Abstract
Most current insect research techniques are ground-based and provide scarce information about flying insects in the planetary boundary layer (PBL), which remains a poorly studied ecological niche. To address this gap, we developed a new insect-sampling method consisting of a fixed-wing drone platform with net traps attached to the fuselage, a mobile design that has optimal aerodynamic characteristics for insect capture in the PBL. We tested the proposed device on 16 flights in Doñana National Park (Spain) with two different trap designs fitted on the fuselage nose and wing. We collected 34 insect specimens belonging to four orders with a representation of twelve families at mean altitudes below 23 m above ground level and sampling altitudes between 9 and 365 m. This drone insect-sampling design constitutes a low-cost and low-impact method for insect monitoring in the PBL, especially in combination with other remote sensing technologies that directly quantify aerial insect abundance but do not provide taxonomic information, opening interesting possibilities for ecology and entomological research, with the possibility of transfer to economically important sectors, such as agriculture and health.
1. Introduction
Insect populations are generally monitored by direct observation or collection using different trap types [1]. These techniques are ground-based and therefore provide no information about insects that fly in the planetary boundary layer (PBL)—also known as atmospheric boundary layer—ranging from tens of meters above ground level (AGL) to several km, depending on the height of low-altitude temperature inversions [2]. There is a lack of knowledge about insect composition, distribution, movements and interactions with other organisms in the PBL ecological niche [3,4], although such information is of considerable interest for agroecology and with respect to efficient management of the benefits and threats associated with insects. Methodological approaches to study insects in the PBL include large, fixed suction traps [5] which allow for sampling at 5–20 m AGL (E.g., https://www.rothamsted.ac.uk/insect-survey, accessed on 30 June 2022); the use of traps attached to manned aircrafts [6], kites [7] and helium balloons [8,9] for sampling up to a few hundred meters AGL; and different types of radar to detect insect concentrations at higher altitudes [10]. LiDAR (light detection and ranging), a remote sensing method based on the use of light in the form of a pulsed laser to measure distances, has only recently been applied in entomology [11,12,13] suggesting promising results in detection of flying insects at altitudes of 3–600 m. The application of such methods is often limited by the high cost of equipment and its maintenance; the complex skills necessary to operate such equipment and interpret results; and limited access to higher PBL altitudes, e.g., for balloons, which tend to drift horizontally; and the logistics associated with operating such tools.
Small drones (aka unmanned aircraft systems (UAS) or remotely piloted aircraft systems (RPAS)) have undergone remarkable development in the last decade and are now integrated in various civilian sectors [14]. Most environmental drone applications rely on aerial images gathered by onboard cameras, enabling study of habitats, animals or plants at high spatial and temporal resolution [15]. The capabilities of drones to perform actions such as collecting or dropping products have been less exploited, although some examples include water collection [16] and aerobiological sampling [17,18].
Drones have recently been used for flying-insects sampling. Of the two main drone designs: rotary-wing and fixed-wing, the preferred choice for this task to date has been the rotary-wing design. These systems, also known as multicopters, use the permanent rotation of their motors to generate the relative air speed needed by the propellers to generate lift [19]. Multicopters can be accurately controlled, hover in place and take-off/land vertically in small patches of terrain. These characteristics make them very popular among users in general and particularly suitable for flying close to the ground or sweeping the vegetation canopy to capture insects [20,21,22,23]. From an aeronautical perspective, at higher altitudes, where the PBL is free of obstacles, a fixed-wing design is ideal because it is aerodynamically more efficient and allows for longer flight duration [24,25], which is necessary to increase the chances of capturing insects at low densities. Fixed-wing drones depend on forward motion for lift, requiring constant forward movement through propulsion systems to create a wind stream to pass through their wings in order to get enough lift to oppose their weight, or can keep flying without propeller rotation as long as relative airspeed is constant [19].
In this work, we present a proof of concept of a fixed-wing drone specifically designed for insect sampling in the PBL. We tested the proposed device with two trap designs attached to the aircraft fuselage and nose. This new tool is intended to contribute to improved understanding of insect diversity and abundance in the PBL, which could be useful for future studies in the fields of agroecology, agriculture and human health, as well as the ultimate development of more effective conservation strategies to help revert global insect decline.
2. Materials and Methods
2.1. Study Area
Our study was carried out in Doñana Natural Area (36°59′ N, 6°26′ W, altitude 0–40 m above sea level, mostly <10 m), which comprises both National and Natural parks and is located on the Atlantic coast of southwestern Spain (Figure 1). With a Mediterranean subhumid climate and marked seasons, Doñana Natural Area hosts three main ecosystems: Mediterranean shrubland/forest, sandy dunes and marshland.
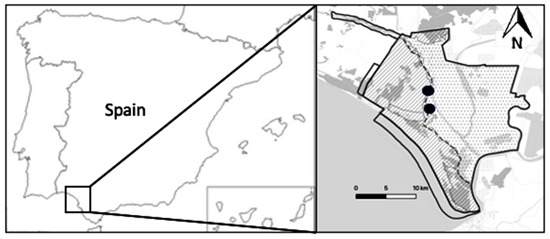
Figure 1.
Study area: Doñana Natural Area, southwestern Spain. Experimental locations are indicated with black dots.
2.2. Drone System
The drone system used for insect sampling was designed and built by members of our team and named “LOCOMOVE” (LOw COst MObile VEhicle) (Figure 2). The design requirements include weight below 5.5 kg to be launched by hand and that the engine is located on upper rear fuselage (“pusher design”) to avoid damage when landing on hard terrain. The aircraft structure is made of composite (fiberglass and carbon fiber), and the wings are made of foam-covered on wood and finished with Oracover. The non-removable parts are glued with epoxy, and the removable parts (e.g., wings) are fixed with screws. The drone has a 210 cm wingspan, a 146 cm length, a maximum takeoff weight of 5.2 kg and an 800 g payload. The engine model is a Hacker A50 12S (https://hackermotorusa.com, accessed on 30 June 2022), and the engine speed controller is a Kontronik Jive 60A (https://www.kontronik.com, accessed on 30 June 2022). The platform is equipped with 14 × 7 propellers and Futaba servos (https://www.futaba.com/, accessed on 30 June 2022). The system is powered by LiPo 6S 10,400 mA batteries for the engine and LiPo 3S 2200 mA batteries for avionics. The drone uses an advanced navigation system (Pixhawk 1, https://ardupilot.org/, accessed on 30 June 2022) for automatic guidance, navigation and control. It includes an estimator that optically fuses the sensor measurements to provide the aircraft status (global and relative position, ground speed, airspeed and attitude) with higher frequency and lower error than using Global Positioning System (GPS) alone. The navigation system enables the use of automatic flight plans specified by flight tasks (e.g., route of waypoints, takeoff, landing, cruise flight and loitering). The ground control station consists of a ruggedized Dell EMC-R135-WG laptop (https://www.dell.com/es-es, accessed on 30 June 2022) with Mission Planner software (https://ardupilot.org/planner/, accessed on 30 June 2022) for autonomous operation and a Futaba T10CAG 2.4 Ghz radio transmitter (https://www.futaba.com/, accessed on 30 June 2022) for manual control. The aerial platform has an approximate cost of EUR 1000, and the ground control station costs approximately EUR 900. At night, we equipped the aircraft with light-emitting diode (LED) lights on the wings and tail sides to maintain an operator-to-drone line of sight. Takeoff was initiated by hand or elastomer catapult, and upon return, the aircraft landed on its belly, which was reinforced to avoid fuselage damage.
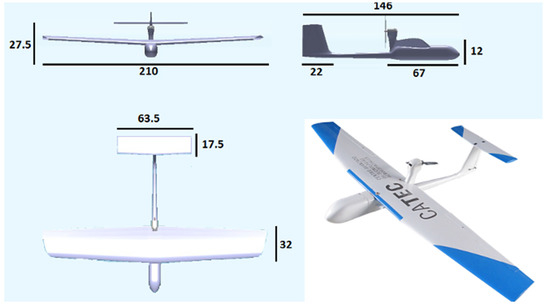
Figure 2.
Fixed-wing LOCOMOVE drone; measurements in cm.
2.3. Drone Insect Traps
We tested two different trap types and locations on the aircraft that were designed to minimize the drag caused by the trap on the aircraft aerodynamics, as well as the influence of the vehicle aerodynamics on insect capture. The first trap design consists of a tunnel with a semiarch shape (16 cm width, 4 cm opening) embedded and centered in the drone fuselage and above the wing structure. We attached a commercial mosquito net to the tunnel edges for insect capture (Figure 3, top right shows the net pulled out in the tunnel for illustrative purposes). This solution is optimal from an aerodynamic point of view because it is integrated in the aircraft profile, minimizing parasite drag. We also tested a second trap design consisting of an aluminum cylinder (3.5 cm radius) with a stocking net attached with a flange that constitutes a type of upper pod. The cylinder is attached above the drone nose with a plastic piece (Figure 3, bottom shows the trap with the lid closed). This trap design placed on the front of the drone nose facilitates a clean air flow in flight, avoiding the possible interference of the fuselage in the air flow, although it produces slightly more parasite drag than the former configuration. The cylinder trap includes a lid activated by a servo, which allows opening or closing of the trap at the desired sampling altitude; we kept this lid open during flights for methodological consistency with the semiarch trap experiments.

Figure 3.
Insect traps details. Top: semiarch design; bottom: cylinder design.
2.4. Drone Flight Description
We performed two drone insect-sampling field campaigns in a Mediterranean shrubland habitat. The first took place in May, with ten flights performed at night (23–1 h local time) and the second in September, with six flights performed in the evening (20–21.30 h local time). Due to the lack of studies about insect densities in the PBL in the area, sampling times were chosen according to observational checks on the previous days, showing that flying insects were more active during those periods. The flights were conducted at altitudes ranging from 9 to 365 m AGL, making 1.5 km radius ellipsoidal/oval tracks around the ground control station where the drone operator was located (Table 1, Figure S1). Drone telemetry was recorded for all except two flights (flights 11 and 12) due to a technical problem, but basic flight parameters for those flights were extracted from field notes.

Table 1.
Insects drone sampling results per flight (* indicates that no telemetry data were available and flight parameters were extracted from field notes). Sampling altitude is above ground level.
3. Results
We collected a total of 34 insect specimens in the 16 drone flights performed during the two field campaigns. The most abundant insect order captured was Coleoptera (50%), followed by Diptera (41%), Hemiptera (6%) and Hymenoptera (3%), with a representation of 12 families in total (Table 1).
During the first campaign using the semiarch trap, we captured 10 individuals belonging to 3 orders (Diptera, Coleoptera and Hymenoptera), with a representation of 3 families. In the second campaign using the cylinder trap, we captured 24 individuals belonging to 3 orders (Coleoptera, Diptera and Hemiptera), with a representation of 10 families.
4. Discussion
The fixed-wing drone design proposed here successfully served to collect a variety of flying insects, providing evidence that the system can be used for aerial sampling in the planetary boundary layer. Given the scarcity of studies assessing insect density in this niche, we were not able to evaluate the capture efficiency of the system against a control, nor the two trap designs against each other, as they were tested in different field campaigns. Nevertheless, the overall collecting method provided a number of sampled specimens consistent with related works: we captured 36 insects during 16 flights (9–365 m AGL); 16 specimens were captured during 18 flights (10–100 m AGL) over rice fields in South Korea [20]; 74 adult leafhoppers were collected during 26 flights (20–40 m) above an alfalfa field in the Unites States of America [18]; and a small number of individuals was recorded using camera transects to monitor nocturnal flying insects in a protected area in Poland, indicating that the density of flying nocturnal insects is very low, even in insect-rich habitats [26]. A larger-scale sampling effort would be necessary to provide reliable abundance estimates, as is the case for many insect and other wildlife-monitoring techniques (e.g., insect light traps, camera traps). We predict the highest potential of drone insect sampling in combination with remote monitoring methods, such as entomology radars [10], LiDAR [27] and camera transects [26], which quantify aerial insect abundance. For example, systematically remote monitoring methods can provide information on times and locations of higher insect concentrations (e.g., migration), and drones could be used to identify them.
From an aeronautical point of view, the drone design presented here performed as specified. Our flight times fit into the longest reported in the insect sampling literature for fixed-wing drones: 30 min [18] and 15 min [17]; and rotary-wing drones: 10–15 min [22,23], 12 min [21] and 5 min [20]. Whereas we opted to manufacture our own platform, similar drones can be found in the market or be built at low cost following a DIY (do-it-yourself) approach. For example, [25] provides a guide to manufacture a fixed-wing platform with 65 min autonomy for approximately USD 995 that could be easily adapted to flying-insects sampling.
The insect traps we tested did not cause any aircraft stability problems such as those mentioned in other drone studies (see [23]). Although both insect traps worked, we concluded that the cylinder design is preferable to the semiarch design, as the former can be easily made using light materials (such as plastic or recycled tin), whereas the latter requires customization of the wing design. Attaching sampling devices to aircrafts dramatically alters their flying qualities [28]; thus, special care must be devoted to their installation within the structure to avoid aerodynamic effects that could perturb insect sampling and drag increments negatively affecting the aircraft performance. For this reason, we recommend that the traps be installed on the plane forward axis and towards the front, where the trap weight can be compensated by adjusting the battery location inside the fuselage on the same axis to maintain the plane center of gravity.
Drones present advantages over other aerial sampling techniques that make them a versatile addition to existing methods [23]. Small drones are portable and can be deployed rapidly, facilitating sampling in difficult access areas and on short notice. This makes them convenient for gathering data according to difficult-to-predict environmental changes that affect insect movements; catastrophic phenomena of interest in ecology and agricultural studies (e.g., pollution, fires); and health emergencies. Small electric drones do not significantly disturb surrounding wildlife when used responsibly [29] and are zero-emission vehicles, both of which are important aspects when surveying sensitive natural areas [15]. The insect specimens sampled with drones can be released after identification if they survive in the sampling nets (some may die or suffer physical damage due to capture pressure), which makes drones a less invasive method relative to other techniques [23]. The major limitations of the expansion of drone insect sampling include legal restrictions that interdict flying beyond the line of sight, at night and over specific locations, constraining the scale of work in several countries. In line with [30], we suggest that operations that do not pose a safety threat should be permitted, which is the case for many protected areas and agricultural fields where insect sampling has more interest.
Our study supports the growing body of evidence that suggests that drones have the potential to revolutionize insect aerial sampling [17,18,20,21,22,23]. The method described here can be applied to ecology and entomology research, representing a valuable tool for assessment of the severity of current insect population decline, with the possibility of transfer to economically important sectors, such as agriculture, where real-time information on insects could contributes to the design of effective strategies for sustainable crop management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/drones6080189/s1, Figure S1: (a–d). Drone paths 1–16 (excluding 11 and 12) showing altitude ranges and mean (black dashed line) with flight dynamics including: total flight distance (FD), total flight time (FT), and mean altitude flown (MA). Telemetry for take-off and landing data was clipped and removed at <10 m for May flights (1–10), and <3 m for September flights (13–16).
Author Contributions
Conceptualization, M.M.-P., J.R.M.-d.D., A.G.P.-L., C.I., F.A., A.V., J.J.N., A.O. and P.J.M.; methodology M.M.-P., J.R.M.-d.D., A.G.P.-L., C.I. and J.J.N.; formal analysis, M.M.-P., J.R.M.-d.D., A.G.P.-L., C.I. and R.J.G.; investigation, M.M.-P., J.R.M.-d.D., A.G.P.-L., C.I., P.J.M. and J.J.N.; resources, J.J.N., A.O. and P.J.M.; data curation, M.M.-P. and R.J.G.; writing—original draft preparation, M.M.-P., J.R.M.-d.D., A.G.P.-L., C.A.S.-B., C.I. and J.J.N.; writing—review and editing, M.M.-P., J.R.M.-d.D., A.G.P.-L., C.I., A.O. and J.J.N.; visualization, M.M.-P., R.J.G. and J.R.M.-d.D.; supervision, J.R.M.-d.D.; project administration, J.J.N. and P.J.M.; funding acquisition, J.J.N., A.O. and P.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
M.M.-P., J.R.M.-d.D., F.A., A.V., J.J.N., A.O. and P.J.M. were funded by Planet Project (European Commission 7th Framework Programme Grant Agreement 257649). Additionally, M.M. -P. was funded by the European Union “NextGenerationEU” (Programa María Zambrano, Ministerio de Universidades, Spain).
Data Availability Statement
Not applicable.
Acknowledgments
Logistical and technical support was provided by ICTS-RBD-CSIC, Ministry of Science and Innovation and co-financed by FEDER Funds. Doñana Natural Area authorities authorized the work conducted in the field (Permit number 2011/25 PLANET). We thank Íñigo Sánchez (Zoo de Jerez, Spain) for his help with insect identification. We thank Allan Anderson Bell and Jose Ramón López for operating the drone.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Grootaert, P.; Pollet, M.; Dekoninck, W.; van Achterberg, C. Sampling insects: General techniques, strategies and remarks. In Manual on Field Recording Techniques and Protocols for All Taxa Biodiversity Inventories and Monitoring 8; Eyman, J., Degreef, J., Hauser, C., Monje, J.C., Samyn, Y., Spiegel, D.V., Eds.; ABC: London, UK, 2010; pp. 377–399. [Google Scholar]
- Westbrook, J.K.; Eyster, R.S. Atmospheric Environment Associated with Animal Flight; Chilson, P., Frick, W., Kelly, J., Liechti, F., Eds.; Aeroecology Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Chapman, J.W.; Nesbit, R.L.; Burgin, L.E.; Reynolds, D.R.; Smith, A.D.; Middleton, D.R.; Hill, J.K. Flight orientation behaviors promote optimal migration trajectories in high-flying insects. Science 2010, 327, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Reynolds, D. High altitude migration of the diamondback moth Plutella xylostella to the U.K. Ecol. Entomol. 2002, 27, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.G.; Taylor, L.R. The development of large suction traps for airborne insects. Ann. Appl. Biol. 1955, 43, 51–62. [Google Scholar] [CrossRef]
- Greenstone, M.H.; Eaton, R.R.; Morgan, C.E. Sampling Aerially Dispersing Arthropods: A High-Volume, Inexpensive, Automobile- and Aircraft-Borne System. J. Econ. Entomol. 1991, 84, 1717–1724. [Google Scholar] [CrossRef]
- Hardy, A.; Milne, P. Studies in the distribution of insects by aerial currents. J. Anim. Ecol. 1938, 7, 199–229. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D.; Smith, E.T.; Woiwod, I.P. An aerial netting study of insects migrating at high altitude over England. Bull. Entomol. Res. 2004, 94, 123–136. [Google Scholar] [CrossRef]
- Fukuyama, K.; Maetô, K.; Kirton, L.G. Field tests of a balloon-suspended trap system for studying insects in the canopy of tropical rainforests. Ecol. Res. 1994, 9, 357–360. [Google Scholar] [CrossRef]
- Chapman, J.; Reynolds, D.; Smith, A. Migratory and foraging movements in beneficial insects: A review of radar monitoring and tracking methods. Int. J. Pest Manag. 2004, 50, 225–232. [Google Scholar] [CrossRef]
- Van Klink, R.; August, T.; Bas, Y.; Bodesheim, P.; Bonn, A.; Fossoy, F.; Høye, T.T.; Jongejans, E.; Menz, M.H.; Miraldo, A.; et al. Emerging technologies revolutionise insect ecology and monitoring. Trends Ecol. Evol. 2022, 130, 26–42. [Google Scholar] [CrossRef]
- Brydegaard, M.; Jansson, S.; Malmqvist, E.; Mlacha, Y.P.; Gebru, A.; Okumu, F.; Killeen, G.F.; Kirkeby, C. Lidar reveals activity anomaly of malaria vectors during pan-African eclipse. Sci. Adv. 2020, 6, eaay5487. [Google Scholar] [CrossRef]
- Brydegaard, M.; Gebru, A.; Kirkeby, C.; Åkesson, S.; Smith, H. Daily evolution of the insect biomass spectrum in an agricultural landscape accessed with lidar. EPJ Web Conf. 2016, 119, 22004. [Google Scholar] [CrossRef] [Green Version]
- De Miguel Molina, B.; Oña, M.S. The drone sector in Europe. In Ethics and Civil Drones; Springer: Cham, Switzerland, 2018; pp. 7–33. [Google Scholar]
- Jiménez López, J.; Mulero-Pázmány, M. Drones for Conservation in Protected Areas: Present and Future. Drones 2019, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Schwarzbach, M.; Laiacker, M.; Mulero-Pazmany, M.; Kondak, K. Remote water sampling using flying robots. In Proceedings of the 2014 International Conference on Unmanned Aircraft Systems (Icuas), Orlando, FL, USA, 27–30 May 2014; pp. 72–76. [Google Scholar]
- Schmale, D.G.; Dingus, B.R.; Reinholtz, C. Development and application of an autonomous unmanned aerial vehicle for precise aerobiological sampling above agricultural fields. J. Field Robot. 2008, 25, 133–147. [Google Scholar] [CrossRef]
- Shields, E.J.; Testa, A.M. Fall migratory flight initiation of the potato leafhopper, Empoasca fabae (Homoptera: Cicadellidae): Observations in the lower atmosphere using remote piloted. Agric. For. Meteorol. 1999, 97, 317–330. [Google Scholar] [CrossRef]
- Paredes, J.A.; Saito, C.; Abarca, M.; Cuellar, F. Study of effects of high-altitude environments on multicopter and fixed-wing UAVs’ energy consumption and flight time. In Proceedings of the 2017 13th IEEE Conference on Automation Science and Engineering (CASE), Xi’an, China, 20–23 August 2017. [Google Scholar]
- Kim, H.G.; Park, J.-S.; Lee, D.-H. Potential of Unmanned Aerial Sampling for Monitoring Insect Populations in Rice Fields. BioOne 2018, 101, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.; Krupke, D.; Burbage, M.; Bhatnagar, S.; Fekete, S.P.; Becker, A.T. Using a UAV for Destructive Surveys of Mosquito Population. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018. [Google Scholar]
- Neufeld, J.; Ryu, J.; Barbour, J. Development of a UAS-based insect scouting method. J. NACAA 2019, 12. [Google Scholar]
- Locken, H.; Fischer, O.W.; Selz, J.; Boppre, M. ‘Drone-Netting’ for Sampling Live Insects. J. Insect Sci. 2020, 20, ieaa086. [Google Scholar] [CrossRef]
- Ollero, A.; Merino, L. Control and perception techniques for aerial robotics. Annu. Rev. Control 2004, 28, 167–178. [Google Scholar] [CrossRef]
- Mesquita, G.P.; Rodriguez-Teijeiro, J.D.; de Oliveira, R.R.; Mulero-Pazmany, M. Steps to build a DIY low-cost fixed-wing drone for biodiversity conservation. PLoS ONE 2021, 16, e0255559. [Google Scholar] [CrossRef]
- Ruczyński, I.; Hałat, Z.; Zegarek, M.; Borowik, T.; Dechmann, D.K.N. Camera transects as a method to monitor high temporal and spatial ephemerality of flying nocturnal insects. Methods Ecol. Evol. 2020, 11, 294–302. [Google Scholar] [CrossRef]
- Kirkeby, C.; Wellenreuther, M.; Brydegaard, M. Observations of movement dynamics of flying insects using high resolution lidar. Sci. Rep. 2016, 6, 29083. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Patel, V.; Woolsey, C.A.; Hovakimyan, N.; Schmale, D. L1 Adaptive Control of a UAV for Aerobiological Sampling. In 2007 American Control Conference; IEEE: Piscataway Township, NJ, USA, 2007; pp. 4660–4665. [Google Scholar]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, L.A.; Jones, T.; Maneschijn, A. Considerations for UAV design and operation in South African airspace. Aeronaut. J. 2006, 110, 695–701. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).