaepman, M. Review of Constituent Retrieval in Optically Deep and Complex Waters from Satellite Imagery. Remote Sens. Environ. 2012, 118, 116–126. [Google Scholar] [CrossRef]
- Gholizadeh, M.H.; Melesse, A.M.; Reddi, L. A Comprehensive Review on Water Quality Parameters Estimation Using Remote Sensing Techniques. Sensors 2016, 16, 1298. [Google Scholar] [CrossRef] [PubMed]
- Dörnhöfer, K.; Oppelt, N. Remote Sensing for Lake Research and Monitoring—Recent Advances. Ecol. Indic. 2016, 64, 105–122. [Google Scholar] [CrossRef]
- Ogashawara, I.; Mishra, D.R.; Gitelson, A.A. Remote Sensing of Inland Waters: Background and Current State-of-the-Art. In Bio-optical Modeling and Remote Sensing of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 97801280465482017. [Google Scholar]
- Li, L.; Song, K. Bio-Optical Modeling of Phycocyanin. In Bio-optical Modeling and Remote Sensing of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 97801280465482017. [Google Scholar]
- Olmanson, L.G.; Brezonik, P.L.; Bauer, M.E. Remote Sensing for Regional Lake Water Quality Assessment: Capabilities and Limitations of Current and Upcoming Satellite Systems. Adv. Watershed Sci. Assess. Handb. Environ. Chem. 2015, 33, 111–140. [Google Scholar] [CrossRef]
- Morel, A.; Prieur, L. Analysis of Variations in Ocean Color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Yacobi, Y.Z.; Rundquist, D.C.; Stark, R.; Han, L.; Etzion, D. Remote Estimation of Chlorophyll Concentration in Productive Waters: Principals, Algorithm Development and Validation. In Proceedings of the NWQMC National Monitoring Conference 2000, Austin, TX, USA, 25–27 April 2000; pp. 149–160. [Google Scholar]
- Neil, C.; Spyrakos, E.; Hunter, P.D.; Tyler, A.N. A Global Approach for Chlorophyll-a Retrieval across Optically Complex Inland Waters Based on Optical Water Types. Remote Sens. Environ. 2019, 229, 159–178. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Lee, Z.; Tucker, C.S. Quantifying Cyanobacterial Phycocyanin Concentration in Turbid Productive Waters: A Quasi-Analytical Approach. Remote Sens. Environ. 2013, 133, 141–151. [Google Scholar] [CrossRef]
- Ogashawara, I.; Mishra, D.R.; Mishra, S.; Curtarelli, M.P.; Stech, J.L. A Performance Review of Reflectance Based Algorithms for Predicting Phycocyanin Concentrations in Inland Waters. Remote Sens. 2013, 5, 4774–4798. [Google Scholar] [CrossRef]
- Yao, H.; Qin, R.; Chen, X. Unmanned Aerial Vehicle for Remote Sensing Applications—A Review. Remote Sens. 2019, 11, 1443. [Google Scholar] [CrossRef]
- Kislik, C.; Dronova, I.; Kelly, M. UAVs in Support of Algal Bloom Research: A Review of Current Applications and Future Opportunities. Drones 2018, 2, 35. [Google Scholar] [CrossRef]
- Koutalakis, P.; Tzoraki, O. UAVs for Hydrologic Scopes: Application of a Low-Cost UAV to Estimate Surface Water Velocity by Using Three Different Image-Based Methods. Drones 2019, 3, 14. [Google Scholar] [CrossRef]
- Koutalakis, P.; Tzoraki, O.; Gkiatas, G.; Zaimes, G.N. Using UAV to capture and record torrent bed and banks, flood debris, and riparian areas. Drones 2020, 4, 77. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Q.; Tian, Y.Q.; Chen, R.F.; Gardner, G.B. Estimation of Chromophoric Dissolved Organic Matter in the Mississippi and Atchafalaya River Plume Regions Using above—Surface Hyperspectral Remote Sensing. J. Geophys. Res. Ocean. 2011, 116, 1–22. [Google Scholar] [CrossRef]
- Dronova, I.; Kislik, C.; Dinh, Z.; Kelly, M. A Review of Unoccupied Aerial Vehicle Use in Wetland Applications: Emerging Opportunities in Approach, Technology, and Data. Drones 2021, 5, 45. [Google Scholar] [CrossRef]
- Tóth, V.Z.; Grósz, J.; Ladányi, M.; Jung, A. A New Lake Algae Detection Method Supported by a Drone-Based Multispectral Camera. Lakes Reserv. Res. Manag. 2021, 26, 1–8. [Google Scholar] [CrossRef]
- Lee, H.; Kang, T.; Pyo, J.; Park, Y.; Kwon, Y.; Cho, S.; Cho, K.; Ahn, M.-H.; Ligaray, M.; Kim, K. High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery. Remote Sens. 2018, 10, 1180. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Pyo, J.C.; Kwon, Y.H.; Duan, H.; Cho, K.H.; Park, Y. Drone-Based Hyperspectral Remote Sensing of Cyanobacteria Using Vertical Cumulative Pigment Concentration in a Deep Reservoir. Remote Sens. Environ. 2020, 236, 111517. [Google Scholar] [CrossRef]
- Cui, M.; Sun, Y.; Huang, C.; Li, M. Water Turbidity Retrieval Based on UAV Hyperspectral Remote Sensing. Water 2022, 14, 128. [Google Scholar] [CrossRef]
- Olivetti, D.; Roig, H.; Martinez, J.; Borges, H. Low-Cost Unmanned Aerial Multispectral Imagery for Siltation Monitoring in Reservoirs. Remote Sens. 2020, 12, 1855. [Google Scholar] [CrossRef]
- Kwon, S.; Seo, I.W.; Noh, H.; Kim, B. Hyperspectral Retrievals of Suspended Sediment Using Cluster-Based Machine Learning Regression in Shallow Waters. Sci. Total Environ. 2022, 833, 155168. [Google Scholar] [CrossRef] [PubMed]
- Windle, A.E.; Silsbe, G.M.; Moses, W. Evaluation of Unoccupied Aircraft System (UAS) Remote Sensing Re Fl Ectance Retrievals for Water Quality Monitoring in Coastal Waters. Front. Environ. Sci. 2021, 9, 674247. [Google Scholar] [CrossRef]
- Román, A.; Tovar-s, A.; Gauci, A.; Deidun, A.; Caballero, I.; Colica, E.; Amico, S.D.; Navarro, G. Water-Quality Monitoring with a UAV-Mounted Multispectral Camera in Coastal Waters. Remote Sens. 2023, 15, 237. [Google Scholar] [CrossRef]
- Aasen, H.; Honkavaara, E.; Lucieer, A.; Zarco-Tejada, P.J. Quantitative Remote Sensing at Ultra-High Resolution with UAV Spectroscopy: A Review of Sensor Technology, Measurement Procedures, and Data Correctionworkflows. Remote Sens. 2018, 10, 1091. [Google Scholar] [CrossRef]
- APHA—Association American Public Health. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA, WWA, WPCR, Eds.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Wójcik, K.A.; Bialik, R.J.; Osińska, M.; Figielski, M. Investigation of Sediment-Rich Glacial Meltwater Plumes Using a High-Resolution Multispectral Sensor Mounted on an Unmanned Aerial Vehicle. Water 2019, 11, 2405. [Google Scholar] [CrossRef]
- Westoby, M.J.; Brasington, J.; Glasser, N.F.; Hambrey, M.J.; Reynolds, J.M. Structure-from-Motion’photogrammetry: A low-cost, effective tool forgeoscience applications. Geomorphology 2012, 179, 14. [Google Scholar] [CrossRef]
- Vydhyanathan, A.; Bellusci, G. XSens Mti-G White Paper: The Next Generation Xsens Motion Trackers for Industrial Applications. XSENS, version 2.0.5. 2018, pp. 1–10. Available online: https://www.xsens.com/hubfs/Downloads/Whitepapers/MTi_whitepaper.pdf (accessed on 2 April 2023).
- Wei, L.; Huang, C.; Zhong, Y.; Wang, Z.; Hu, X.; Lin, L. Inland Waters Suspended Solids Concentration Retrieval Based on PSO-LSSVM for UAV-Borne Hyperspectral Remote Sensing Imagery. Remote Sens. 2019, 11, 1455. [Google Scholar] [CrossRef]
- Overstreet, B.T.; Legleiter, C.J. Removing Sun Glint from Optical Remote Sensing Images of Shallow Rivers. Earth Surf. Process. Landforms 2017, 42, 318–333. [Google Scholar] [CrossRef]
- Zeng, C.; Richardson, M.; King, D.J. The Impacts of Environmental Variables on Water Reflectance Measured Using a Lightweight Unmanned Aerial Vehicle (UAV)-Based Spectrometer System. ISPRS J. Photogramm. Remote Sens. 2017, 130, 217–230. [Google Scholar] [CrossRef]
- Mobley, C.D. Estimation of the Remote-Sensing Reflectance from above-Surface Measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef]
- Bracewell, R. The Fourier Transform. Sci. Am. 1989, 260, 86–95. [Google Scholar] [CrossRef]
- Ogashawara, I. Terminology and Classification of Bio-Optical Algorithms. Remote Sens. Lett. 2015, 6, 613–617. [Google Scholar] [CrossRef]
- Ansper, A.; Alikas, K. Retrieval of Chlorophyll a from Sentinel-2 MSI Data for the European Union Water Framework Directive Reporting Purposes. Remote Sens. 2019, 11, 64. [Google Scholar] [CrossRef]
- Statistics, M. Bootstrap Methods: Another Look at the Jackknife. Statistics 2008, 7, 1–26. [Google Scholar]
- Kulesa, A.; Krzywinski, M.; Blainey, P.; Altman, N. Sampling Distributions and the Bootstrap: The Bootstrap Can Be Used to Assess Uncertainty of Sample Estimates. Nat. Methods 2015, 12, 477–478. [Google Scholar] [CrossRef]
- Ministério Do Meio Ambiente, Conselho Nacional Do Meio Ambiente Resolução Nº 430, DE 13 DE MAIO DE 2011. Available online: https://anmlegis.datalegis.inf.br/action/ActionDatalegis.php?acao=abrirTextoAto&link=S&tipo=RES&numeroAto=00000430&seqAto=000&valorAno=2011&orgao=CONAMA/MMA&cod_modulo=405&cod_menu=6783 (accessed on 13 May 2021).
- Moses, W.J.; Sterckx, S.; Montes, M.J.; De Keukelaere, L.; Knaeps, E. Atmospheric Correction for Inland Waters. In Bio-optical Modeling and Remote Sensing of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 97801280465482017. [Google Scholar]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 3rd ed.; Kirk, J.T.O., Ed.; University Press: Cambridge, UK, 2011. [Google Scholar]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands, 1993; p. 222. [Google Scholar]
- Cicerelli, R.E.; Galo, M.D.L.B.T.; Roig, H.L. Multisource Data for Seasonal Variability Analysis of Cyanobacteria in a Tropical Inland Aquatic Environment. Mar. Freshw. Res. 2017, 68, 2344–2354. [Google Scholar] [CrossRef]
- Yan, Y.; Bao, Z.; Shao, J. Phycocyanin Concentration Retrieval in Inland Waters: A Comparative Review of the Remote Sensing Techniques and Algorithms. J. Great Lakes Res. 2018, 44, 748–755. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Satellite Estimation of Chlorophyll-a Concentration Using the Red and NIR Bands of MERISThe Azov Sea Case Study. IEEE Geosci. Remote Sens. Lett. 2009, 6, 845–849. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gurlin, D.; Moses, W.J.; Barrow, T. A Bio-Optical Algorithm for the Remote Estimation of the Chlorophyll-a Concentration in Case 2 Waters. Environ. Res. Lett. 2009, 4, 2–7. [Google Scholar] [CrossRef]
- Moses, W.J.; Gitelson, A.A.; Perk, R.L.; Gurlin, D.; Rundquist, D.C.; Leavitt, B.C.; Barrow, T.M.; Brakhage, P. Estimation of Chlorophyll- a Concentration in Turbid Productive Waters Using Airborne Hyperspectral Data. Water Res. 2011, 46, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, R.; Duan, H.; Loiselle, S.; Xu, J. A Spectral Decomposition Algorithm for Estimating Chlorophyll-a Concentrations in Lake Taihu, China. Remote Sens. 2014, 6, 5090–5106. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Estimation of Chlorophyll- a Concentration in Case II Waters Using MODIS and MERIS Data—Successes and Challenges. Environ. Res. Lett. 2009, 4, 045005. [Google Scholar] [CrossRef]
- Gower, J.; King, S.; Borstad, G.; Brown, L. Detection of Intense Plankton Blooms Using the 709 Nm Band of the MERIS Imaging Spectrometer. Int. J. Remote Sens. 2005, 26, 2005–2012. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R. Normalized Difference Chlorophyll Index: A Novel Model for Remote Estimation of Chlorophyll-a Concentration in Turbid Productive Waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Price, K.P. Harmful Algal Bloom Characterization at Ultra-High Spatial and Temporal Resolution Using Small Unmanned Aircraft Systems. Toxins 2015, 7, 1065–1078. [Google Scholar] [CrossRef]
- Yang, W.; Matsushita, B.; Chen, J.; Fukushima, T.; Ma, R. An Enhanced Three-Band Index for Estimating Chlorophyll-a in Turbid Case-II Waters: Case Studies of Lake Kasumigaura, Japan, and Lake Dianchi, China. IEEE Geosci. Remote Sens. Lett. 2010, 7, 655–659. [Google Scholar] [CrossRef]
- Alawadi, F. Detection of Surface Algal Blooms Using the Newly Developed Algorithm Surface Algal Bloom Index (SABI). Remote Sens. Ocean. Sea Ice Large Water Reg. 2010, 7825, 782506. [Google Scholar] [CrossRef]
- Rouse, J.W.J.; Haas, R.H.; Deering, D.W.; Shell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC Type III Final Report; NASA/GSFC: Greenbelt, MD, USA, 1974; p. 371.
- Jang, S.W.; Yoon, H.J.; Kwak, S.N.; Sohn, B.Y.; Kim, S.G.; Kim, D.H. Algal Bloom Monitoring Using UAVs Imagery. Adv. Sci. Technol. Lett. 2016, 138, 30–33. [Google Scholar] [CrossRef]
- Xu, F.; Gao, Z.; Jiang, X.; Shang, W.; Ning, J.; Song, D.; Ai, J. A UAV and S2A Data-Based Estimation of the Initial Biomass of Green Algae in the South Yellow Sea. Mar. Pollut. Bull. 2018, 128, 408–414. [Google Scholar] [CrossRef]
- Brivio, P.A.; Giardino, C.; Zilioli, E. Determination of Chlorophyll Concentration Changes in Lake Garda Using an Image-Based Radiative Transfer Code for Landsat TM Images. Int. J. Remote Sens. 2001, 22, 487–502. [Google Scholar] [CrossRef]
- Gilerson, A.; Zhou, J.; Hlaing, S.; Ioannou, I.; Gross, B.; Moshary, F.; Ahmed, S. Fluorescence Component in the Reflectance Spectra from Coastal Waters. II. Performance of Retrieval Algorithms. Opt. Express 2008, 16, 2446. [Google Scholar] [CrossRef] [PubMed]
- Le, C.; Li, Y.; Zha, Y.; Sun, D.; Huang, C.; Lu, H. A Four-Band Semi-Analytical Model for Estimating Chlorophyll a in Highly Turbid Lakes: The Case of Taihu Lake, China. Remote Sens. Environ. 2009, 113, 1175–1182. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Evers-King, H.; Robertson Lain, L. Distinguishing Cyanobacteria from Algae in Optically Complex Inland Waters Using a Hyperspectral Radiative Transfer Inversion Algorithm. Remote Sens. Environ. 2020, 248, 111981. [Google Scholar] [CrossRef]
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J.; Loftin, K.A.; Meredith, A. Measurement of Cyanobacterial Bloom Magnitude Using Satellite Remote Sensing. Sci. Rep. 2019, 9, 18310. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Ruiz-Verdú, A.; Domínguez-Gómez, J.A.; Peña-Martinez, R.; Peters, S.W.M.; Gons, H.J. Influence of Phytoplankton Pigment Composition on Remote Sensing of Cyanobacterial Biomass. Remote Sens. Environ. 2007, 106, 414–427. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Schluchter, W.M. A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach. Remote Sens. 2009, 1, 758–775. [Google Scholar] [CrossRef]
- Schalles, J.F.; Yacobi, Y.Z. Remote Detection and Seasonal Patterns of Phycocyanin, Carotenoid and Chlorophyll Pigments in Eutrophic Waters. Ergeb. Limnol. 2000, 55, 153–168. [Google Scholar]
- Woźniak, M.; Bradtke, K.M.; Darecki, M.; Krȩzel, A. Empirical Model for Phycocyanin Concentration Estimation as an Indicator of Cyanobacterial Bloom in the Optically Complex Coastalwaters of the Baltic Sea. Remote Sens. 2016, 8, 212. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Shi, K.; Li, Z.; Song, K. A Semi-Analytical Algorithm for Remote Estimation of Phycocyanin in Inland Waters. Sci. Total Environ. 2012, 435–436, 141–150. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Présing, M.; Kovács, A.W.; Preston, T. Spectral Discrimination of Phytoplankton Colour Groups: The Effect of Suspended Particulate Matter and Sensor Spectral Resolution. Remote Sens. Environ. 2008, 112, 1527–1544. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Duan, H.; Cannizzaro, J.; Ma, R. A Novel MERIS Algorithm to Derive Cyanobacterial Phycocyanin Pigment Concentrations in a Eutrophic Lake: Theoretical Basis and Practical Considerations. Remote Sens. Environ. 2014, 154, 298–317. [Google Scholar] [CrossRef]
- Kudela, R.M.; Palacios, S.L.; Austerberry, D.C.; Accorsi, E.K.; Guild, L.S.; Torres-Perez, J. Application of Hyperspectral Remote Sensing to Cyanobacterial Blooms in Inland Waters. Remote Sens. Environ. 2015, 167, 196–205. [Google Scholar] [CrossRef]
- Dash, P.; Walker, N.D.; Mishra, D.R.; Hu, C.; Pinckney, J.L.; D’Sa, E.J. Estimation of Cyanobacterial Pigments in a Freshwater Lake Using OCM Satellite Data. Remote Sens. Environ. 2011, 115, 3409–3423. [Google Scholar] [CrossRef]
- Gitelson, A. The Peak near 700 Nm on Radiance Spectra of Algae and Water: Relationships of Its Magnitude and Position with Chlorophyll concentration. Int. J. Remote Sens. 1992, 13, 3367–3373. [Google Scholar] [CrossRef]
- Jiang, G.; Loiselle, S.A.; Yang, D.; Ma, R.; Su, W.; Gao, C. Remote Estimation of Chlorophyll a Concentrations over a Wide Range of Optical Conditions Based on Water Classification from VIIRS Observations. Remote Sens. Environ. 2020, 241, 111735. [Google Scholar] [CrossRef]
- Tavares, M.H.; Lins, R.C.; Harmel, T.; Fragoso, C.R., Jr.; Martínez, J.-M.; Motta-Marques, D. Atmospheric and Sunglint Correction for Retrieving Chlorophyll-a in a Productive Tropical Estuarine-Lagoon System Using Sentinel-2 MSI Imagery. ISPRS J. Photogramm. Remote Sens. 2021, 174, 215–236. [Google Scholar] [CrossRef]
- Castro, C.C.; Antonio, J.; Delgado, J.; Alejandro, B.; Hinojo, S.; Luis, J.; Arango, C.; Andr, F.; Tuya, C.; Ramon, D. An UAV and Satellite Multispectral Data Approach to Monitor Water Quality in Small Reservoirs. Remote Sens. 2020, 12, 1514. [Google Scholar] [CrossRef]
- Salem, S.I.; Higa, H.; Kim, H.; Kobayashi, H.; Oki, K.; Oki, T. Assessment of Chlorophyll-a Algorithms Considering Different Trophic Statuses and Optimal Bands. Sensors 2017, 17, 1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lavender, S.; Muller, J.P.; Walton, D.; Karlson, B.; Kronsell, J. Determination of Phytoplankton Abundances (Chlorophyll-a) in the Optically Complex Inland Water—The Baltic Sea. Sci. Total Environ. 2017, 601–602, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zou, J.; Li, Y.; Yang, H.; Shi, K.; Li, J.; Wang, Y.; Chena, X.; Zheng, F. Assessment of NIR-Red Algorithms for Observation of Chlorophyll-a in Highly Turbid Inland Waters in China. ISPRS J. Photogramm. Remote Sens. 2014, 93, 29–39. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, W.; Tian, Y.Q.; Yu, Q.; Zheng, Y.; Huang, L. Remote Estimation of Colored Dissolved Organic Matter and Chlorophyll-a in Lake Huron Using Sentinel-2 Measurements. J. Appl. Remote Sens. 2017, 11, 1. [Google Scholar] [CrossRef]
- Lins, R.C.; Martinez, J.M.; da Motta Marques, D.; Cirilo, J.A.; Fragoso, C.R. Assessment of Chlorophyll-a Remote Sensing Algorithms in a Productive Tropical Estuarine-Lagoon System. Remote Sens. 2017, 9, 516. [Google Scholar] [CrossRef]
- Watanabe, F.; Alcântara, E.; Rodrigues, T.; Rotta, L.; Bernardo, N.; Imai, N. Remote Sensing of the Chlorophyll-a Based on OLI/Landsat-8 and MSI/Sentinel-2a (Barra Bonita Reservoir, Brazil). An. Acad. Bras. Cienc. 2018, 90, 1987–2000. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, C.; Akbar, A.; Xue, Y.; Zhou, Y. Spectral and Spatial Feature Integrated Ensemble Learning Method for Grading Urban River Network Water Quality. Remote Sens. 2021, 13, 4591. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Davis, T.W.; Wynne, T.T.; Graham, J.L.; Loftin, K.A.; Johengen, T.H.; Gossiaux, D.; Palladino, D.; Burtner, A. Challenges for Mapping Cyanotoxin Patterns from Remote Sensing of Cyanobacteria. Harmful Algae 2016, 54, 160–173. [Google Scholar] [CrossRef]
- Klemas, V. Remote Sensing of Algal Blooms: An Overview with Case Studies. J. Coast. Res. 2012, 278, 34–43. [Google Scholar] [CrossRef]
- Ruiz-Verdú, A.; Simis, S.G.H.; de Hoyos, C.; Gons, H.J.; Peña-Martínez, R. An Evaluation of Algorithms for the Remote Sensing of Cyanobacterial Biomass. Remote Sens. Environ. 2008, 112, 3996–4008. [Google Scholar] [CrossRef]








| Parameters | Mean * | Median * | Min * | Max * | Std. Deviation * |
|---|---|---|---|---|---|
| Chl-a (µg/L) | 116.31 | 113.85 | 14.30 | 290.70 | 80.81 |
| Green algae (µg/L) | 81.18 | 84.55 | 13.98 | 193.90 | 48.25 |
| Blue–green algae (µg/L) | 22.23 | 3.10 | 0.00 | 112.50 | 35.36 |
| Diatoms (µg/L) | 8.54 | 5.50 | 0.09 | 43.10 | 10.27 |
| Cryptophyta (µg/L) | 4.38 | 0.01 | 0.00 | 20.30 | 6.63 |
| Yellow substances (µg/L) | 0.24 | 0.00 | 0.00 | 3.29 | 0.75 |
| pH | 8.79 | 9.10 | 6.30 | 9.80 | 0.89 |
| Conductivity (µS/cm) | 49.43 | 51.00 | 14.40 | 90.10 | 18.95 |
| Turbidity (FNU) | 40.70 | 15.90 | 2.50 | 366.10 | 82.56 |
| Temperature (°C) | 25.68 | 25.80 | 24.00 | 26.80 | 0.75 |
| Code | Model Algorithm | Nano-Hyperspec | Parrot Sequoia | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | MAE | R2 | RMSE | MAE | |||
| Two-band NIR and Red models | ||||||||
| 1 | SR716/SR676 | 0.87 | 32.8 | 25.7 | Not applicable | |||
| 2 | SR709/SR665 | 0.86 | 33.1 | 26.8 | Not applicable | [24] | ||
| 3 | SR705/SR665 | 0.84 | 36.8 | 29.8 | Not applicable | [50] | ||
| 4 | SR740/SR665 * | 0.81 | 38.7 | 29.8 | 0.59 | 57.7 | 42.0 | [51] |
| 5 | SR666−1 × SR704 | 0.81 | 39.7 | 32 | Not applicable | [52] | ||
| 6 | SR665−1 × SR783 * | 0.78 | 39.7 | 32.8 | 0.28 | 72.5 | 52.4 | [41] |
| Three-band NIR and Red models | ||||||||
| 7 | (SR666−1 − SR704−1) × SR723 | 0.86 | 32.9 | 25.6 | Not applicable | [52] | ||
| 8 | (SR665−1 − SR705−1) × SR740 | 0.84 | 35.0 | 27.0 | Not applicable | [53] | ||
| 9 | (SR665−1 − SR705−1) × SR783 * | 0.83 | 36.9 | 28.2 | 0.44 | 65.5 | 46.6 | [51] |
| 10 | (1/SR670 − 1/SR710) × SR750 | 0.83 | 36.1 | 28.0 | Not applicable | [54] | ||
| 11 | (SR665−1 − SR708−1) × SR753 | 0.84 | 36.2 | 28.1 | Not applicable | [55] | ||
| 12 | (1/SR660 − 1/SR708) × SR755 | 0.83 | 37.3 | 29.0 | Not applicable | [23] | ||
| Index models | ||||||||
| 13 | FLH: SR680 − [SR665 + (SR708/SR665) × ((λ680 − λ665)/(λ708 − λ665))] | 0.86 | 35.2 | 28.5 | Not applicable | [56] | ||
| 14 | NDCI: (SR708 − SR665)/(SR708 + SR665) * | 0.82 | 38.6 | 31.4 | 0.72 | 47.6 | 37.0 | [57] |
| 15 | BNDVI: (N − B)/(N + B) | 0.67 | 52.2 | 42.1 | Not applicable | [58] | ||
| 16 | INDEX: (SR665−1 − SR708−1)/(SR753−1 + SR708−1) | 0.67 | 53.2 | 39.7 | Not applicable | [59] | ||
| 17 | SABI: (N − R)/(B + G) | 0.6 | 56.8 | 46.5 | Not applicable | [60] | ||
| 18 | NDVI: (N − R)/(N + R) | 0.56 | 59.9 | 49.3 | 0.66 | 52.1 | 41.0 | [61] |
| 19 | AI: ((SR850 − SR660)/(SR850 + SR660)) + ((SR850 − SR625)/(SR850 + SR625)) | 0.56 | 61.3 | 51.6 | Not applicable | [62] | ||
| 20 | GNDVI: (N − G)/(N + G) | 0.36 | 73.8 | 61.6 | 0.40 | 68.1 | 56.7 | [17] |
| 21 | NGRDI: (G − R)/(G + R) | 0.29 | 74.8 | 61.6 | 0.37 | 73.1 | 57.9 | [63] |
| 22 | KIVU: (B − R)/G | 0.20 | 82.9 | 68.0 | Not applicable | [64] | ||
| 23 | GLI: (2 × G − R − B)/(2 × G + R + B) | 0.14 | 82.8 | 70.3 | Not applicable | [65] | ||
| 24 | NGBDI: (G − B)/(G + B) | −0.05 | 89.3 | 74.8 | Not applicable | [66] | ||
| 25 | EXG: 2 × G − R − B | 0 | 91.7 | 76.9 | Not applicable | [67] | ||
| Semi-analytical models | ||||||||
| 26 | [35.7 × (SR708/SR665) − 19.3]1.124 * | 0.86 | 34.3 | 27.6 | Not applicable | [65] | ||
| 27 | (SR662 − SR693)/(SR740 + SR705) * | 0.14 | 89.4 | 72.3 | Not applicable | [66] | ||
| Code | Model Algorithm | Nano-Hyperspec | Parrot Sequoia | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | MAE | R2 | RMSE | MAE | |||
| Two-Band Models | ||||||||
| 28 | SR708/SR622 | 0.73 | 13.5 | 9.5 | Not applicable | [23] | ||
| 29 | SR710/SR620 | 0.64 | 14.0 | 10.2 | Not applicable | [24] | ||
| 30 | SR709/SR600 | 0.55 | 15.1 | 10.8 | Not applicable | [68] | ||
| 31 | SR700/SR600 | 0.71 | 20.0 | 13.5 | Not applicable | [70] | ||
| 32 | SR724/SR600 | 0.04 | 21.0 | 14.2 | Not applicable | [15] | ||
| 33 | SR650/SR625 | 0.61 | 28.9 | 21.9 | Not applicable | [71] | ||
| 34 | SR724/SR660 * | 0.39 | 34.6 | 24.5 | 0.47 | 35.1 | 25.5 | [72] |
| Three-band NIR and Red models | ||||||||
| 35 | (SR624−1 − SR600−1) × SR725 | 0.83 | 12.1 | 9.0 | Not applicable | [73] | ||
| 36 | (1/SR622 − 1/SR708) × SR755 * | 0.56 | 15.6 | 10.8 | 0.27 | 37.2 | 26.7 | [23] |
| 37 | (SR615−1 − SR600−1) × SR725 | 0.65 | 15.5 | 11.5 | Not applicable | [74] | ||
| Index models | ||||||||
| 38 | PCI: SR555 − (SR555 − SR665)/(λ655 − λ555) × (λ630 − λ555) − SR630 | 0.52 | 19.8 | 14.1 | Not applicable | [75] | ||
| 39 | SLH: SR714 − [SR654 + ((SR754 − SR654)/(λ754 − λ654)) × (λ714 − λ654)] | 0.68 | 33.4 | 22.8 | Not applicable | [76] | ||
| 40 | CI: SR681 − SR665 − (SR709 − SR665) × ((λ681 − λ665))/(λ709 − λ665)) | 0.64 | 32.5 | 22.7 | Not applicable | [76] | ||
| 41 | (SR556 − SR510)/(λ556 − λ510) | 0.51 | 38.8 | 27.8 | Not applicable | [77] | ||
| Devices | Weight (kg) | Cost in USD (×1000) | Devices | Weight (kg) | Cost in USD (×1000) |
|---|---|---|---|---|---|
| Parrot Sequoia with irradiance sensor | 0.25 | 4 | Headwall Nano-Hyperspec Package | 0.68 | 80 |
| DJI Phantom 4 UAV | 0.9 | 2 | |||
| UAV Battery | 0.47 | 0.1 | DJI Matrice 600 UAV | 5.9 | 4 |
| Blank row | |||||
| Pix4Dmapper Software | 2 | Battery package | 4.1 | 1 | |
| 3D support | 0.1 | 0.2 | |||
| DJI GIMBAL RONIM MX | 2.2 | 1.5 | |||
| 3D support | 0.1 | 0.1 | |||
| Total | 1.7 | 8.6 | Total | ~13 | 86.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivetti, D.; Cicerelli, R.; Martinez, J.-M.; Almeida, T.; Casari, R.; Borges, H.; Roig, H. Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming. Drones 2023, 7, 410. https://doi.org/10.3390/drones7070410
Olivetti D, Cicerelli R, Martinez J-M, Almeida T, Casari R, Borges H, Roig H. Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming. Drones. 2023; 7(7):410. https://doi.org/10.3390/drones7070410
Chicago/Turabian StyleOlivetti, Diogo, Rejane Cicerelli, Jean-Michel Martinez, Tati Almeida, Raphael Casari, Henrique Borges, and Henrique Roig. 2023. "Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming" Drones 7, no. 7: 410. https://doi.org/10.3390/drones7070410
APA StyleOlivetti, D., Cicerelli, R., Martinez, J.-M., Almeida, T., Casari, R., Borges, H., & Roig, H. (2023). Comparing Unmanned Aerial Multispectral and Hyperspectral Imagery for Harmful Algal Bloom Monitoring in Artificial Ponds Used for Fish Farming. Drones, 7(7), 410. https://doi.org/10.3390/drones7070410








