Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
- To identify mammal species in the field, such as red deer, roe deer, foxes, badgers, stone martens, European hares, and domestic cows, and their sensitivity to drone noise;
- To test the possibility of recognizing the sex of red deer and determining the sex ratio in a population;
- To test the possibility of distinguishing red deer calves from adults to determine reproduction.
2. Materials and Methods
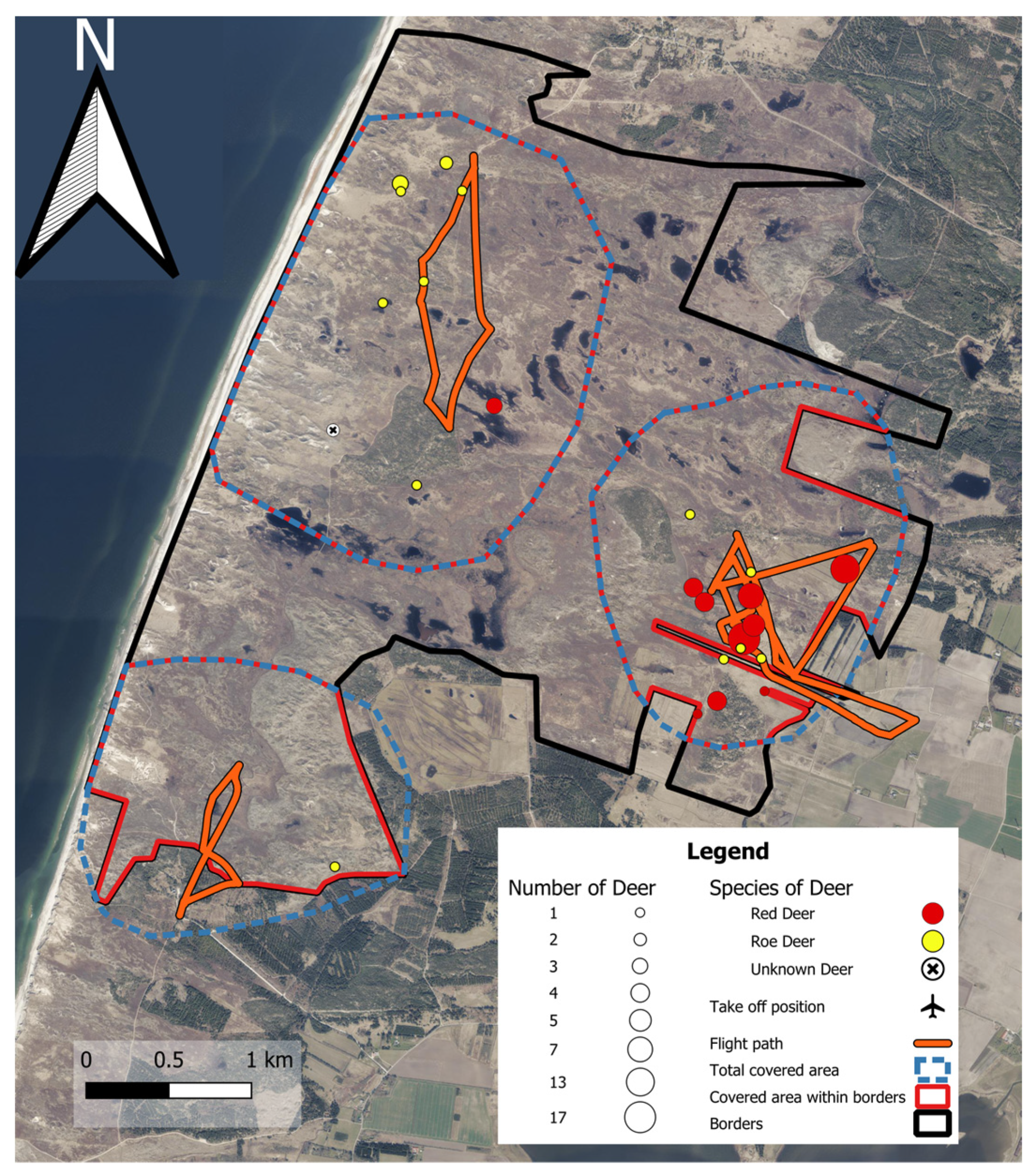
2.1. Image Collection and Study Areas
2.2. Data Collection
2.3. Species Identification and Drone Tolerance
2.4. Red and Roe Deer Counts in Lyngby Hede
3. Results
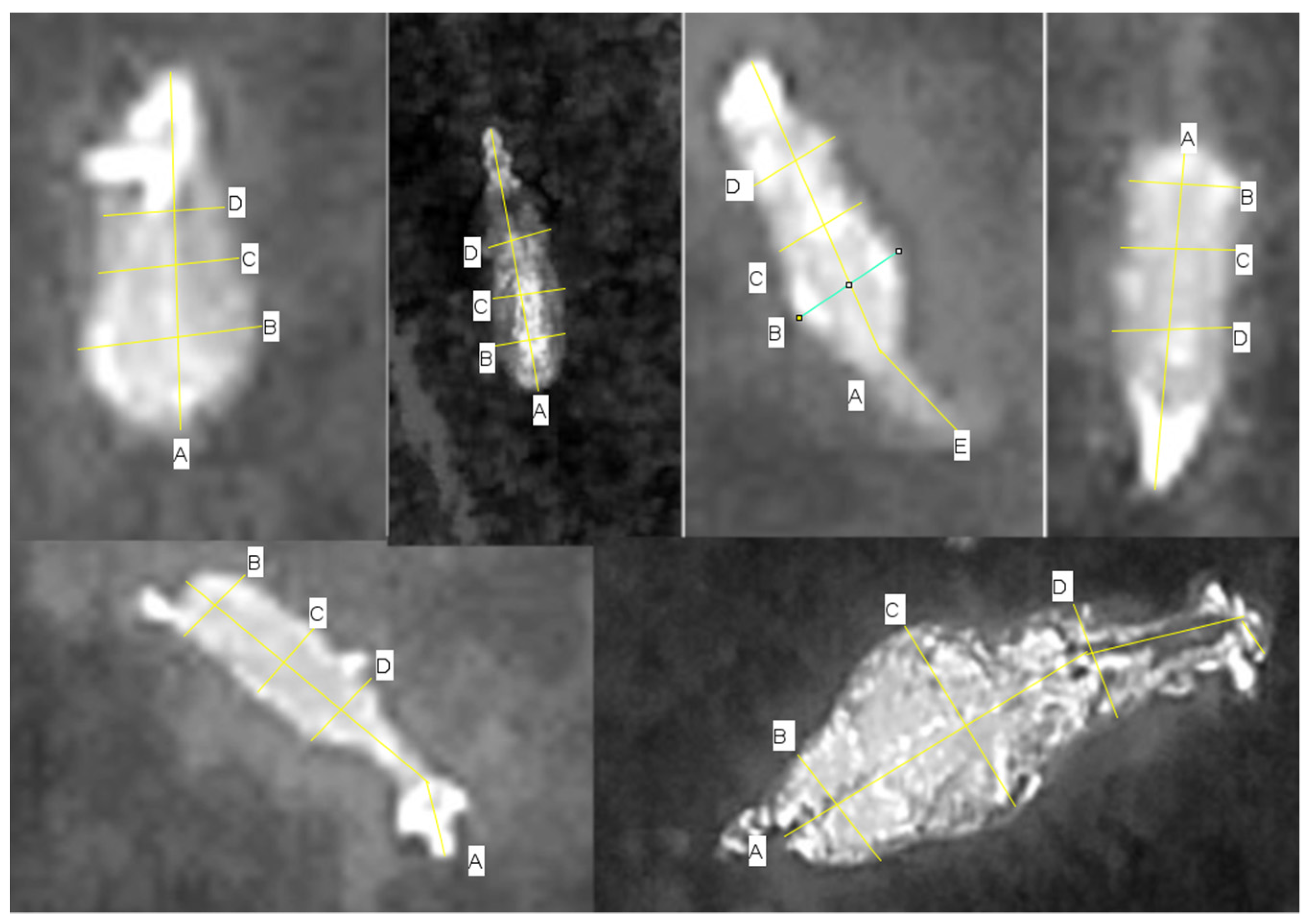
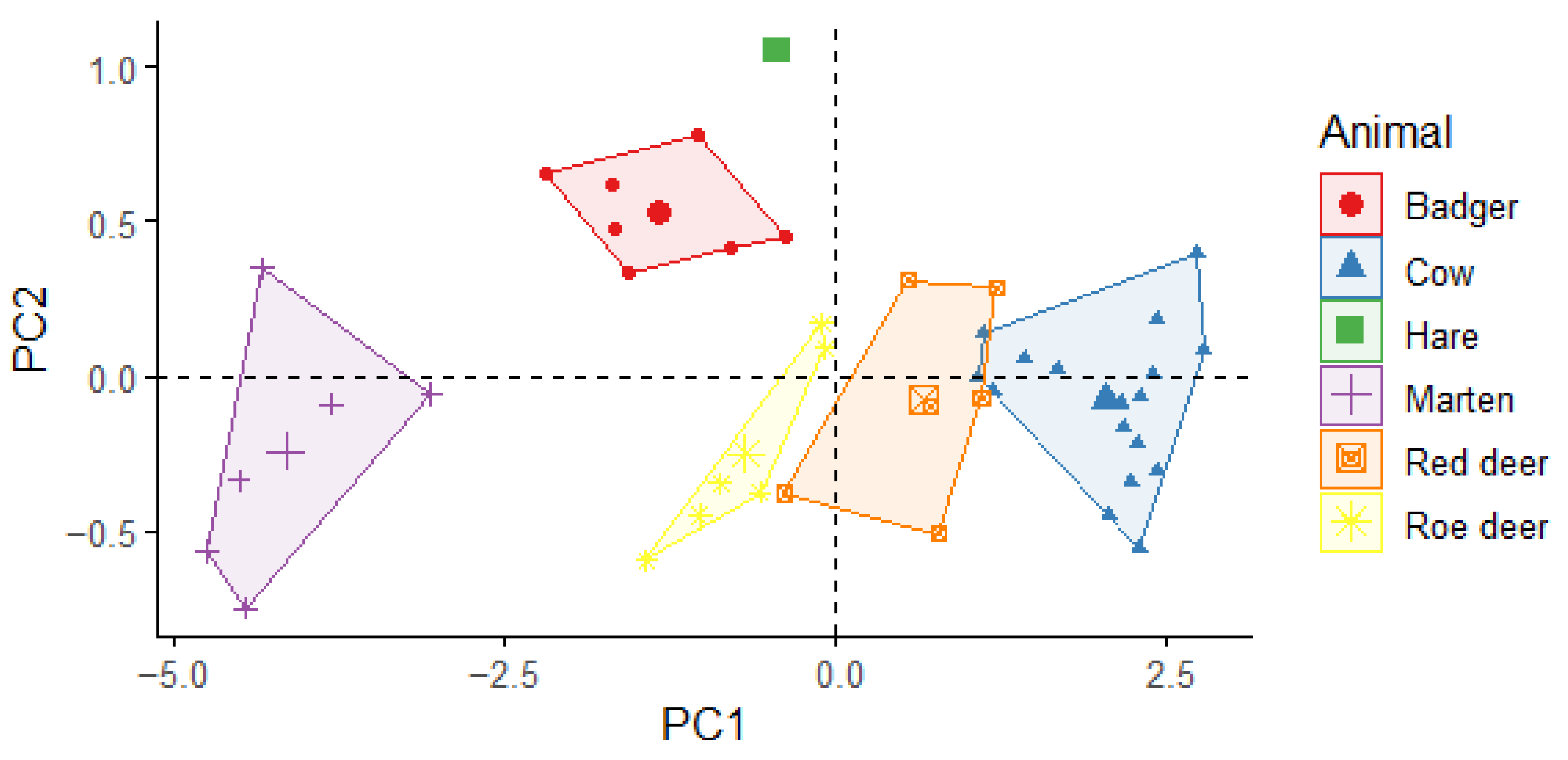
3.1. Thermal Body Measures for Species Recognition
3.2. Characteristics Used for Recognising Species
3.3. Sex and Maturity Recognition of Red Deer
3.4. Disturbance and Flight Altitude
3.5. Species and Population Observed at the Moor, Lyngby Hede
3.6. Sex and Maturity of Red Deer
4. Discussion
4.1. The Parameters Needed for Species Recognition and Population Studies
4.2. Disturbance by the Drone
4.3. Limitations and Challenges Using Drone Survey for Monitoring Mammals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Ethics
Conflicts of Interest
References
- Jones, J.P.; Asner, G.P.; Butchart, S.H.; Karanth, K.U. The ‘why’, ‘what’ and ‘how’ of monitoring for conservation. In Key Topics in Conservation Biology 2; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 327–343. [Google Scholar]
- Mitchell, C.; Fox, A.D.; Harradine, J.; Clausager, I.B. Measures of annual breeding success amongst Eurasian Wigeon Anas penelope. Bird. Study 2008, 55, 43–51. [Google Scholar] [CrossRef]
- Kahlert, J.; Fox, A.D.; Heldbjerg, H.; Asferg, T.; Sunde, P. Functional responses of human hunters to their prey—Why harvest statistics may not always reflect changes in prey population abundance. Wildl. Biol. 2015, 21, 294–302. [Google Scholar] [CrossRef]
- Christensen, T.K.; Haugaard, L. Fallow Deer in Denmark—Status for Population and Yield 2017 (in Danish Dåvildt I Danmark—Status for Betand Og Udbytte 2017). 2017. Available online: https://dce.au.dk/fileadmin/dce.au.dk/Udgivelser/Notater_2017/DAAVILDT_I_DANMARK.pdf (accessed on 8 July 2023).
- Webbon, C.C.; Baker, P.J.; Harris, S. Faecal density counts for monitoring changes in red fox numbers in rural Britain. J. Appl. Ecol. 2004, 41, 768–779. [Google Scholar] [CrossRef]
- Aubry, P.; Pontier, D.; Aubineau, J.; Berger, F.; Léonard, Y.; Mauvy, B.; Marchandeau, S. Monitoring population size of mammals using a spotlight-count-based abundance index: How to relate the number of counts to the precision? Ecol. Ind. 2012, 18, 599–607. [Google Scholar] [CrossRef]
- Princée, F.P.G. Ecological Models. In Exploring Studbooks for Wildlife Management and Conservation, 1st ed.; Springer: Cham, Switzerland, 2016; Volume XVII, p. 291. [Google Scholar]
- Delisle, Z.J.; Flaherty, E.A.; Nobbe, M.R.; Wzientek, C.M.; Swihart, R.K. Next-generation camera trapping: Systematic review of historic trends suggests keys to expanded research applications in ecology and conservation. Front. Ecol. Evol. 2021, 9, 617996. [Google Scholar] [CrossRef]
- Ingberman, B.; Fusco-Costa, R.; de Araujo Monteiro-Filho, E.L. Population survey and demographic features of a coastal island population of Alouatta clamitans in Atlantic Forest, southeastern Brazil. Int. J. Primatol. 2009, 30, 1–14. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Ruette, S.; Stahl, P.; Albaret, M. Applying distance-sampling methods to spotlight counts of foxes. J. Appl. Ecol. 2003, 40, 32–43. [Google Scholar] [CrossRef]
- Garel, M.; Bonenfant, C.; Hamann, J.; Klein, F.; Gaillard, J. Are abundance indices derived from spotlight counts reliable to monitor red deer Cervus elaphus populations? Wildl. Biol. 2010, 16, 77–84. [Google Scholar] [CrossRef]
- Corlatti, L.; Gugiatti, A.; Pedrotti, L. Spring spotlight counts provide reliable indices to track changes in population size of mountain-dwelling red deer Cervus elaphus. Wildl. Biol. 2016, 22, 268–276. [Google Scholar] [CrossRef]
- Strauß, E.; Grauer, A.; Bartel, M.; Klein, R.; Wenzelides, L.; Greiser, G.; Muchin, A.; Nösel, H.; Winter, A. The German wildlife information system: Population densities and development of European Hare (Lepus europaeus PALLAS) during 2002–2005 in Germany. Eur. J. Wildl. Res. 2008, 54, 142–147. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Sabino-Marques, H.; Barbosa, S.; Costa, P.; Encarnação, C.; Alpizar-Jara, R.; Pita, R.; Beja, P.; Mira, A.; Searle, J.B. Genetic non-invasive sampling (gNIS) as a cost-effective tool for monitoring elusive small mammals. Eur. J. Wildl. Res. 2018, 64, 46. [Google Scholar] [CrossRef]
- Skrbinšek, T.; Luštrik, R.; Majić-Skrbinšek, A.; Potočnik, H.; Kljun, F.; Jelenčič, M.; Kos, I.; Trontelj, P. From science to practice: Genetic estimate of brown bear population size in Slovenia and how it influenced bear management. Eur. J. Wildl. Res. 2019, 65, 29. [Google Scholar] [CrossRef]
- Ebert, C.; Sandrini, J.; Welter, B.; Thiele, B.; Hohmann, U. Estimating red deer (Cervus elaphus) population size based on non-invasive genetic sampling. Eur. J. Wildl. Res. 2021, 67, 27. [Google Scholar] [CrossRef]
- Collier, B.A.; Ditchkoff, S.S.; Raglin, J.B.; Smith, J.M. Detection probability and sources of variation in white-tailed deer spotlight surveys. J. Wildl. Manag. 2007, 71, 277–281. [Google Scholar] [CrossRef]
- Burton, A.C.; Neilson, E.; Moreira, D.; Ladle, A.; Steenweg, R.; Fisher, J.T.; Bayne, E.; Boutin, S. Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 2015, 52, 675–685. [Google Scholar] [CrossRef]
- Nakashima, Y.; Fukasawa, K.; Samejima, H. Estimating animal density without individual recognition using information derivable exclusively from camera traps. J. Appl. Ecol. 2018, 55, 735–744. [Google Scholar] [CrossRef]
- Chrétien, L.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Ito, T.Y.; Miyazaki, A.; Koyama, L.A.; Kamada, K.; Nagamatsu, D. Antler detection from the sky: Deer sex ratio monitoring using drone-mounted thermal infrared sensors. Wildl. Biol. 2022, 2022, e01034. [Google Scholar] [CrossRef]
- Rahman, D.A.; Setiawan, Y.; Wijayanto, A.K.; Rahman, A.A.A.F.; Martiyani, T.R. An experimental approach to exploring the feasibility of unmanned aerial vehicle and thermal imaging in terrestrial and arboreal mammals research. E3S Web Conf. 2020, 211, 02010. [Google Scholar] [CrossRef]
- Howell, L.G.; Clulow, J.; Jordan, N.R.; Beranek, C.T.; Ryan, S.A.; Roff, A.; Witt, R.R. Drone thermal imaging technology provides a cost-effective tool for landscape-scale monitoring of a cryptic forest-dwelling species across all population densities. Wildl. Res. 2021, 49, 66–78. [Google Scholar] [CrossRef]
- Lethbridge, M.; Stead, M.; Wells, C. Estimating kangaroo density by aerial survey: A comparison of thermal cameras with human observers. Wildl. Res. 2019, 46, 639–648. [Google Scholar] [CrossRef]
- Gallego, D.; Sarasola, J.H. Using drones to reduce human disturbance while monitoring breeding status of an endangered raptor. Remote Sens. Ecol. Conserv. 2021, 7, 550–561. [Google Scholar] [CrossRef]
- Israel, M. A UAV-based roe deer fawn detection system. Int. Arch. Photogramm. Remote Sens. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Dunstan, A.; Robertson, K.; Fitzpatrick, R.; Pickford, J.; Meager, J. Use of unmanned aerial vehicles (UAVs) for mark-resight nesting population estimation of adult female green sea turtles at Raine Island. PLoS ONE 2020, 15, e0228524. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Evaluation of an off-the-shelf Unmanned Aircraft System for Surveying Flocks of Geese. Waterbirds 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Wilson, J.; Amano, T.; Fuller, R.A. Using Drones to Survey Shorebirds. Univ. Qld. 2022, III, 25. [Google Scholar]
- Pagon, N.; Grignolio, S.; Pipia, A.; Bongi, P.; Bertolucci, C.; Apollonio, M. Seasonal variation of activity patterns in roe deer in a temperate forested area. Chronobiol. Int. 2013, 30, 772–785. [Google Scholar] [CrossRef]
- Ensing, E.P.; Ciuti, S.; de Wijs, F.A.; Lentferink, D.H.; Ten Hoedt, A.; Boyce, M.S.; Hut, R.A. GPS based daily activity patterns in European red deer and North American elk (Cervus elaphus): Indication for a weak circadian clock in ungulates. PLoS ONE 2014, 9, e106997. [Google Scholar] [CrossRef]
- Fløjgaard, C.; Haugaard, L.; de Barba, M.; Taberlet, P.; Ejrnæs, R. A DNA-Based Survey of Diet Choice in Red Deer in Klelund Dyrehave. A Survey of the Spacial and Timely Variation in the Diet Choice of Red Deer (In Danish Fødevalg Hos Krondyr i Klelund Dyrehave. Undersøgelse af Den Rumlige og Tidsmæssige Variation i Krondyrenes Fødevalg). DCE National center for Environment and Energy, Aarhus University. 2016, 190, p. 60. Available online: https://dce.au.dk/udgivelser/vr/nr-151-200/abstracts/no-190-a-dna-based-study-of-food-choices-by-red-deer-in-klelund-dyrhave (accessed on 14 December 2022).
- Bowers, S.; Gandy, S.; Dickerson, T.; Brown, C.; Strauch, T.; Neuendorff, D.; Randel, R.; Willard, S. Evaluating velvet antler growth in red deer stags (Cervus elaphus) using hand-held and digital infrared thermography. Can. J. Anim. Sci. 2010, 90, 13–21. [Google Scholar] [CrossRef]
- Hedearealer mellem Lyngby og Flade Sø. Available online: https://naturstyrelsen.dk/drift-og-pleje/driftsplanlaegning/thy/omraadeplaner/hedearealer-mellem-lyngby-og-flade-soe/ (accessed on 14 December 2022).
- R Core Team R: A Language and Environment for Statistical Computing, version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.K.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Le, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 15 July 2023).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Bray, J.; Maxwell, S. Introduction to Multivariate Analysis of Variance. In Multivariate Analysis of Variance; Sullivan, J.L., Ed.; Sage Publications, Inc.: Newbury Park, CA, USA, 1985; Volume 54, pp. 7–13. [Google Scholar]
- Esri ArcGis Desktop: Release 10.8.1.4362. 2020. Available online: https://desktop.arcgis.com/en/arcmap/latest/get-started/main/get-started-with-arcmap.htm (accessed on 20 September 2022).
- Tarugara, A.; Clegg, B.W.; Gandiwa, E.; Muposhi, V.K.; Wenham, C.M. Measuring body dimensions of leopards ( Panthera pardus ) from camera trap photographs. PeerJ 2019, 7, e7630. [Google Scholar] [CrossRef]
- Marcus Rowcliffe, J.; Carbone, C.; Jansen, P.A.; Kays, R.; Kranstauber, B. Quantifying the sensitivity of camera traps: An adapted distance sampling approach. Methods Ecol. Evol. 2011, 2, 464–476. [Google Scholar] [CrossRef]
- Dalla Corte, A.P.; Rex, F.E.; Almeida, D.R.A.d.; Sanquetta, C.R.; Silva, C.A.; Moura, M.M.; Wilkinson, B.; Zam-brano, A.M.A.; Cunha Neto, E.M.d.; Veras, H.F. Measuring individual tree diameter and height using GatorEye High-Density UAV-Lidar in an integrated crop-livestock-forest system. Remote Sens. 2020, 12, 863. [Google Scholar] [CrossRef]
- Cui, S.; Chen, D.; Sun, J.; Chu, H.; Li, C.; Jiang, Z. A simple use of camera traps for photogrammetric estimation of wild animal traits. J. Zool. 2020, 312, 12–20. [Google Scholar] [CrossRef]
- Ansell, W.F.H. Standardisation of Field Data on Mammals. Zool. Afr. 1965, 1, 97–113. [Google Scholar] [CrossRef]
- Bresiński, W. Grouping tendencies in roe deer under agrocenosis conditions. Acta Theriol 1982, 27, 427–447. [Google Scholar] [CrossRef]
- Jarnemo, A.; Jansson, G.; Månsson, J. Temporal variations in activity patterns during rut–implications for survey techniques of red deer, Cervus elaphus. Wildl. Res. 2017, 44, 106–113. [Google Scholar] [CrossRef]
- Hewison, A.J.M.; Gaillard, J.M.; Angibault, J.M.; van Laere, G.; Vincent, J.P. The influence of density on post-weaning winter growth in roe deer Capreolus capreolus fawns. J. Zool. 2002, 257, 303–309. [Google Scholar] [CrossRef]
- Bennitt, E.; Bartlam-Brooks, H.L.A.; Hubel, T.Y.; Wilson, A.M. Terrestrial mammalian wildlife responses to Unmanned Aerial Systems approaches. Sci. Rep. 2019, 9, 2142. [Google Scholar] [CrossRef]
- Bracha, H.S. Freeze, flight, fight, fright, faint: Adaptationist perspectives on the acute stress response spectrum. CNS Spectr. 2004, 9, 679–685. [Google Scholar] [CrossRef]
- Tablado, Z.; Jenni, L. Determinants of uncertainty in wildlife responses to human disturbance. Biol. Rev. 2017, 92, 216–233. [Google Scholar] [CrossRef]
- Wäldchen, J.; Mäder, P. Machine learning for image based species identification. Methods Ecol. Evol. 2018, 9, 2216–2225. [Google Scholar] [CrossRef]
- Park, G.; Lee, Y.; Yoon, Y.; Ahn, J.; Lee, J.; Jang, Y. Machine Learning-Based Species Classification Methods Using DART-TOF-MS Data for Five Coniferous Wood Species. Forests 2022, 13, 1688. [Google Scholar] [CrossRef]
- Hey, T.; Butler, K.; Jackson, S.; Thiyagalingam, J. Machine learning and big scientific data. Philos. Trans. R. Soc. A 2020, 37, 20190054. [Google Scholar] [CrossRef]
- Lindenfors, P.; Gittleman, J.L.; Jones, K.E. Sexual size dimorphism in mammals. Sex Size Gend. Roles Evol. Stud. Sex. Size Dimorphism 2007, 16, 26. [Google Scholar]
- Marti, I.; Ryser-Degiorgis, M. Morphometric characteristics of free-ranging Eurasian lynx Lynx lynx in Switzerland and their suitability for age estimation. Wildl. Biol. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Dawson, T.J.; Norton, M.A.; Rodoreda, S.; Abbott, S.K.; McLeod, S.R. The burden of size and growth for the juveniles of large mammalian herbivores: Structural and functional constraints in the feeding biology of juveniles relative to adults in red kangaroos, Osphranter rufus. Ecol. Evol. 2021, 11, 9062–9078. [Google Scholar] [CrossRef]
- Schwarz, J.M.; McCarthy, M.M. Steroid-induced sexual differentiation of the developing brain: Multiple pathways, one goal. J. Neurochem. 2008, 105, 1561–1572. [Google Scholar] [CrossRef]
- McPherson, F.J.; Chenoweth, P.J. Mammalian sexual dimorphism. Anim. Reprod. Sci. 2012, 131, 109–122. [Google Scholar]
- Swanson, E.M.; McElhinny, T.L.; Dworkin, I.; Weldele, M.L.; Glickman, S.E.; Holekamp, K.E. Ontogeny of sexual size dimorphism in the spotted hyena (Crocuta crocuta). J. Mammal. 2013, 94, 1298–1310. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Wang, N.; Li, Z.; Heller, R.; Liu, R.; Zhao, Y.; Han, J.; Pan, X.; Zheng, Z.; et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science 2019, 364, eaav6335. [Google Scholar] [CrossRef]



| Species | Attentive | Fleeing | Altitude (m) | dB | Observed Change |
|---|---|---|---|---|---|
| Marten 1 | No | No | 120 | 55 | |
| Yes | No | 109 | 55 | Froze | |
| Marten 2 | No | No | 90–120 | ~61.5–55 | |
| Yes | No | 80 | 60 | Froze | |
| Marten 3 | No | No | 60–120 | 60–55 | |
| Yes | No | 55 | ~61.5 | Froze | |
| Hare | Yes | No | 116 | 55 | Ears moving |
| Badger | No | No | 120 | 55 | |
| No | No | 110 | 55 | ||
| No | No | 100 | 55 | ||
| No | No | 90 | 57.5 | ||
| No | No | 70 | 60 | ||
| No | No | 55 | ~61.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsen, H.L.; Møller-Lassesen, K.; Enevoldsen, E.M.E.; Madsen, S.B.; Obsen, M.T.; Povlsen, P.; Bruhn, D.; Pertoldi, C.; Pagh, S. Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals. Drones 2023, 7, 680. https://doi.org/10.3390/drones7110680
Larsen HL, Møller-Lassesen K, Enevoldsen EME, Madsen SB, Obsen MT, Povlsen P, Bruhn D, Pertoldi C, Pagh S. Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals. Drones. 2023; 7(11):680. https://doi.org/10.3390/drones7110680
Chicago/Turabian StyleLarsen, Hanne Lyngholm, Katrine Møller-Lassesen, Esther Magdalene Ellersgaard Enevoldsen, Sarah Bøgh Madsen, Maria Trier Obsen, Peter Povlsen, Dan Bruhn, Cino Pertoldi, and Sussie Pagh. 2023. "Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals" Drones 7, no. 11: 680. https://doi.org/10.3390/drones7110680
APA StyleLarsen, H. L., Møller-Lassesen, K., Enevoldsen, E. M. E., Madsen, S. B., Obsen, M. T., Povlsen, P., Bruhn, D., Pertoldi, C., & Pagh, S. (2023). Drone with Mounted Thermal Infrared Cameras for Monitoring Terrestrial Mammals. Drones, 7(11), 680. https://doi.org/10.3390/drones7110680









