The Drone Revolution of Shark Science: A Review
Abstract
1. Overview
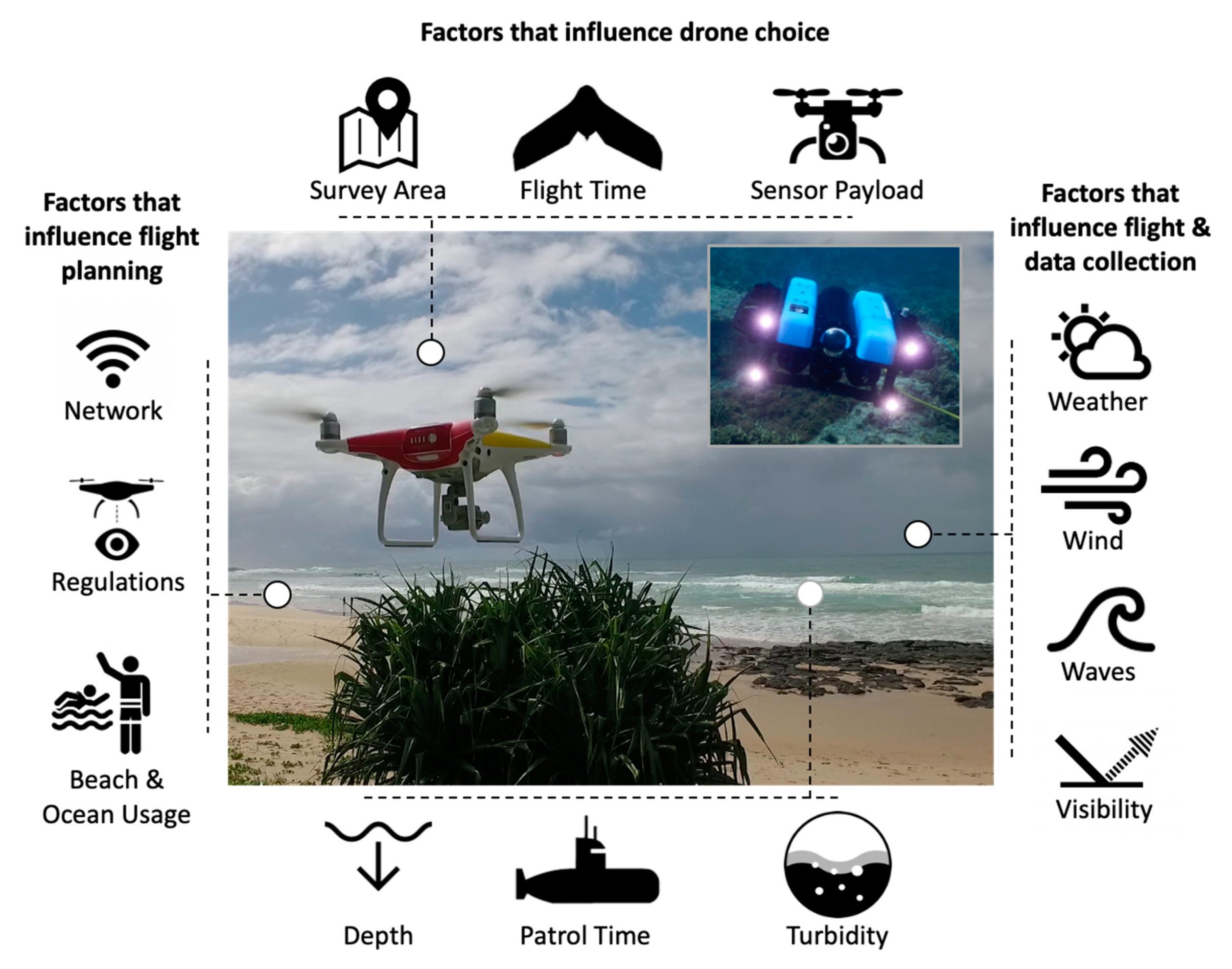
2. Drones for Studying Sharks
3. Drone Research Areas
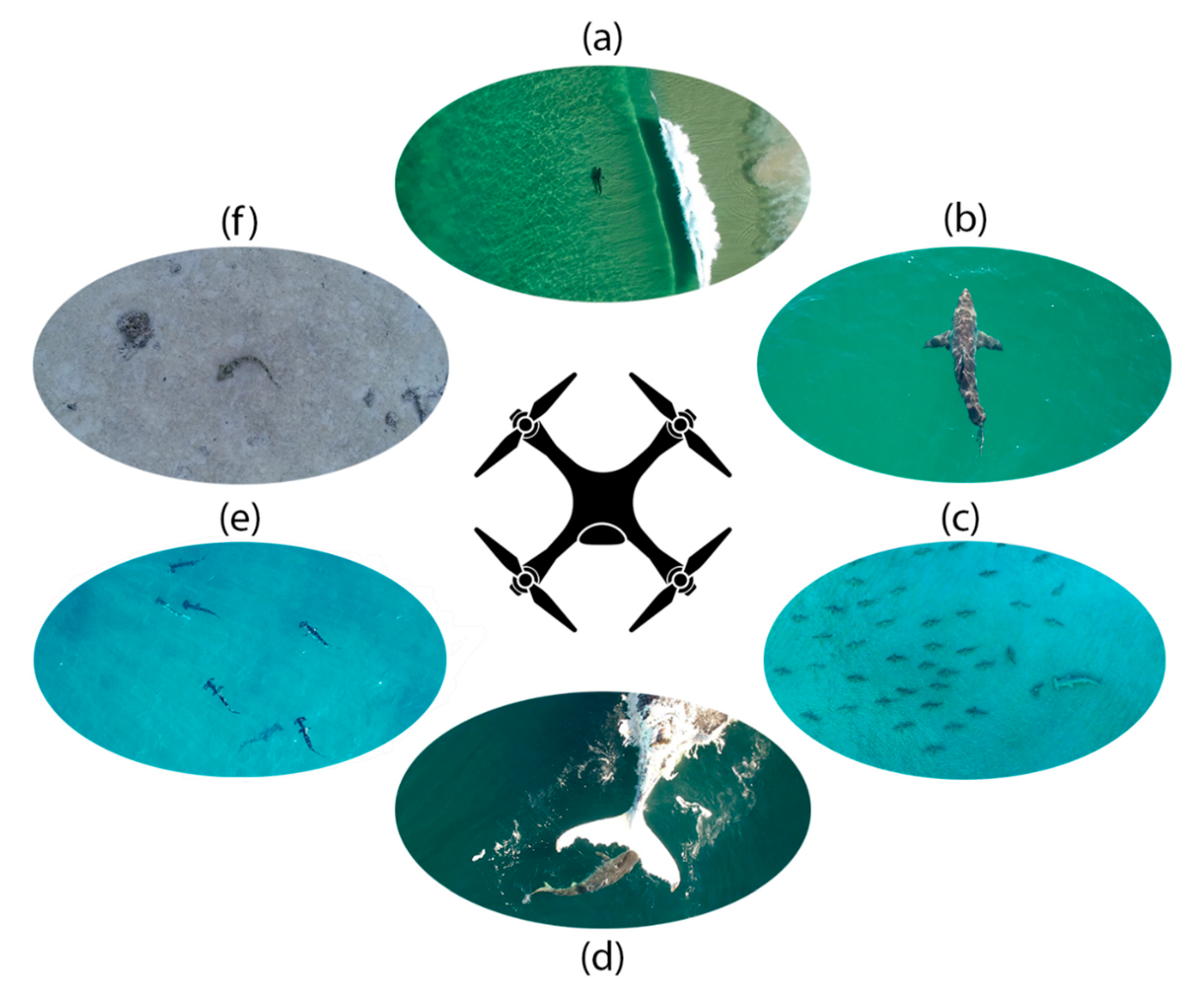
3.1. Drones as a Tool for Shark Hazard Reduction
3.2. Drone Studies of Shark Predation Events
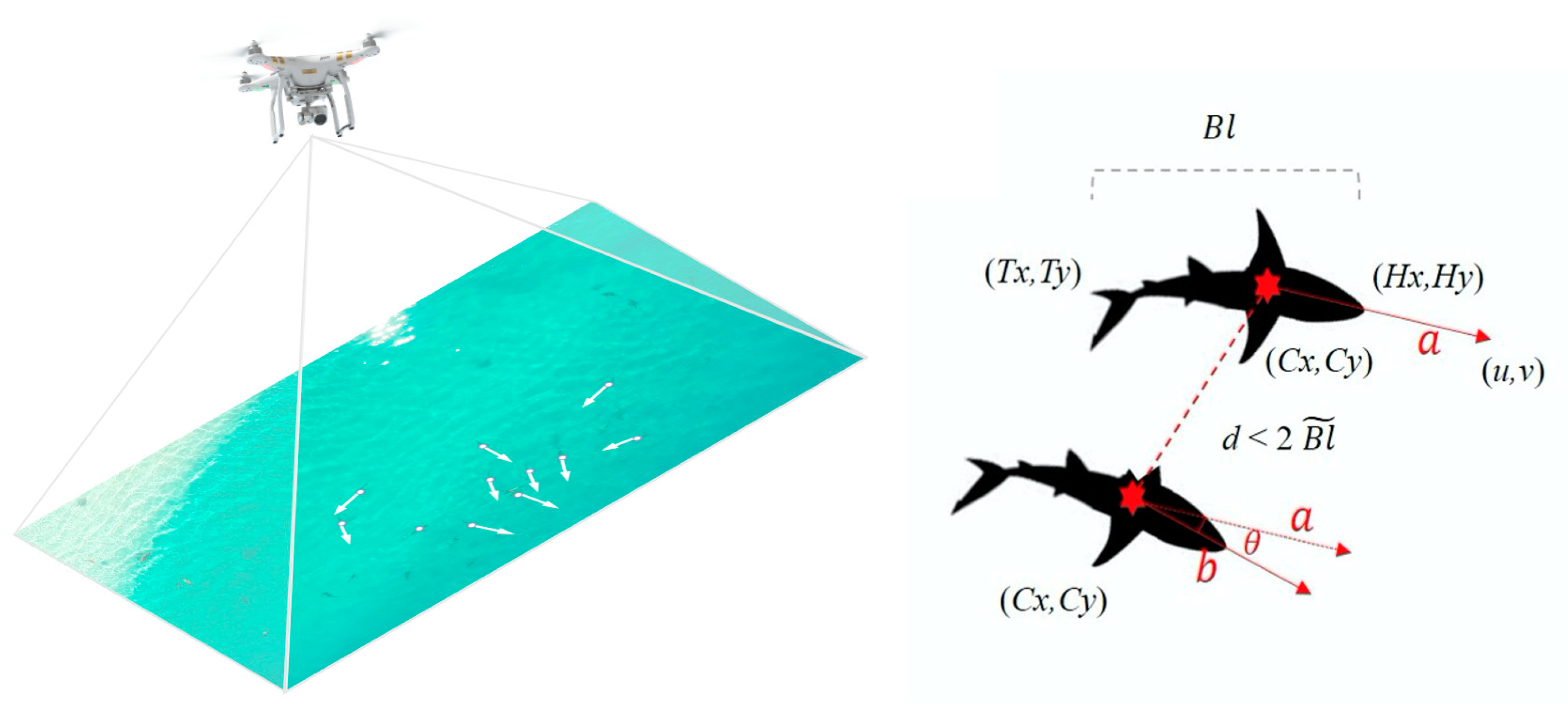
3.3. Drone Studies of Shark Behaviour and Social Interactions
3.4. Shark Behaviour around Whale Carcasses
3.5. Drone Research of Pelagic Shark Aggregations
3.6. Drone Studies of Reef Sharks
4. Enabling Technologies for Future Drone-Based Shark Research
4.1. Alternative Sensors on Drones for Shark Research
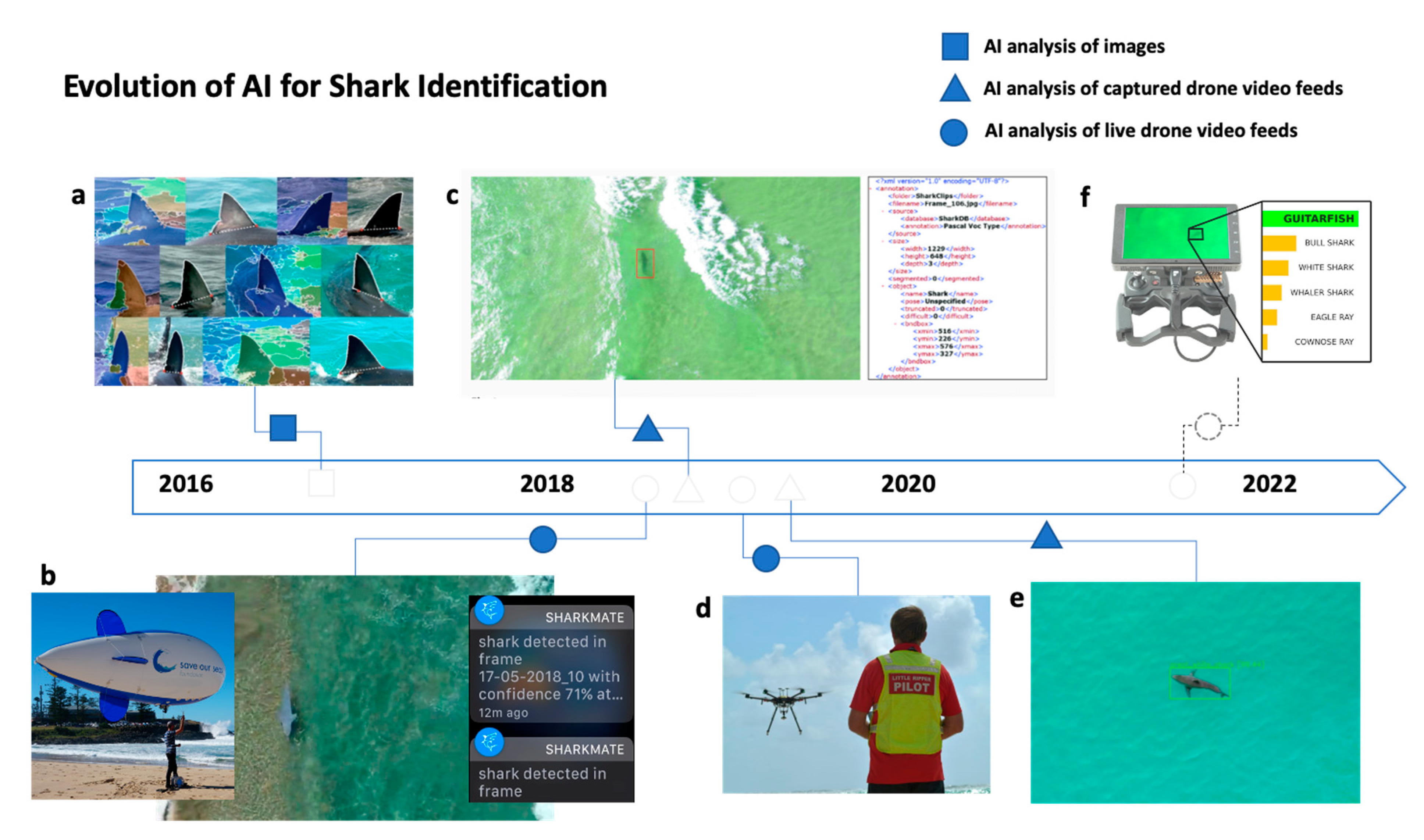
4.2. Artificial Intelligence for Shark Monitoring, Detection, and Alerting
4.3. The Potential of Underwater Drones
5. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, A. It’s okay to call them drones. J. Unmanned Veh. Syst. 2014, 2, iii–v. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Kelaher, B.P. The potential for unmanned aerial vehicles (UAVs) to conduct marine fauna surveys in place of manned aircraft. ICES J. Mar. Sci. 2018, 75, 1–8. [Google Scholar] [CrossRef]
- Kiszka, J.J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Rieucau, G.; Kiszka, J.J.; Castillo, J.C.; Mourier, J.; Boswell, K.M.; Heithaus, M.R. Using unmanned aerial vehicle (UAV) surveys and image analysis in the study of large surface-associated marine species: A case study on reef sharks Carcharhinus melanopterus shoaling behaviour. J. Fish Biol. 2018, 93, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Frixione, M.G.; García, M.D.; Gauger, M.F.W. Drone imaging of elasmobranchs: Whale sharks and golden cownose rays co-occurrence in a zooplankton hot-spot in southwestern Sea of Cortez. Food Webs 2020, 24, e00155. [Google Scholar] [CrossRef]
- Skomal, G.B.; Hoyos-Padilla, E.M.; Kukulya, A.; Stokey, R. Subsurface observations of white shark Carcharodon carcharias predatory behavior using an autonomous underwater vehicle. J. Fish Biol. 2015, 87, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S. Using Autonomous Underwater Vehicles to Assess the Habitat Use and Swimming Behavior of White Sharks (Carcharodon carcharias). Master’s Thesis, University of Massachusetts, Dartmouth, MA, USA, 2018. [Google Scholar]
- Ho, C.; Joly, K.; Nosal, A.P.; Lowe, C.G.; Clark, C.M. Predicting Coordinated Group Movements of Sharks with Limited Observations using AUVs. In Proceedings of the Symposium on Applied Computing, Marrakech, Morocco, 3–7 April 2017; pp. 289–296. [Google Scholar]
- Clark, C.M.; Forney, C.; Manii, E.; Shinzaki, D.; Gage, C.; Farris, M.; Lowe, C.G.; Moline, M. Tracking and following a tagged leopard shark with an autonomous underwater vehicle. J. Field Robot. 2013, 30, 309–322. [Google Scholar] [CrossRef]
- Hensel, E.; Wenclawski, S.; Layman, C.A. Using a small, consumer-grade drone to identify and count marine megafauna in shallow habitats. Lat. Am. J. Aquat. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Doan, M.D.; Kajiura, S.M. Adult blacktip sharks (Carcharhinus limbatus) use shallow water as a refuge from great hammerheads (Sphyrna mokarran). J. Fish Biol. 2020, 96, 1530–1533. [Google Scholar] [CrossRef]
- Benavides, M.T.; Fodrie, F.J.; Johnston, D.W. Shark detection probability from aerial drone surveys within a temperate estuary. J. Unmanned Veh. Syst. 2020, 8, 44–56. [Google Scholar] [CrossRef]
- Packard, G.E.; Kukulya, A.; Austin, T.; Dennett, M.; Littlefield, R.; Packard, G.; Purcell, M.; Stokey, R. Continuous autonomous tracking and imaging of white sharks and basking sharks using a REMUS-100 AUV. In Proceedings of the 2013 Ocean Sciences Meeting, San Diego, CA, USA, 23–27 September 2013; pp. 1–5. [Google Scholar]
- Fortuna, J.; Ferreira, F.; Gomes, R.; Ferreira, S.; Sousa, J. Using low cost open source UAVs for marine wild life monitoring—Field report. IFAC Proc. 2013, 2, 291–295. [Google Scholar] [CrossRef]
- Hawkes, L.A.; Exeter, O.; Henderson, S.M.; Kerry, C.; Kukulya, A.; Rudd, J.; Whelan, S.; Yoder, N.; Witt, M.J. Autonomous underwater videography and tracking of basking sharks. Anim. Biotelem. 2020, 8, 29. [Google Scholar] [CrossRef]
- Gore, M.; Abels, L.; Wasik, S.; Saddler, L.; Ormond, R. Are close-following and breaching behaviours by basking sharks at aggregation sites related to courtship? J. Mar. Biol. Assoc. UK 2019, 99, 681–693. [Google Scholar] [CrossRef]
- Dines, S.; Gennari, E. First observations of white sharks (Carcharodon carcharias) attacking a live humpback whale (Megaptera novaeangliae). Mar. Freshw. Res. 2020, 71, 1205–1210. [Google Scholar] [CrossRef]
- Lea, J.S.E.; Daly, R.; Leon, C.; Daly, C.A.K.; Clarke, C.R. Life after death: Behaviour of multiple shark species scavenging a whale carcass. Mar. Freshw. Res. 2019, 70, 302–306. [Google Scholar] [CrossRef]
- López, N.A.; McAuley, R.; Meeuwig, J. Identification of the southernmost aggregation of juvenile scalloped hammerhead sharks (Sphyrna lewini) in Australia. 2021; in prepare. [Google Scholar]
- Gallagher, A.J.; Papastamatiou, Y.P.; Barnett, A. Apex predatory sharks and crocodiles simultaneously scavenge a whale carcass. J. Ethol. 2018, 36, 205–209. [Google Scholar] [CrossRef]
- Raoult, V.; Tosetto, L.; Williamson, J.E. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones 2018, 2, 37. [Google Scholar] [CrossRef]
- Tucker, J.P.; Vercoe, B.; Santos, I.R.; Dujmovic, M.; Butcher, P.A. Whale carcass scavenging by sharks. Glob. Ecol. Conserv. 2019, 19, e00655. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing white shark (Carcharodon carcharias) behaviour along coastal beaches for conservation-focused shark mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Tucker, J.P.; Colefax, A.P.; Santos, I.R.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. White shark behaviour altered by stranded whale carcasses: Insights from drones and implications for beach management Ocean Coast. Manag. 2021, 200, 105477. [Google Scholar]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Reliability of marine faunal detections in drone-based monitoring. Ocean Coast. Manag. 2019, 174, 108–115. [Google Scholar] [CrossRef]
- Colefax, A.P.; Butcher, P.A.; Pagendam, D.E.; Kelaher, B.P. Comparisons of localised distributions of white, bull, and tiger sharks using three tech-based methods. Ocean Coast. Manag. 2020, 198, 105366. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Walsh, A.J.; Purcell, C.R.; Pagendam, D.E.; Cagnazzi, D.D.B.; Butcher, P.A. Utility of spectral band selection from drone-based hyperspectral imagery for improving detectability of submerged marine fauna. Biol. Conserv. 2021, submitted. [Google Scholar]
- Kelaher, B.P.; Colefax, A.P.; Tagliafico, A.; Bishop, M.J.; Giles, A.; Butcher, P.A. Assessing variation in assemblages of large marine fauna off ocean beaches using drones. Mar. Freshw. Res. 2019, 71, 68–77. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Peddemors, V.M.; Hoade, B.; Colefax, A.P.; Butcher, P.A. Comparison of sampling precision for nearshore marine wildlife using unmanned and manned aerial surveys. J. Unmanned Veh. Syst. 2019, 8, 30–43. [Google Scholar] [CrossRef]
- Saqib, M.; Khan, S.D.; Sharma, N.; Scully-Power, P.; Butcher, P.; Colefax, A.; Blumenstein, M. Real-time drone surveillance and population estimation of marine animals from aerial imagery. In Proceedings of the 2018 International Conference on Image and Vision Computing New Zealand, Auckland, New Zealand, 19–21 November 2018; pp. 1–6. [Google Scholar]
- Sharma, N.; Scully-Power, P.; Blumenstein, M. Shark Detection from Aerial Imagery Using Region-Based CNN, a Study; Mitrovic, T., Xue, B., Li, X., Eds.; AI 2018: Advances in Artificial Intelligence; Springer International Publishing: Cham, Switzerland, 2018; pp. 224–236. [Google Scholar]
- Gorkin, R., III; Adams, K.; Berryman, M.J.; Aubin, S.; Li, W.; Davis, A.R.; Barthelemy, J. Sharkeye: Real-Time Autonomous Personal Shark Alerting via Aerial Surveillance. Drones 2020, 4, 18. [Google Scholar] [CrossRef]
- Butcher, P.; Piddock, T.; Colefax, A.; Hoade, B.; Peddemors, V.; Borg, L.; Cullis, B. Beach safety: Can drones provide a platform for sighting sharks? Wildl. Res. 2019, 46, 701–712. [Google Scholar] [CrossRef]
- Raoult, V.; Tosetto, L.; Harvey, C.; Nelson, T.M.; Reed, J.; Parikh, A.; Chan, A.J.; Smith, T.M.; Williamson, J.E. Remotely operated vehicles as alternatives to snorkellers for video-based marine research. J. Exp. Mar. Biol. Ecol. 2020, 522, 1–10. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, 1–34. [Google Scholar] [CrossRef]
- Roff, G.; Brown, C.J.; Priest, M.A.; Mumby, P.J. Decline of coastal apex shark populations over the past half century. Commun. Biol. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Pepin-Neff, C.L.; Wynter, T. Reducing fear to influence policy preferences: An experiment with sharks and beach safety policy options. Mar. Policy 2018, 88, 222–229. [Google Scholar] [CrossRef]
- Chirayath, V.; Earle, S.A. Drones that see through waves—Preliminary results from airborne fluid lensing for centimetre-scale aquatic conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26 (Suppl. 2), 237–250. [Google Scholar] [CrossRef]
- Ferguson, M.C.; Angliss, R.P.; Kennedy, A.; Lynch, B.; Willoughby, A.; Helker, V.; Brower, A.A.; Clarke, J.T. Performance of manned and unmanned aerial surveys to collect visual data and imagery for estimating arctic cetacean density and associated uncertainty. J. Unmanned Veh. Syst. 2018, 6, 128–154. [Google Scholar] [CrossRef]
- Robbins, W.D.; Peddemors, V.M.; Kennelly, S.J.; Ives, M.C. Experimental evaluation of shark detection rates by aerial observers. PLoS ONE 2014, 9, e83456. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.; Apps, K.; Butcher, P.A.; Weiler, B.; Luke, H.; Colefax, A.P. Beach-user perceptions and attitudes towards drone surveillance as a shark mitigation tool. Mar. Policy 2020, 120, 104127. [Google Scholar] [CrossRef]
- Provost, E.J.; Butcher, P.A.; Colefax, A.P.; Coleman, M.A.; Curley, B.G.; Kelaher, B.P. Using drones to quantify beach users across a range of environmental conditions. J. Coast. Conserv. 2019, 23, 633–642. [Google Scholar] [CrossRef]
- Giles, A.B.; Butcher, P.A.; Colefax, A.P.; Pagendam, D.E.; Kelaher, B.P. Responses of bottlenose dolphins (Tursiops spp.) to small drones. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 1–8. [Google Scholar] [CrossRef]
- Klimley, A.P.; Anderson, S.D.; Pyle, P.; Henderson, R.P. Spatiotemporal Patterns of White Shark (Carcharodon carcharias) Predation at the South Farallon Islands, California. Copeia 1992, 3, 680–690. [Google Scholar] [CrossRef]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise Levels of Multi-Rotor Unmanned Aerial Vehicles with Implications for Potential Underwater Impacts on Marine Mammals. Front. Mar. Sci. 2016, 3, 277. [Google Scholar] [CrossRef]
- Porter, M.E.; Ruddy, B.R.; Kajiura, S.M. Volitional Swimming Kinematics of Blacktip Sharks, Carcharhinus limbatus, in the Wild. Drones 2020, 4, 78. [Google Scholar] [CrossRef]
- Kajiura, S.M.; Tellman, S.L. Quantification of massive seasonal aggregations of blacktip sharks (Carcharhinus limbatus) in southeast Florida. PLoS ONE 2016, 11, e0150911. [Google Scholar] [CrossRef] [PubMed]
- Raoult, V.; Broadhurst, M.K.; Peddemors, V.M.; Williamson, J.E.; Gaston, T.F. Resource use of great hammerhead sharks (Sphyrna mokarran) off eastern Australia. J. Fish Biol. 2019, 95, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.; Butcher, P.A.; Colefax, A.P.; Clark, G.F.; Kelaher, B.P. Variation in cownose ray Rhinoptera neglecta abundance and group size on the central east coast of Australia. J. Fish Biol. 2020, 96, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Torney, C.J.; Lamont, M.; Debell, L.; Angohiatok, R.J.; Leclerc, L.-M.; Berdahl, A.M. Inferring the rules of social interaction in migrating caribou. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170385. [Google Scholar] [CrossRef]
- Harris, J.M.; Nelson, J.A.; Rieucau, G.; Broussard, W.P. Use of Drones in Fishery Science. Trans. Am. Fish. Soc. 2019, 148, 687–697. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Patterson, T.A.; Bradford, R.W.; Butcher, P.A. Spatiotemporal distribution patterns of immature Australasian white sharks (Carcharodon carcharias). Sci. Rep. 2020, 10, 10169. [Google Scholar] [CrossRef]
- Curtis, T.H.; Kelly, J.; Menard, K.; Laroche, R.; Jones, R.; Klimley, A.P. Observations on the behavior of White Sharks scavenging from a Whale carcass at Point Reyes, California. Calif. Fish Game 2006, 92, 113–124. [Google Scholar]
- Clua, E.; Chauvet, C.; Read, T.; Werry, J.M.; Lee, S.Y. Behavioural patterns of a Tiger Shark (Galeocerdo cuvier) feeding aggregation at a Blue Whale carcass in Prony Bay, New Caledonia. Mar. Freshw. Behav. Physiol. 2013, 46, 1–20. [Google Scholar] [CrossRef]
- Dicken, M. First observations of young of the year and juvenile Great White Sharks (Carcharodon carcharias) scavenging from a whale carcass. Mar. Freshw. Res. 2008, 59, 596–602. [Google Scholar] [CrossRef]
- Tucker, J.P.; Santos, I.R.; Crocetti, S.; Butcher, P. Whale carcass strandings on beaches: Management challenges, research needs, and examples from Australia. Ocean Coast. Manag. 2018, 163, 323–338. [Google Scholar] [CrossRef]
- Tucker, J.P.; Santos, I.R.; Davis, K.L.; Butcher, P.A. Whale carcass leachate plumes in beach groundwater: A potential shark attractant to the surf? Mar. Pollut. Bull. 2019, 140, 219–226. [Google Scholar] [CrossRef]
- Fowler, S. The Conservation Status of Migratory Sharks; UNEP/CMS Secretariat: Bonn, Germany, 2014; 30p. [Google Scholar]
- Gallagher, A.J.; Hammerschlag, N.; Danylchuk, A.J.; Cooke, S.J. Shark recreational fisheries: Status, challenges, and research needs. Ambio 2017, 46, 385–398. [Google Scholar] [CrossRef]
- Fields, A.T.; Fischer, G.A.; Shea, S.K.H.; Zhang, H.; Abercrombie, D.L.; Feldheim, K.A.; Babcock, E.A.; Chapman, D.D. Species composition of the international shark fin trade assessed through a retail-market survey in Hong Kong. Conserv. Biol. 2018, 32, 376–389. [Google Scholar] [CrossRef]
- Dent, F.; Clarke, S. State of the global market for shark products. In FAO Fisheries and Aquaculture Technical Paper No. 590; FAO: Rome, Italy, 2015; 187p. [Google Scholar]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.G.; Jiao, Y.; Cortés, E. Stock Assessment of Scalloped Hammerheads in the Western North Atlantic Ocean and Gulf of Mexico. North Am. J. Fish. Manag. 2009, 29, 1406–1417. [Google Scholar] [CrossRef]
- Hutchings, J.A.; Myers, R.A.; García, V.B.; Lucifora, L.O.; Kuparinen, A. Life-history correlates of extinction risk and recovery potential. Ecol. Appl. 2012, 22, 1061–1067. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Baum, J.K.; Clarke, S.; Compagno, L.J.V.; Cortés, E.; Domingo, A.; Fordham, S.; Fowler, S.; Francis, M.P.; Gibson, C.; et al. You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 459–482. [Google Scholar] [CrossRef]
- Ketchum, J.T.; Galván-Magaña, F.; Klimley, A.P. Segregation and foraging ecology of whale sharks, Rhincodon typus, in the southwestern Gulf of California. Environ. Biol. Fishes 2013, 96, 779–795. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Mildward, N.E. Utilisation of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ. Biol. Fishes 1993, 37, 337–345. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A. Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar. Biol. 2005, 147, 1239–1249. [Google Scholar] [CrossRef]
- Rowat, D.; Brooks, K.; March, A.; McCarten, C.; Jouannet, D.; Riley, L.; Jeffreys, G.; Perri, M.; Vely, M.; Pardigon, B. Long-term membership of whale sharks (Rhincodon typus) in coastal aggregations in Seychelles and Djibouti. Mar. Freshw. Res. 2011, 62, 621–627. [Google Scholar] [CrossRef]
- Nalesso, E.; Hearn, A.; Sosa-Nishizaki, O.; Steiner, T.; Antoniou, A.; Reid, A.; Bessudo, S.; Soler, G.; Klimley, P.; Lara, F.; et al. Movements of scalloped hammerhead sharks (Sphyrna lewini) at Cocos Island, Costa Rica and between oceanic islands in the Eastern Tropical Pacific. PLoS ONE 2019, 14, e0213741. [Google Scholar] [CrossRef]
- Mucientes, G.R.; Queiroz, N.; Sousa, L.L.; Tarroso, P.; Sims, D.W. Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol. Lett. 2009, 5, 156–159. [Google Scholar] [CrossRef]
- Clarke, S.C.; McAllister, M.K.; Milner-Gulland, E.J.; Kirkwood, G.P.; Michielsens, C.G.J.; Agnew, D.J.; Pikitch, E.K.; Nakano, H.; Shivji, M.S. Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 2006, 9, 1115–1126. [Google Scholar] [CrossRef]
- Graham, F.; Rynne, P.; Estevanez, M.; Luo, J.; Ault, J.S.; Hammerschlag, N. Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Divers. Distrib. 2016, 22, 534–546. [Google Scholar] [CrossRef]
- Queiroz, N.; Humphries, N.E.; Mucientes, G.; Hammerschlag, N.; Lima, F.P.; Scales, K.L.; Miller, P.I.; Sousa, L.L.; Seabra, R.; Sims, D.W. Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc. Natl. Acad. Sci. USA 2016, 113, 1582–1587. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Sharks of the World; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Gallagher, A.J.; Klimley, A.P. The biology and conservation status of the large hammerhead shark complex: The great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fish. 2018, 28, 777–794. [Google Scholar] [CrossRef]
- Bessudo, S.; Soler, G.A.; Klimley, P.A.; Ketchum, J.; Arauz, R.; Hearn, A.; Guzmán, A.; Calmettes, B. Vertical and horizontal movements of the scalloped hammerhead shark (Sphyrna lewini) round Malpelo and Cocos Islands (Tropical Eastern Pacific) using satellite telemetry. Bull. Mar. Coast. Res. 2011, 40, 91–106. [Google Scholar]
- Hammerschlag, N.; Gallagher, A.J.; Lazarre, D.M.; Slonim, C. Range extension of the endangered great hammerhead shark Sphyrna mokarran in the Northwest Atlantic: Preliminary data and significance for conservation. Endanger. Species Res. 2011, 13, 111–116. [Google Scholar] [CrossRef]
- Santos, C.; Coehlo, R. Migrations and habitat use of the smooth hammerhead shark (Sphyrna zygaena) in the Atlantic Ocean. PLoS ONE 2018, 13, e0198664. [Google Scholar] [CrossRef]
- Roemer, R.P.; Gallagher, A.J.; Hammerschlag, N. Shallow water tidal flat use and associated specialized foraging behavior of the great hammerhead shark (Sphyrna mokarran). Mar. Freshw. Behav. Physiol. 2016, 49, 235–249. [Google Scholar] [CrossRef]
- Hearn, A.; Ketchum, J.; Klimley, A.P.; Espinoza, E.; Peñaherrera, C. Hotspots within hotspots? Hammerhead shark movements around Wolf Island, Galapagos Marine Reserve. Mar. Biol. 2010, 157, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.P. Distribution, habitat and movement of juvenile smooth hammerhead sharks (Sphyrna zygaena) in northern New Zealand. N. Z. J. Mar. Freshw. Res. 2016, 50, 506–525. [Google Scholar] [CrossRef]
- Brown, K.T.; Seeto, J.; Lal, M.M.; Miller, C.E. Discovery of an important aggregation area for endangered scalloped hammerhead sharks, Sphyrna lewini, in the Rewa River estuary, Fiji Islands. Pac. Conserv. Biol. 2016, 22, 242–248. [Google Scholar] [CrossRef]
- Duncan, K.M.; Holland, K.N. Habitat use, growth rates and dispersal patterns of juvenile scalloped hammerhead sharks Sphyrna lewini in a nursery habitat. Mar. Ecol. Prog. Ser. 2006, 312, 211–221. [Google Scholar] [CrossRef]
- Jennings, R.D. Seasonal abundance of hammerhead sharks off Cape Canaveral, Florida. Copeia 1985, 223–225. [Google Scholar] [CrossRef]
- Kenney, R.D.; Owen, R.E.; Winn, H.E. Shark distributions off the Northeast United States from Marine Mammal Surveys. Copeia 1985, 1985, 220–223. [Google Scholar] [CrossRef]
- Dicken, M.L.; Booth, A.J. Surveys of white sharks (Carcharodon carcharias) off bathing beaches in Algoa Bay, South Africa. Mar. Freshw. Res. 2013, 64, 530–539. [Google Scholar] [CrossRef]
- Laran, S.; Authier, M.; Van Canneyt, O.; Dorémus, G.; Watremez, P.; Ridoux, V. A comprehensive survey of pelagic megafauna: Their distribution, densities, and taxonomic richness in the tropical Southwest Indian ocean. Front. Mar. Sci. 2017, 4, 139. [Google Scholar] [CrossRef]
- Ducatez, S. Which sharks attract research? Analyses of the distribution of research effort in sharks reveal significant non-random knowledge biases. Rev. Fish Biol. Fish. 2019, 29, 355–367. [Google Scholar] [CrossRef]
- Osgood, G.; Baum, J. Reef sharks: Recent advances in ecological understanding to inform conservation. J. Fish Biol. 2015, 87, 1489–1523. [Google Scholar] [CrossRef] [PubMed]
- Heupel, M.; Simpfendorfer, C. Using acoustic monitoring to evaluate MPAs for shark nursery areas: The importance of long-term data. Mar. Technol. Soc. J. 2005, 39, 10–18. [Google Scholar] [CrossRef]
- Heupel, M.R.; Lédée, E.J.; Simpfendorfer, C.A. Telemetry reveals spatial separation of co-occurring reef sharks. Mar. Ecol. Prog. Ser. 2018, 589, 179–192. [Google Scholar] [CrossRef]
- Cagua, E.F.; Berumen, M.L.; Tyler, E. Topography and biological noise determine acoustic detectability on coral reefs. Coral Reefs 2013, 32, 1123–1134. [Google Scholar] [CrossRef]
- Whitmarsh, S.K.; Fairweather, P.G.; Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish Biol. Fish. 2017, 27, 53–73. [Google Scholar] [CrossRef]
- Barker, S.M.; Peddemors, V.M.; Williamson, J.E. A video and photographic study of aggregation, swimming and respiratory behaviour changes in the Grey Nurse Shark (Carcharias taurus) in response to the presence of SCUBA divers. Mar. Freshw. Behav. Physiol. 2011, 44, 75–92. [Google Scholar] [CrossRef]
- Smith, K.; Scarr, M.; Scarpaci, C. Grey nurse shark (Carcharias taurus) diving tourism: Tourist compliance and shark behaviour at Fish Rock, Australia. Environ. Manag. 2010, 46, 699–710. [Google Scholar] [CrossRef]
- Joyce, K.; Duce, S.; Leahy, S.; Leon, J.X.; Maier, S. Principles and practice of acquiring drone based image data in marine environments. Mar. Freshw. Res. 2018, 70, 952–963. [Google Scholar] [CrossRef]
- Carlisle, A.B.; Starr, R.M. Habitat use, residency, and seasonal distribution of female leopard sharks Triakis semifasciata in Elkhorn Slough, California. Mar. Ecol. Prog. Ser. 2009, 380, 213–228. [Google Scholar] [CrossRef]
- Nakano, H.; Matsunaga, H.; Okamoto, H.; Okazaki, M. Acoustic tracking of bigeye thresher shark Alopias superciliosus in the eastern Pacific Ocean. Mar. Ecol. Prog. Ser. 2003, 265, 255–261. [Google Scholar] [CrossRef]
- Kessel, S.T.; Gruber, S.; Gledhill, K.; Bond, M.; Perkins, R.G. Aerial survey as a tool to estimate abundance and describe distribution of a carcharhinid species, the lemon shark, Negaprion brevirostris. J. Mar. Biol. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Bennett, M.K.; Younes, N.; Joyce, K. Automating Drone Image Processing to Map Coral Reef Substrates Using Google Earth Engine. Drones 2020, 4, 50. [Google Scholar] [CrossRef]
- Casella, E.; Collin, A.; Harris, D.; Ferse, S.; Bejarano, S.; Parravicini, V.; Hench, J.L.; Rovere, A. Mapping coral reefs using consumer-grade drones and structure from motion photogrammetry techniques. Coral Reefs 2017, 36, 269–275. [Google Scholar] [CrossRef]
- Kabiri, K.; Rezai, H.; Moradi, M. A drone-based method for mapping the coral reefs in the shallow coastal waters–case study: Kish Island, Persian gulf. Earth Sci. Inform. 2020, 13, 1265–1274. [Google Scholar] [CrossRef]
- Chabot, D. Trends in drone research and applications as the Journal of Unmanned Vehicle Systems turns five. J. Unmanned Veh. Syst. 2018, 6, vi–xv. [Google Scholar] [CrossRef]
- Hardin, P.J.; Lulla, V.; Jensen, R.R.; Jensen, J.R. Small Unmanned Aerial Systems (sUAS) for environmental remote sensing: Challenges and opportunities revisited. GISci. Remote Sens. 2018, 56, 309–322. [Google Scholar] [CrossRef]
- Johnston, D.W. Unoccupied aircraft systems in marine science and conservation. Annu. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef]
- Letnes, P.A.; Hansen, I.M.; Aas, L.M.S.; Eide, I.; Pettersen, R.; Tassara, L.; Receveur, J.; le Floch, S.; Guyomarch, J.; Camus, L.; et al. Underwater hyperspectral classification of deep sea corals exposed to 2-methylnaphthalene. PLoS ONE 2019, 14, e0209960. [Google Scholar] [CrossRef]
- Chennu, A.; Farber, P.; De’ath, G.; de Beer, D.; Fabricius, K.E. A diver-operated hyperspectral imaging and topographic surveying system for automated mapping of benthic habitats. Sci. Rep. 2017, 7, 7122. [Google Scholar] [CrossRef]
- Colefax, A. Developing the Use of Drones for Non-Destructive Shark Management and Beach Safety. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2020. [Google Scholar]
- Hodgson, J.C.; Baylis, S.M.; Mott, R.; Herrod, A.; Clarke, R.H. Precision wildlife monitoring using unmanned aerial vehicles. Sci. Rep. 2016, 6, 22574. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef] [PubMed]
- Seymour, A.C.; Dale, J.; Hammill, M.; Halpin, P.N.; Johnston, D.W. Automated detection and enumeration of marine wildlife using unmanned aircraft systems (UAS) and thermal imagery. Sci. Rep. 2017, 7, 45127. [Google Scholar] [CrossRef] [PubMed]
- Spaan, D.; Burke, C.; McAree, O.; Aureli, F.; Rangel-Rivera, C.E.; Hutschenreiter, A.; Longmore, S.N.; McWhirter, P.R.; Wich, S.A. Thermal Infrared Imaging from Drones Offers a Major Advance for Spider Monkey Surveys. Drones 2019, 3, 34. [Google Scholar] [CrossRef]
- Horton, T.W.; Hauser, N.; Cassel, S.; Klaus, K.F.; Fettermann, T.; Key, N. Doctor Drone: Non-invasive Measurement of Humpback Whale Vital Signs Using Unoccupied Aerial System Infrared Thermography. Front. Mar. Sci. 2019, 6, 466. [Google Scholar] [CrossRef]
- Thomas, G.L.; Thorne, R.E. Night-time predation by Steller sea lions. Nature 2001, 411, 1013. [Google Scholar] [CrossRef] [PubMed]
- Schoonmaker, J.S.; Podobna, Y.; Boucher, C.D. Electro-optical approach for airborne marine mammal surveys and density estimations. U.S. Navy J. Underw. Acoust. 2011, 61, 668–985. [Google Scholar]
- Blount, C.; Schoonmaker, J.; Saggese, S.; Oakley, D. An Innovative Method for Obtaining High Detection Rates of Sharks on Ocean Beaches; A Report for Shark Alert Pty Ltd.; Cardno: Sydney, NSW, Australia, 2016; 35p. [Google Scholar]
- Fretwell, P.T.; Staniland, I.J.; Forcada, J. Whales from space: Counting southern right whales by satellite. PLoS ONE 2014, 9, e88655. [Google Scholar] [CrossRef]
- Parsons, M.; Bratanov, D.; Gaston, K.J.; Gonzalez, F. UAVs, Hyperspectral Remote Sensing, and Machine Learning Revolutionizing Reef Monitoring. Sensors 2018, 18, 2026. [Google Scholar] [CrossRef]
- Burke, C.; Rashman, M.F.; McAree, O.; Hambrecht, L.; Longmore, S.N.; Piel, A.K.; Wich, S.A. Addressing environmental and atmospheric challenges for capturing high-precision thermal infrared data in the field of astro-ecology. In Proceedings Volume 10709, High Energy, Optical, and Infrared Detectors for Astronomy VIII; SPIE Astronomical Telescopes + Instrumentation: Austin, TX, USA, 2018. [Google Scholar]
- Hambrecht, L.; Brown, R.P.C.; Piel, A.K.; Wich, S.A. Detecting ‘poachers’ with drones: Factors influencing the probability of detection with TIR and RGB imaging in miombo woodlands, Tanzania. Biol. Conserv. 2019, 233, 109–117. [Google Scholar] [CrossRef]
- Hodgson, J.; Mott, R.; Baylis, S.; Pham, T.; Wotherspoon, S.; Kilpatrick, A.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Burr, P.; Samiappan, S.; Hathcock, L.; Moorhead, R.; Dorr, B. Estimating waterbird abundance on catfish aquaculture ponds using an unmanned aerial system. Hum. Wildl. Interact. 2019, 13. [Google Scholar] [CrossRef]
- Eikelboom, J.; Wind, J.; Ven, E.; Kenana, M.; Schroder, B.; Knegt, H.; Langevelde, F.; Prins, H. Improving the precision and accuracy of animal population estimates with aerial image object detection. Methods Ecol. Evol. 2019, 10, 1875–1887. [Google Scholar] [CrossRef]
- Sandino, J.; Gonzalez, F. A novel approach for invasive weeds and vegetation surveys using UAS and Artificial Intelligence. In Proceedings of the 2018 23rd International Conference on Methods Models in Automation Robotics (MMAR), Międzyzdroje, Poland, 27–30 August 2018; pp. 515–520. [Google Scholar]
- Nevalainen, O.; Honkavaara, E.; Tuominen, S.; Viljanen, N.; Hakala, T.; Yu, X.; Hyyppä, J.; Saari, H.; Pölönen, I.; Imai, N.; et al. Individual tree detection and classification with UAV-based photogrammetric point clouds and hyperspectral imaging. Remote Sens. 2017, 9, 185. [Google Scholar] [CrossRef]
- Sandino, J.; Pegg, G.; Gonzalez, F.; Smith, G. Aerial Mapping of forests affected by pathogens using UAVs, hyperspectral sensors, and artificial intelligence. Sensors 2018, 18, 944. [Google Scholar] [CrossRef] [PubMed]
- Geraeds, M.; van Emmerik, T.; de Vries, R.; Ab Razak, M.S. Riverine plastic litter monitoring using unmanned aerial vehicles (UAVs). Remote Sens. 2019, 11, 2045. [Google Scholar] [CrossRef]
- Dujon, A.; Schofield, G. Importance of machine learning for enhancing ecological studies using information-rich imagery. Endanger. Species Res. 2019, 39, 91–104. [Google Scholar] [CrossRef]
- Maire, F.; Alvarez, L.M.; Hodgson, A. Automating marine mammal detection in aerial images captured during wildlife surveys: A deep learning approach. In AI 2015: Advances in Artificial Intelligence; Pfahringer, B., Renz, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 379–385. [Google Scholar]
- Dharmawan, W.; Nambo, H. End-to-End Xception model implementation on Carla Self Driving Car in moderate dense environment. In Proceedings of the 2019 2nd Artificial Intelligence and Cloud Computing Conference, AICCC 2019, Kobe, Japan, 21–23 December 2019; Association for Computing Machinery: New York, NY, USA, 2019; pp. 139–143. [Google Scholar]
- Sanil, N.; Rakesh, V.; Mallapur, R.; Ahmed, M.R. Deep learning techniques for obstacle detection and avoidance in driverless cars. In Proceedings of the 2020 International Conference on Artificial Intelligence and Signal Processing (AISP), Vellore, India, 10–12 January 2020; pp. 1–4. [Google Scholar]
- Ismail, W.N.; Hassan, M.M.; Alsalamah, H.A.; Fortino, G. CNN-Based health model for regular health factors analysis in internet-of-medical things environment. IEEE Access 2020, 8, 52541–52549. [Google Scholar] [CrossRef]
- Ditria, E.M.; Lopez-Marcano, S.; Sievers, M.; Jinks, E.L.; Brown, C.J.; Connolly, R.M. Automating the analysis of fish abundance using object detection: Optimizing animal ecology with deep learning. Front. Mar. Sci. 2020, 7, 429. [Google Scholar] [CrossRef]
- Fernandes, A.F.; Turra, E.M.; de Alvarenga, É.R.; Passafaro, T.L.; Lopes, F.B.; Alves, G.F.; Singh, V.; Rosa, G.J. Deep Learning image segmentation for extraction of fish body measurements and prediction of body weight and carcass traits in Nile tilapia. Comput. Electron. Agric. 2020, 170, 105274. [Google Scholar] [CrossRef]
- Hughes, B.; Burghardt, T. Automated visual fin identification of individual great white sharks. Int. J. Comput. Vis. 2017, 122, 542. [Google Scholar] [CrossRef]
- Gonda, F.; Kaynig, V.; Jones, T.R.; Haehn, D.; Lichtman, J.W.; Parag, T.; Pfister, H. ICON: An Interactive Approach to Train Deep Neural Networks for Segmentation of Neuronal Structures. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 327–331. [Google Scholar]
- Smith, A.G.; Han, E.; Petersen, J.; Olsen, N.A.F.; Giese, C.; Athmann, M.; Dresbøll, D.B.; Thorup-Kristensen, K. RootPainter: Deep learning segmentation of biological images with corrective annotation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kellenberger, B.; Marcos, D.; Lobry, S.; Tuia, D. Half a percent of labels is enough: Efficient animal detection in UAV imagery using deep CNNs and active learning. IEEE Trans. Geosci. Remote Sens. 2019, 57, 9524–9533. [Google Scholar] [CrossRef]
- Chirayath, V.; Li, A. Next-Generation optical sensing technologies for exploring ocean worlds—NASA FluidCam, MiDAR, and NeMO-Net. Front. Mar. Sci. 2019, 6, 521. [Google Scholar] [CrossRef]
- Gray, P.C.; Bierlich, K.C.; Mantell, S.A.; Friedlaender, A.S.; Goldbogen, J.A.; Johnston, D.W. Drones and convolutional neural networks facilitate automated and accurate cetacean species identification and photogrammetry. Methods Ecol. Evol. 2019, 10, 1490–1500. [Google Scholar] [CrossRef]
- Lowe, C.G.; White, C.F.; Clark, C.M. Use of autonomous vehicles for tracking and surveying of acoustically tagged elasmobranchs. In Shark Research: Emerging Technologies and Applications for the Field and Laboratory; Carrier, J., Heithaus, M., Simpfendorfer, C., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Eiler, J.H.; Grothues, T.M.; Dobarro, J.A.; Masuda, M.M. Comparing autonomous underwater vehicle (AUV) and vessel-based tracking performance for locating acoustically tagged fish. Mar. Fish. Rev. 2013, 75, 27–42. [Google Scholar] [CrossRef]
- Goudey, C.A.; Consi, T.; Manley, J.; Graham, M.; Donovan, B.; Kiley, L. A robotic boat for autonomous fish tracking. Mar. Technol. Soc. J. 1998, 32, 47. [Google Scholar]
- Grothues, T.; Dobarro, J.; Eiler, J. Collecting, interpreting, and merging fish telemetry data from an AUV: Remote sensing from an already remote platform. In Proceedings of the 2010 Autonomous Underwater Vehicles Symposium, Monterey, CA, USA, 1–3 September 2010; Volume 136, pp. 1–9. [Google Scholar]
- Grothues, T.; Dobarro, J.; Ladd, J.; Higgs, A.; Niezgoda, G.; Miller, D. Use of a multi-sensored AUV to telemeter tagged Atlantic sturgeon and map their spawning habitat in the Hudson River, USA. In Proceedings of the 2008 Autonomous Underwater Vehicles Symposium, Woods Hole, MA, USA, 13–14 October 2008; pp. 1–7. [Google Scholar]
- Raoult, V.; Williamson, J.E.; Smith, T.M.; Gaston, T.F. Effects of on-deck holding conditions and air exposure on post-release behaviours of sharks revealed by a remote operated vehicle. J. Exp. Mar. Biol. Ecol. 2019, 511, 10–18. [Google Scholar] [CrossRef]
- White, C.F.; Lin, Y.; Clark, C.M.; Lowe, C.G. Human vs robot: Comparing the viability and utility of autonomous underwater vehicles for the acoustic telemetry tracking of marine organisms. J. Exp. Mar. Biol. Ecol. 2016, 485, 112–118. [Google Scholar] [CrossRef]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, N.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.; Pirotta, V.; et al. Operational protocols for the Use of Drones in Marine Animal Research. J. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
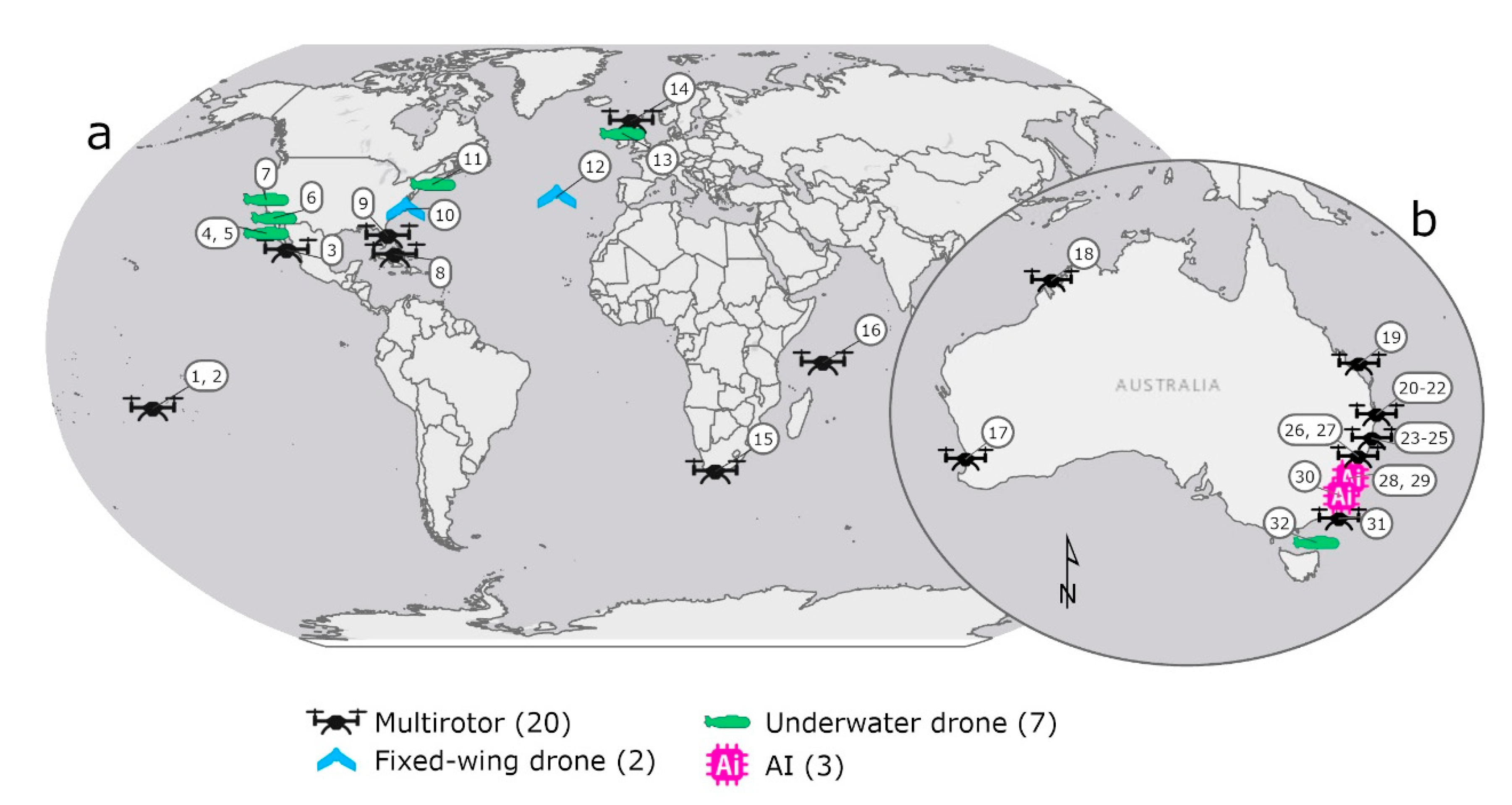

| ID | Location | Drone | Model | Focus | Reference |
|---|---|---|---|---|---|
| 1 | Moorea, French Polynesia | Multirotor | DJI Phantom 2 | Shark density | Kiszka et al. [3] |
| 2 | Moorea, French Polynesia | Multirotor | DJI Phantom 2 | Shoaling behaviour | Rieucau et al. [4] |
| 3 | Bahia de la Paz, Baja, Mexico | Multirotor | DJI Spark | Co-occurrence | Frixione et al. [5] |
| 4 | Guadalupe Island, Mexico | Underwater drone (AUV) | REMUS-100 | Shark behaviour | Skomal et al. [6] |
| 5 | Guadalupe Island, Mexico | Underwater drone (AUV) | REMUS-100 | Fine scale movements | Gabriel [7] |
| 6 | La Jolla, CA, USA | Underwater drone (AUV) | Not-specified | Group movements | Ho et al. [8] |
| 7 | SeaPlane Lagoon, CA, USA | Underwater drone (AUV) | Oceanserver IVER2 | Shark movements | Clark et al. [9] |
| 8 | Bahamas, USA | Multirotor | DJI Phantom 2+ | Detectability | Hensel et al. [10] |
| 9 | Florida SE Coast, USA | Multirotor | DJI Phantom 4 Pro | Predatory avoidance behaviour | Doan and Kajiura [11] |
| 10 | Beaufort, NC, USA | Fixed-wing drone | eBee | Detectability of shark analogues | Benavides et al. [12] |
| 11 | Cape Cod, MA, USA | Underwater drone (AUV) | REMUS-100 | Shark movements | Packard et al. [13] |
| 12 | Faial Island, Azores, Portugal | Fixed-wing drone | Skywalker X8 | Detectability of aggregations | Fortuna et al. [14] |
| 13 | Sea of the Hebrides, UK | Underwater drone (AUV) | REMUS-100 | Sub-surface behaviour | Hawkes et al. [15] |
| 14 | Sea of the Hebrides, UK | Multirotor | DJI Phantom 3 Pro | Social behaviour | Gore et al. [16] |
| 15 | Mossel Bay, South Africa | Multirotor | DJI Phantom 3 and 4 | Whale hunting behaviour | Dines and Gennari [17] |
| 16 | D’Arros and St Joseph, Seychelles | Multirotor | DJI Phantom 4 | Whale scavenging/hunting behaviour | Lea et al. [18] |
| 17 | Shoalwater, WA, Australia | Multirotor | DJI Mavic Pro | Shoaling behaviour | López et al. [19] |
| 18 | Kimberly, WA, Australia | Multirotor | DJI Phantom 4 | Whale scavenging/hunting behaviour | Gallagher et al. [20] |
| 19 | Heron Island, Queensland, Australia | Multirotor | DJI Phantom 3 Pro | Shark movement tracking | Raoult et al. [21] |
| 20 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Whale scavenging/hunting behaviour | Tucker et al. [22] |
| 21 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Swimming behaviour | Colefax et al. [23] |
| 22 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Swimming behaviour | Tucker et al. [24] |
| 23 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Detection probability | Colefax et al. [25] |
| 24 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Detection probability | Colefax et al. [26] |
| 25 | NSW Coast, Australia | Multirotor | DJI Matrice | Detection probability | Colefax et al. [27] |
| 26 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Faunal richness | Kelaher et al. [28] |
| 27 | NSW Coast, Australia | Multirotor | DJI Phantom 4 | Helicopter v drone for shark detection | Kelaher et al. [29] |
| 28 | NSW Coast, Australia | Artificial intelligence | Not-specified | Detection probability | Saqib et al. [30] |
| 29 | NSW Coast, Australia | Artificial intelligence | Not-specified | Detection probability | Sharma et al. [31] |
| 30 | NSW Coast, Australia | Artificial intelligence | Blimp-based system | Shark surveillance | Gorkin III et al. [32] |
| 31 | NSW Coast, Australia | Multirotor | DJI Inspire 1 | Beach safety | Butcher et al. [33] |
| 32 | Flinders Island, Tasmania, Australia | Underwater drone (ROV) | BlueROV 2 | Post-release behaviour | Raoult et al. [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butcher, P.A.; Colefax, A.P.; Gorkin, R.A.; Kajiura, S.M.; López, N.A.; Mourier, J.; Purcell, C.R.; Skomal, G.B.; Tucker, J.P.; Walsh, A.J.; et al. The Drone Revolution of Shark Science: A Review. Drones 2021, 5, 8. https://doi.org/10.3390/drones5010008
Butcher PA, Colefax AP, Gorkin RA, Kajiura SM, López NA, Mourier J, Purcell CR, Skomal GB, Tucker JP, Walsh AJ, et al. The Drone Revolution of Shark Science: A Review. Drones. 2021; 5(1):8. https://doi.org/10.3390/drones5010008
Chicago/Turabian StyleButcher, Paul A., Andrew P. Colefax, Robert A. Gorkin, Stephen M. Kajiura, Naima A. López, Johann Mourier, Cormac R. Purcell, Gregory B. Skomal, James P. Tucker, Andrew J. Walsh, and et al. 2021. "The Drone Revolution of Shark Science: A Review" Drones 5, no. 1: 8. https://doi.org/10.3390/drones5010008
APA StyleButcher, P. A., Colefax, A. P., Gorkin, R. A., Kajiura, S. M., López, N. A., Mourier, J., Purcell, C. R., Skomal, G. B., Tucker, J. P., Walsh, A. J., Williamson, J. E., & Raoult, V. (2021). The Drone Revolution of Shark Science: A Review. Drones, 5(1), 8. https://doi.org/10.3390/drones5010008






