Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays
Abstract
1. Introduction
2. Opportunities for Ray Research Using Drones
2.1. Drones as a Tool to Monitor Ray Behaviour
2.2. Drones as a Mapping Tool
3. Current Challenges for Ray Research Using Drones
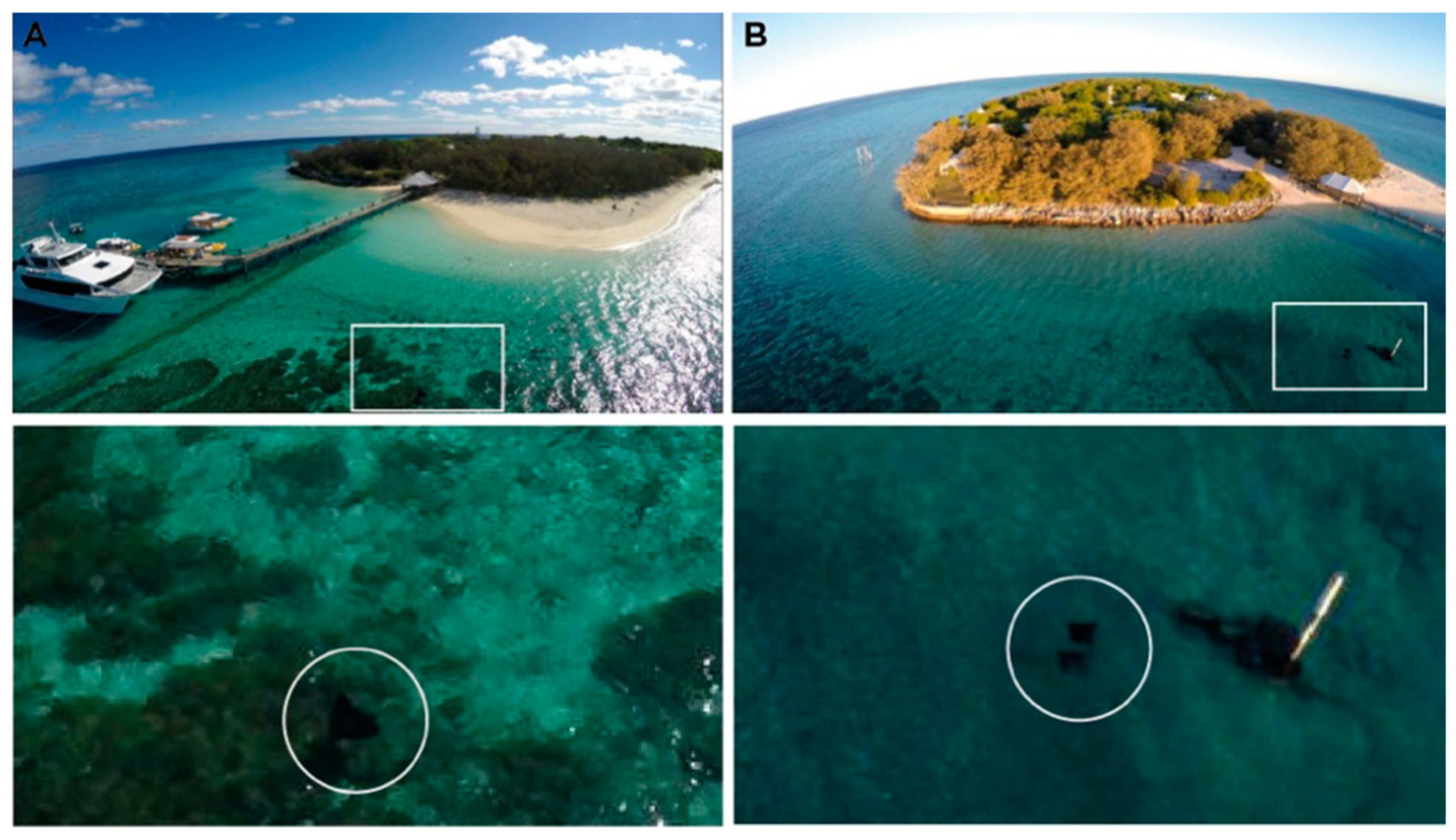
3.1. Challenges with Monitoring Natural Ray Behaviour Using Drones
3.2. Overcoming Challenges in Monitoring Natural Ray Behaviour Using Drones
3.3. Challenges with Drone-Based Habitat Mapping
3.4. Overcoming Drone-Based Habitat Mapping Challenges
4. Broader Issues for the Application of Drones in Ray Research
4.1. Regulatory Issues
| Approach | Definition | Example |
|---|---|---|
| Outright or Effective Ban | Do not allow commercial flight of drones at all, or enforce requirements that are practically unattainable | Egypt permits government approved commercial flights, though permission has never been explicitly given |
| VLOS (Visual Line Of Sight) Dependent | Commercial flights are permissible while maintaining a VLOS of the drone, with some countries allowing exceptions to constant VLOS according to appropriate accreditation and relevant permissions | Australia enforces a set of standard operating conditions including VLOS, allowing exceptions for formally licensed pilots with an operating certificate from the Civil Aviation Safety Authority |
| Permissive | Legislative regulations are reasonable and relatively unrestrictive, with appropriate avenues implemented to attain required permissions, licensing and registration | Sweden has clear and attainable certification requirements that safely enable the commercial use of drones |
4.2. Overcoming Regulatory Issues
4.3. Technical and Operational Issues
4.4. Overcoming Technical and Operational Issues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levitis, D.A.; Lidicker, W.Z.; Freund, G. Behavioural biologists do not agree on what constitutes behaviour. Anim. Behav. 2009, 78, 103–110. [Google Scholar] [CrossRef]
- Katzner, T.E.; Arlettaz, R. Evaluating Contributions of Recent Tracking-Based Animal Movement Ecology to Conservation Management. Front. Ecol. Evol. 2020, 7, 519. [Google Scholar] [CrossRef]
- Ogburn, M.B.; Harrison, A.-L.; Whoriskey, F.G.; Cooke, S.J.; Mills Flemming, J.E.M.; Torres, L.G. Addressing Challenges in the Application of Animal Movement Ecology to Aquatic Conservation and Management. Front. Mar. Sci. 2017, 4, 70. [Google Scholar] [CrossRef]
- Cooke, S.J.; Martins, E.G.; Struthers, D.P.; Gutowsky, L.F.G.; Power, M.; Doka, S.E.; Dettmers, J.M.; Crook, D.A.; Lucas, M.C.; Holbrook, C.M.; et al. A moving target—incorporating knowledge of the spatial ecology of fish into the as-sessment and management of freshwater fish populations. Environ. Monit. Assess. 2016, 188, 239. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Merriner, J.V. Food Habits and Feeding Behavior of the Cownose Ray, Rhinoptera bonasus, in Lower Chesapeake Bay. Estuaries 1985, 8, 305–310. [Google Scholar] [CrossRef]
- Perryman, R.J.Y.; Venables, S.K.; Tapilatu, R.F.; Marshall, A.D.; Brown, C.; Franks, D.W. Social preferences and network structure in a population of reef manta rays. Behav. Ecol. Sociobiol. 2019, 73, 114. [Google Scholar] [CrossRef]
- Schluessel, V.; Bennett, M.B.; Collin, S.P. Diet and reproduction in the white-spotted eagle ray Aetobatus narinari from Queensland, Australia and the Penghu Islands, Taiwan. Mar. Freshw. Res. 2010, 61, 1278–1289. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R. Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef]
- Schwartz, F.J. Mass Migratory Congregations and Movements of Several Species of Cownose Rays, Genus Rhinoptera: A World-Wide Review. J. Elisha Mitchell Sci. Soc. 1990, 106, 10–13. Available online: www.jstor.org/stable/24333475 (accessed on 28 December 2020).
- Gray, A.E.; Mulligan, T.J.; Hannah, R.W. Food habits, occurrence, and population structure of the bat ray, Myliobatis californica, in Humboldt Bay, California. Environ. Biol. Fishes 1997, 49, 227–238. [Google Scholar] [CrossRef]
- Goodman, M.A.; Conn, P.B.; Fitzpatrick, E. Seasonal Occurrence of Cownose Rays (Rhinoptera bonasus) in North Carolina’s Estuarine and Coastal Waters. Chesap. Sci. 2010, 34, 640–651. [Google Scholar] [CrossRef]
- Le Port, A.; Lavery, S.D.; Montgomery, J.C. Conservation of coastal stingrays: Seasonal abundance and population structure of the short-tailed stingray Dasyatis brevicaudata at a Marine Protected Area. ICES J. Mar. Sci. 2012, 69, 1427–1435. [Google Scholar] [CrossRef]
- Ajemian, M.J.; Powers, S.P. Towed-float satellite telemetry tracks large-scale movement and habitat connectivity of myliobatid stingrays. Environ. Boil. Fishes 2014, 97, 1067–1081. [Google Scholar] [CrossRef]
- Ramsden, S.; Cotton, C.F.; Curran, M.C. Using acoustic telemetry to assess patterns in the seasonal residency of the Atlantic stingray Dasyatis sabina. Environ. Boil. Fishes 2016, 100, 89–98. [Google Scholar] [CrossRef]
- Stewart, J.; Smith, T.; Marshall, G.; Abernathy, K.; Fonseca-Ponce, I.; Froman, N.; Stevens, G. Novel applications of animal-borne Crittercams reveal thermocline feeding in two species of manta ray. Mar. Ecol. Prog. Ser. 2019, 632, 145–158. [Google Scholar] [CrossRef]
- Frixione, M.G.; García, M.D.J.G.; Gauger, M.F. Drone imaging of elasmobranchs: Whale sharks and golden cownose rays co-occurrence in a zooplankton hot-spot in southwestern Sea of Cortez. Food Webs 2020, 24, e00155. [Google Scholar] [CrossRef]
- Oleksyn, S.; Tosetto, L.; Raoult, V.; Williamson, J.E. Drone-Based Tracking of the Fine-Scale Movement of a Coastal Stingray (Bathytoshia brevicaudata). Remote. Sens. 2020, 13, 40. [Google Scholar] [CrossRef]
- Butcher, P.A.; Colefax, A.P.; Gorkin, R.A.; Kajiura, S.M.; López, N.A.; Mourier, J.; Purcell, C.R.; Skomal, G.B.; Tucker, J.P.; Walsh, A.J.; et al. The Drone Revolution of Shark Science: A Review. Drones 2021, 5, 8. [Google Scholar] [CrossRef]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, D.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.B.; Pirotta, V.; et al. Operational Protocols for the Use of Drones in Marine Animal Research. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Kiszka, J.; Mourier, J.; Gastrich, K.; Heithaus, M.R. Using unmanned aerial vehicles (UAVs) to investigate shark and ray densities in a shallow coral lagoon. Mar. Ecol. Prog. Ser. 2016, 560, 237–242. [Google Scholar] [CrossRef]
- Chen, C.-H.; Liu, K.-H. Stingray detection of aerial images with region-based convolution neural network. In Proceedings of the 2017 IEEE International Conference on Consumer Electronics—Taiwan (ICCE-TW), Taipei, Taiwan, 12–14 June 2017; pp. 175–176. [Google Scholar]
- Hensel, E.; Wenclawski, S.; Layman, C. Using a small, consumer grade drone to identify and count marine megafauna in shallow habitats. Lat. Am. J. Aquat. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Saqib, M.; Khan, S.D.; Sharma, N.; Scully-Power, P.; Butcher, P.; Colefax, A.P.; Blumenstein, M. Real-Time Drone Surveillance and Population Estimation of Marine Animals from Aerial Imagery. In Proceedings of the 2018 International Conference on Image and Vision Computing New Zealand (IVCNZ), Auckland, New Zealand, 19–21 November 2018; pp. 1–6. [Google Scholar]
- Kelaher, B.P.; Peddemors, V.M.; Hoade, B.; Colefax, A.P.; Butcher, P.A. Comparison of sampling precision for nearshore marine wildlife using unmanned and manned aerial surveys. J. Unmanned Veh. Syst. 2020, 8, 30–43. [Google Scholar] [CrossRef]
- Kelaher, B.P.; Colefax, A.P.; Tagliafico, A.; Bishop, M.J.; Giles, A.; Butcher, P.A. Assessing variation in assemblages of large marine fauna off ocean beaches using drones. Mar. Freshw. Res. 2020, 71, 68. [Google Scholar] [CrossRef]
- Tagliafico, A.; Butcher, P.A.; Colefax, A.P.; Clark, G.F.; Kelaher, B.P. Variation in cownose ray Rhinoptera neglecta abundance and group size on the central east coast of Australia. J. Fish Biol. 2019, 96, 427–433. [Google Scholar] [CrossRef]
- Gorkin, R.A.; Adams, K.R.; Berryman, M.J.; Aubin, S.; Li, W.; Davis, A.R.; Barthelemy, J. Sharkeye: Real-Time Autonomous Personal Shark Alerting via Aerial Surveillance. Drones 2020, 4, 18. [Google Scholar] [CrossRef]
- Green, E.P.; Mumby, P.J.; Edwards, A.J.; Clark, C.D. A review of remote sensing for the assessment and management of tropical coastal resources. Coast. Manag. 1996, 24, 1–40. [Google Scholar] [CrossRef]
- Hamylton, S.M. Mapping coral reef environments: A review of historical methods, recent advances and future opportuni-ties. Prog. Phys. Geogr. 2017, 41, 803–833. [Google Scholar] [CrossRef]
- Joyce, K.E.; Duce, S.; Leahy, S.M.; Leon, J.; Maier, S.W. Principles and practice of acquiring drone-based image data in ma-rine environments. Mar. Freshw. Res. 2019, 70, 952. [Google Scholar] [CrossRef]
- Bennett, M.K.; Younes, N.; Joyce, K.E. Automating Drone Image Processing to Map Coral Reef Substrates Using Google Earth Engine. Drones 2020, 4, 50. [Google Scholar] [CrossRef]
- Yang, B.; Hawthorne, T.L.; Hessing-Lewis, M.; Duffy, E.J.; Reshitnyk, L.Y.; Feinman, M.; Searson, H. Developing an Intro-ductory UAV/Drone Mapping Training Program for Seagrass Monitoring and Research. Drones 2020, 4, 70. [Google Scholar] [CrossRef]
- Fiori, L.; Doshi, A.; Martinez, E.; Orams, M.B.; Bollard-Breen, B. The Use of Unmanned Aerial Systems in Marine Mammal Research. Remote. Sens. 2017, 9, 543. [Google Scholar] [CrossRef]
- Schofield, G.; Esteban, N.; Katselidis, K.A.; Hays, G.C. Drones for research on sea turtles and other marine vertebrates—A review. Biol. Conserv. 2019, 238, 108214. [Google Scholar] [CrossRef]
- Brisson-Curadeau, É.; Bird, D.; Burke, C.; Fifield, D.A.; Pace, P.; Sherley, R.B.; Elliott, K.H. Seabird species vary in behavioural response to drone census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef] [PubMed]
- Raoult, V.; Tosetto, L.; Williamson, J.E. Drone-Based High-Resolution Tracking of Aquatic Vertebrates. Drones 2018, 2, 37. [Google Scholar] [CrossRef]
- Colefax, A.P.; Kelaher, B.P.; Pagendam, D.E.; Butcher, P.A. Assessing White Shark (Carcharodon carcharias) Behavior Along Coastal Beaches for Conservation-Focused Shark Mitigation. Front. Mar. Sci. 2020, 7, 268. [Google Scholar] [CrossRef]
- Landeo-Yauri, S.; Ramos, S.; Castelblanco-Martínez, E.A.; Niño-Torres, D.N.; Searle, L. Using small drones to pho-to-identify Antillean manatees: A novel method for monitoring an endangered marine mammal in the Caribbean Sea. Endanger. Species Res. 2020, 41, 79–90. [Google Scholar] [CrossRef]
- Raoult, V.; Gaston, T. Rapid biomass and size-frequency estimates of edible jellyfish populations using drones. Fish. Res. 2018, 207, 160–164. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.; Wotherspoon, S.; Kilpatrick, A.D.; Segaran, R.R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Ventura, D.; Bruno, M.; Jona-Lasinio, G.; Belluscio, A.; Ardizzone, G. A low-cost drone based application for identifying and mapping of coastal fish nursery grounds. Estuar. Coast. Shelf Sci. 2016, 171, 85–98. [Google Scholar] [CrossRef]
- Duffy, J.P.; Pratt, L.; Anderson, K.; Land, P.E.; Shutler, J.D. Spatial assessment of intertidal seagrass meadows using opti-cal imaging systems and a lightweight drone. Estuar. Coast. Shelf Sci. 2018, 200, 169–180. [Google Scholar] [CrossRef]
- Ridge, J.T.; Johnston, D.W. Unoccupied Aircraft Systems (UAS) for Marine Ecosystem Restoration. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Casella, E.; Collin, A.; Harris, D.; Ferse, S.; Bejarano, S.; Parravicini, V.; Hench, J.L.; Rovere, A. Mapping coral reefs using consumer-grade drones and structure from motion photogrammetry techniques. Coral Reefs 2017, 36, 269–275. [Google Scholar] [CrossRef]
- Kabiri, K.; Rezai, H.; Moradi, M. A drone-based method for mapping the coral reefs in the shallow coastal waters—case study: Kish Island, Persian Gulf. Earth Sci. Inform. 2020, 13, 1265–1274. [Google Scholar] [CrossRef]
- Williamson, J.E.; Duce, S.; Joyce, K.E.; Raoult, V. Putting sea cucumbers on the map: Projected holuthurian bioturbation rates on a coral reef scale. Coral Reefs 2020. accepted. [Google Scholar]
- Windle, A.E.; Poulin, S.K.; Johnston, D.W.; Ridge, J.T. Rapid and Accurate Monitoring of Intertidal Oyster Reef Habitat Using Unoccupied Aircraft Systems and Structure from Motion. Remote. Sens. 2019, 11, 2394. [Google Scholar] [CrossRef]
- Fisher, R.A.; Call, G.C.; Grubbs, R.D. Cownose Ray (Rhinoptera bonasus) Predation Relative to Bivalve Ontogeny. J. Shellfish Res. 2011, 30, 187–196. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Kelleher, C.; Baker, E.A.; Lautz, L.K. Relative information from thermal infrared imagery via unoccu-pied aerial vehicle informs simulations and spatially-distributed assessments of stream temperature. Sci. Total Environ. 2019, 661, 364–374. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Kelleher, C.; Malcolm, I.A.; Caldwell, S.; Hannah, D. Assessing the potential of drone-based thermal infrared imagery for quantifying river temperature heterogeneity. Hydrol. Process. 2019, 33, 1152–1163. [Google Scholar] [CrossRef]
- Schlaff, A.M.; Heupel, M.R.; Simpfendorfer, C.A. Influence of environmental factors on shark and ray movement, behaviour and habitat use: A review. Rev. Fish Biol. Fish. 2014, 24, 1089–1103. [Google Scholar] [CrossRef]
- MacArthur, R.A.; Geist, V.; Johnston, R.H. Cardiac and Behavioral Responses of Mountain Sheep to Human Disturbance. J. Wildl. Manag. 1982, 46, 351. [Google Scholar] [CrossRef]
- Richardson, W.J.; Würsig, B. Influences of man-made noise and other human actions on cetacean behaviour. Mar. Freshw. Behav. Physiol. 1997, 29, 183–209. [Google Scholar] [CrossRef]
- Wegdell, F.; Hammerschmidt, K.; Fischer, J. Conserved alarm calls but rapid auditory learning in monkey responses to novel flying objects. Nat. Ecol. Evol. 2019, 3, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Brunton, E.A.; Bolin, J.A.; Leon, J.X.; Burnett, S.E. Leon Fright or Flight? Behavioural Responses of Kangaroos to Drone-Based Monitoring. Drones 2019, 3, 41. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Vincent, J.B.; Werden, L.K.; Tanner, J.C.; Laske, T.G.; Iaizzo, P.A.; Garshelis, D.L.; Fieberg, J. Bears Show a Physiological but Limited Behavioral Response to Unmanned Aerial Vehicles. Curr. Biol. 2015, 25, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.F.; Hall, G.P.; McDonald, P.G. Evaluation of unmanned aerial vehicle shape, flight path and camera type for waterfowl surveys: Disturbance effects and species recognition. PeerJ 2016, 4, e1831. [Google Scholar] [CrossRef]
- Christiansen, F.; Rojano-Doñate, L.; Madsen, P.T.; Bejder, L. Noise Levels of Multi-Rotor Unmanned Aerial Vehicles with Implications for Potential Underwater Impacts on Marine Mammals. Front. Mar. Sci. 2016, 3, 277. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef]
- Ramos, E.A.; Maloney, B.; Magnasco, M.O.; Reiss, D. Bottlenose dolphins and antillean manatees respond to small mul-ti-rotor unmanned aerial systems. Front. Mar. Sci. 2018, 5, 316. [Google Scholar] [CrossRef]
- Fettermann, T.; Fiori, L.; Bader, M.; Doshi, A.; Breen, D.; Stockin, K.A.; Bollard, B. Behaviour reactions of bottlenose dolphins (Tursiops truncatus) to multirotor Unmanned Aerial Vehicles (UAVs). Sci. Rep. 2019, 9, 8558. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.R.; Gibbs, L.; Knott, N.A.; Broad, A.; Hing, M.; Taylor, M.D.; Davis, A.R. Coexisting with sharks: A novel, so-cially acceptable and non-lethal shark mitigation approach. Sci. Rep. 2020, 10, 17497. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.D.; Peddemors, V.M.; Kennelly, S.J. Assessment of Shark Sighting Rates by Aerial Beach Patrols; NSW Department of Primary Industries: Cronulla, Australia, 2012.
- Westgate, A.; Koopman, H.; Siders, Z.A.; Wong, S.; Ronconi, R. Population density and abundance of basking sharks Cetorhinus maximus in the lower Bay of Fundy, Canada. Endanger. Species Res. 2014, 23, 177–185. [Google Scholar] [CrossRef]
- Butcher, P.; Piddocke, T.P.; Colefax, A.P.; Hoade, B.; Peddemors, V.M.; Borg, L.; Cullis, B.R. Beach safety: Can drones provide a platform for sighting sharks? Wildl. Res. 2019, 46, 701. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef]
- Ruiz-García, D.; Adams, K.; Brown, H.; Davis, A.R. Determining Stingray Movement Patterns in a Wave-Swept Coastal Zone Using a Blimp for Continuous Aerial Video Surveillance. Fishes 2020, 5, 31. [Google Scholar] [CrossRef]
- Duffy, J.P.; Cunliffe, A.M.; Debell, L.; Sandbrook, C.G.; Wich, S.A.; Shutler, J.D.; Myers-Smith, I.H.; Varela, M.R.; Anderson, K. Location, location, location: Considerations when using lightweight drones in challenging environments. Remote. Sens. Ecol. Conserv. 2018, 4, 7–19. [Google Scholar] [CrossRef]
- Turner, D.; Lucieer, A.; Watson, C. An Automated Technique for Generating Georectified Mosaics from Ultra-High Resolu-tion Unmanned Aerial Vehicle (UAV) Imagery, Based on Structure from Motion (SfM) Point Clouds. Remote Sens. 2012, 4, 1392–1410. [Google Scholar] [CrossRef]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote. Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef]
- Parsons, M.; Bratanov, D.; Gaston, K.J.; Gonzalez, F. UAVs, Hyperspectral Remote Sensing, and Machine Learning Revolu-tionizing Reef Monitoring. Sensors 2018, 18, 2026. [Google Scholar] [CrossRef]
- Roelfsema, C.M.; Phinn, S.R. Integrating field data with high spatial resolution multispectral satellite imagery for cali-bration and validation of coral reef benthic community maps. J. Appl. Remote Sens. 2010, 4, 043527. [Google Scholar] [CrossRef]
- Chirayath, V.; Earle, S.A. Drones that see through waves—Preliminary results from airborne fluid lensing for centime-tre-scale aquatic conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 237–250. [Google Scholar] [CrossRef]
- Chirayath, V.; Li, A. Next-Generation Optical Sensing Technologies for Exploring Ocean Worlds—NASA FluidCam, MiDAR, and NeMO-Net. Front. Mar. Sci. 2019, 6, 521. [Google Scholar] [CrossRef]
- Dujon, A.M.; Schofield, G. Importance of machine learning for enhancing ecological studies using information-rich im-agery. Endanger. Species Res. 2019, 39, 91–104. [Google Scholar] [CrossRef]
- Kilfoil, J.P.; Rodriguez-Pinto, I.; Kiszka, J.J.; Heithaus, M.R.; Zhang, Y.; Roa, C.C.; Ailloud, L.E.; Campbell, M.D.; Wirsing, A.J. Using unmanned aerial vehicles and machine learning to improve sea cucumber density estimation in shallow habitats. ICES J. Mar. Sci. 2020, 77, 2882–2889. [Google Scholar] [CrossRef]
- Rossi, L.; Mammi, I.; Pelliccia, F. UAV-Derived Multispectral Bathymetry. Remote. Sens. 2020, 12, 3897. [Google Scholar] [CrossRef]
- Chabot, D. Trends in drone research and applications as theJournal of Unmanned Vehicle Systemsturns five. J. Unmanned Veh. Syst. 2018, 6, vi–xv. [Google Scholar] [CrossRef]
- Ogden, L.E. Drone Ecology. BioScience 2013, 63, 776. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Small unmanned aircraft: Precise and convenient new tools for surveying wetlands. J. Unmanned Veh. Syst. 2013, 1, 15–24. [Google Scholar] [CrossRef]
- Wallace, P.J.; Martin, R.; White, I. Keeping pace with technology: Drones, disturbance and policy deficiency. J. Environ. Plan. Manag. 2017, 61, 1271–1288. [Google Scholar] [CrossRef]
- Tyokumbur, E.T. Review of Potential Ecological Impacts of Peaceful Robotic Drone Use and Policy Implications for Developing Countries. Am. J. Environ. Policy Manag. 2015, 4, 67–71. [Google Scholar]
- Walther, J.C.; PytlikZillig, L.M.; Detweiler, C.; Houston, A. How people make sense of drones used for atmospheric science (and other purposes): Hopes, concerns, and recommendations. J. Unmanned Veh. Syst. 2019, 7, 219–234. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Kaminski, M.E. Drone federalism: Civilian drones and the things they carry. 4 California Law Review Circuit 57. 2013. Available online: https://ssrn.com/abstract=2257080 (accessed on 29 December 2020).
- Allan, B.M.; Ierodiaconou, D.; Nimmo, D.G.; Herbert, M.; Ritchie, E.G. Free as a drone: Ecologists can add UAVs to their toolbox. Front. Ecol. Environ. 2015, 13, 354–355. [Google Scholar] [CrossRef]
- Hugenholtz, C.; Brown, O.; Walker, J.; Barchyn, T.; Nesbit, P.; Kucharczyk, M.; Myshak, S. Spatial Accuracy of UAV-Derived Orthoimagery and Topography: Comparing Photogrammetric Models Processed with Direct Geo-Referencing and Ground Control Points. Geomatica 2016, 70, 21–30. [Google Scholar] [CrossRef]
- Jones, T. International Commercial Drone Regulation and Drone Delivery Services; RAND: Santa Monica, CA, USA, 2017; No. RR-1718/3-RC. [Google Scholar]
- Powell, D.M.C.; Spencer, M.B.; Holland, D.; Broadbent, E.; Petrie, K.J. Pilot fatigue in short-haul operations: Effects of number of sectors, duty length, and time of day. Aviat. Space Environ. Med. 2007, 78, 698–701. [Google Scholar]
- Gregory, K.B.; Winn, W.; Johnson, K.; Rosekind, M.R. Pilot fatigue survey: Exploring fatigue factors in air medical oper-ations. Air Med. J. 2010, 29, 309–319. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Evaluation of an off-the-shelf Unmanned Aircraft System for Surveying Flocks of Geese. Waterbirds 2012, 35, 170–174. [Google Scholar] [CrossRef]
- Debell, L.; Anderson, K.; Brazier, R.; King, N.; Jones, L. Water resource management at catchment scales using lightweight UAVs: Current capabilities and future perspectives. J. Unmanned Veh. Syst. 2016, 4, 7–30. [Google Scholar] [CrossRef]
- Fujii, K.; Higuchi, K.; Rekimoto, J. Endless Flyer: A Continuous Flying Drone with Automatic Battery Replacement. In Proceedings of the 2013 IEEE 10th International Conference on Ubiquitous Intelligence and Computing and 2013 IEEE 10th International Conference on Autonomic and Trusted Computing, Washington, DC, USA, 18–21 December 2013; pp. 216–223. [Google Scholar]
- Abd-Elrahman, A.; Pearlstine, L.; Percival, F. Development of pattern recognition algorithm for automatic bird detection from unmanned aerial vehicle imagery. Surv. Land Inf. Sci. 2005, 65, 37. [Google Scholar]
- Vayssade, J.-A.; Arquet, R.; Bonneau, M. Automatic activity tracking of goats using drone camera. Comput. Electron. Agric. 2019, 162, 767–772. [Google Scholar] [CrossRef]
- Redmon, J.; Farhadi, A. YOLO9000: Better, Faster, Stronger. In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 6517–6525. [Google Scholar]
- Redmon, J.; Farhadi, A. Yolov3: An incremental improvement. arXiv 2018, arXiv:1804.02767. [Google Scholar]

| Research Focus | Details | Species | Publication |
|---|---|---|---|
| Abundance | Conducted transects to assess abundance over different habitats | Pink whipray (Himantura fai) | Kiszka et al. 2016 [22] |
| Methodology testing | Assessing the effectiveness of a neural network at real-time stingray detection from drone footage | Not reported | Chen and Liu (2017) [23] |
| Abundance | Identifying and counting marine megafauna in shallow habitats | Southern stingray (Dasyatis americana) Spotted eagle ray (Aetobatus narinari) | Hensel et al. (2018) [24] |
| Methodology testing | Assessing the effectiveness of deep learning object detectors in the surveillance and estimation of marine animals from drone footage in real time | Not reported | Saqib et al. (2018) [25] |
| Abundance | Compared precision of real-time helicopter and drone counts, as well as post-hoc analysis of drone footage | Not reported in abstract | Kelaher et al. (2019a) [26] |
| Abundance | Assessing variation in assemblages of large marine fauna off ocean beaches using drones | Australian cownose ray (Rhinoptera neglecta) Spotted eagle ray (Aetobatus narinari) Souther eagle ray (Myliobatus spp.) Devil ray (Mobulidae) | Kelaher et al. (2019b) [27] |
| Abundance | Monitoring the occurrence and shape of schools of cownose rays | Australian cownose ray (Rhinoptera neglecta) | Tagliafico et al. (2019) [28] |
| Feeding behaviour Distribution | Drone imaging of occurrence and feeding behaviour | Golden cownose ray (Rhinoptera steindachneri) | Frixione et al. (2020) [17] |
| Methodology | Real-time autonomous shark alerting using cloud-hosted machine learning detection algorithms | Not reported | Gorkin et al. (2020) [29] |
| Fine-scale movement and behaviour | Monitoring impacts of biotic and abiotic factors on stingray movement and behaviour | Short-tail stingray (Bathytoshia brevicaudata) | Oleksyn et al. (2021) [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksyn, S.; Tosetto, L.; Raoult, V.; Joyce, K.E.; Williamson, J.E. Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays. Drones 2021, 5, 12. https://doi.org/10.3390/drones5010012
Oleksyn S, Tosetto L, Raoult V, Joyce KE, Williamson JE. Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays. Drones. 2021; 5(1):12. https://doi.org/10.3390/drones5010012
Chicago/Turabian StyleOleksyn, Semonn, Louise Tosetto, Vincent Raoult, Karen E. Joyce, and Jane E. Williamson. 2021. "Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays" Drones 5, no. 1: 12. https://doi.org/10.3390/drones5010012
APA StyleOleksyn, S., Tosetto, L., Raoult, V., Joyce, K. E., & Williamson, J. E. (2021). Going Batty: The Challenges and Opportunities of Using Drones to Monitor the Behaviour and Habitat Use of Rays. Drones, 5(1), 12. https://doi.org/10.3390/drones5010012








