1. Introduction
Hydrogels, as hydrophilic polymer networks, exhibit characteristic water uptake behavior, making them particularly interesting for sensor and actuator setups. As water diffusivity is the time-limiting step in swelling [
1], application in thin films with low thickness is crucial for achieving fast response times. Employing stimuli-responsive monomers (e.g.,
N-Isopropylacryl-amide, NIPAAm) facilitates temperature-dependent swelling. NIPAAm-based hydrogels undergo a phase transition at their lower critical solution temperature (LCST), above which they repel out water and hence shrink. Initiated Chemical Vapor Deposition (iCVD), as a solvent-free technique, makes it possible to coat complex 3D structures conformally with controlled composition and thickness. Both these factors have been found to affect shape and position of the LCST transition [
2]. Based on these findings, structural properties of thin films of varying composition and thickness were investigated and deemed responsible for the change in LCST.
2. Materials and Methods
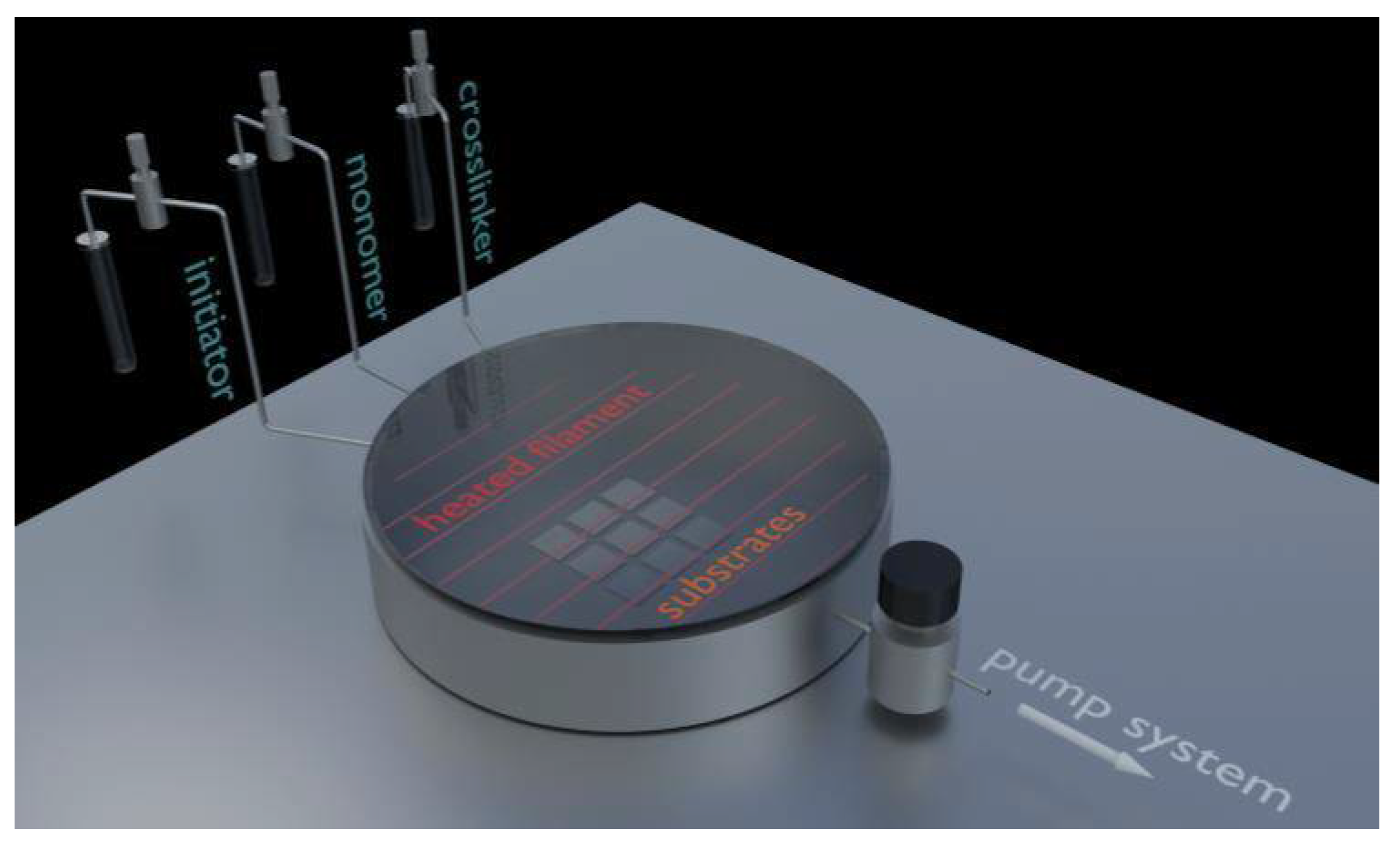
A series of thin films were deposited by iCVD. The depositions were done in a cylindrical reaction chamber (360 mm diameter, 55 mm height; see
Figure 1). Single-side polished crystalline silicon wafers (Siegert Wafer Gmbh, Aachen, Germany) with a 1.5–2 nm thick native oxide are used as substrates. These are placed on the bottom of the reaction chamber, which serves as a temperature controlled sample stage and was kept at 35 °C for all samples. The initiator (di-
tert-butyl peroxide, TBPO; Aldrich, Wien, Austria) is flown into the reaction chamber at flow rates set via a needle valve (Swagelok, Solon, OH, USA). Monomer (N-isopropylacrylamide, NIPAAm; Aldrich, Germany) and cross-linker (di(ethylene glycol) divinyl ether, DEGDVE; Aldrich, Germany) are held in glass jars at set temperatures (85 °C and 70 °C, respectively) and are also flown into the reaction chamber through a heated mixing line (90 °C) at flow rates set via needle valves (Swagelok, USA). Controlling the flow rates and their ratio also determines the chemical composition of the deposited thin films. A heated filament (200 °C) consisting of Ni-Cr wires (Goodfellow, Wrexham, UK) is mounted 25 mm above the substrates, at a temperature sufficient to form initiator radicals without affecting the monomer molecules. Monomer and cross-linker molecules are adsorbed on the substrate, where the initiator radicals promote surface polymerization. The working pressure (250 mTorr) is controlled by a throttle valve (MKS Instruments, Andover, MA, USA) and a Duo 5 M rotary vane pump (MKS Instruments, USA). Laser interferometry with a He-Ne laser (λ = 633 nm; Thorlabs, Newton, NJ, USA) is used to monitor the film thickness in situ through a removable quartz glass lid. With deposition rate also being determined by the individual flow rates, the deposition time is used to control the deposited film thickness.
Film thickness and optical properties of the films were investigated by means of spectroscopic ellipsometry (M-2000S, J. A. Woollam, Lincoln, NE, USA) in a wavelength range of 370–1000 nm. All swelling experiments in water were performed in a temperature controlled liquid stage (J. A. Woollam, USA) in deionized water. The optical model chosen for these studies consists of a c-Si semi-infinite layer at the bottom (temperature dependent), a 1.6 nm thick native SiO2 layer in the middle and the polymer film on top. The polymer layer was modelled with a Cauchy function and an Urbach tail accounting for adsorption at low wavelength, with the surrounding medium being set to H2O with temperature dependent optical properties. For the temperature dependent swelling experiments, the liquid stage was pre-cooled to 10 °C, before applying a temperature ramp to 50 °C at a heating rate of 0.5 °C/min and recording the respective signal. Beforehand, the deposited films were rinsed for 30 s with deionized water for equilibration. Furthermore, as the first and second heating experiment show differences in shape and position of the transition, the third heating experiment was used for the determination of the LCST, as all the further heating ramps give reproducible results; this effect is attributed to the removal of loosely attached material and monomer/oligomer inclusion. The thickness and refractive index measurements in N2 atmosphere and at 30% relative humidity (rh) were performed in a THMS600 temperature stage (Linkam, Tadworth, UK) at room temperature (~25 °C), with the N2 and water vapor being supplied from a custom built mixing setup. The relative humidity in the sample stage was monitored by a SHT15 humidity sensor (Sensirion, Staefa, Switzerland); the ellipsometry measurements were carried out after equilibration, where the sample thickness would not change more than 0.5 nm in 5 min. For evaluation of the optical data, the same model as in the liquid case was used, but with air (n ≈ 1) as the ambient material.
3. Results
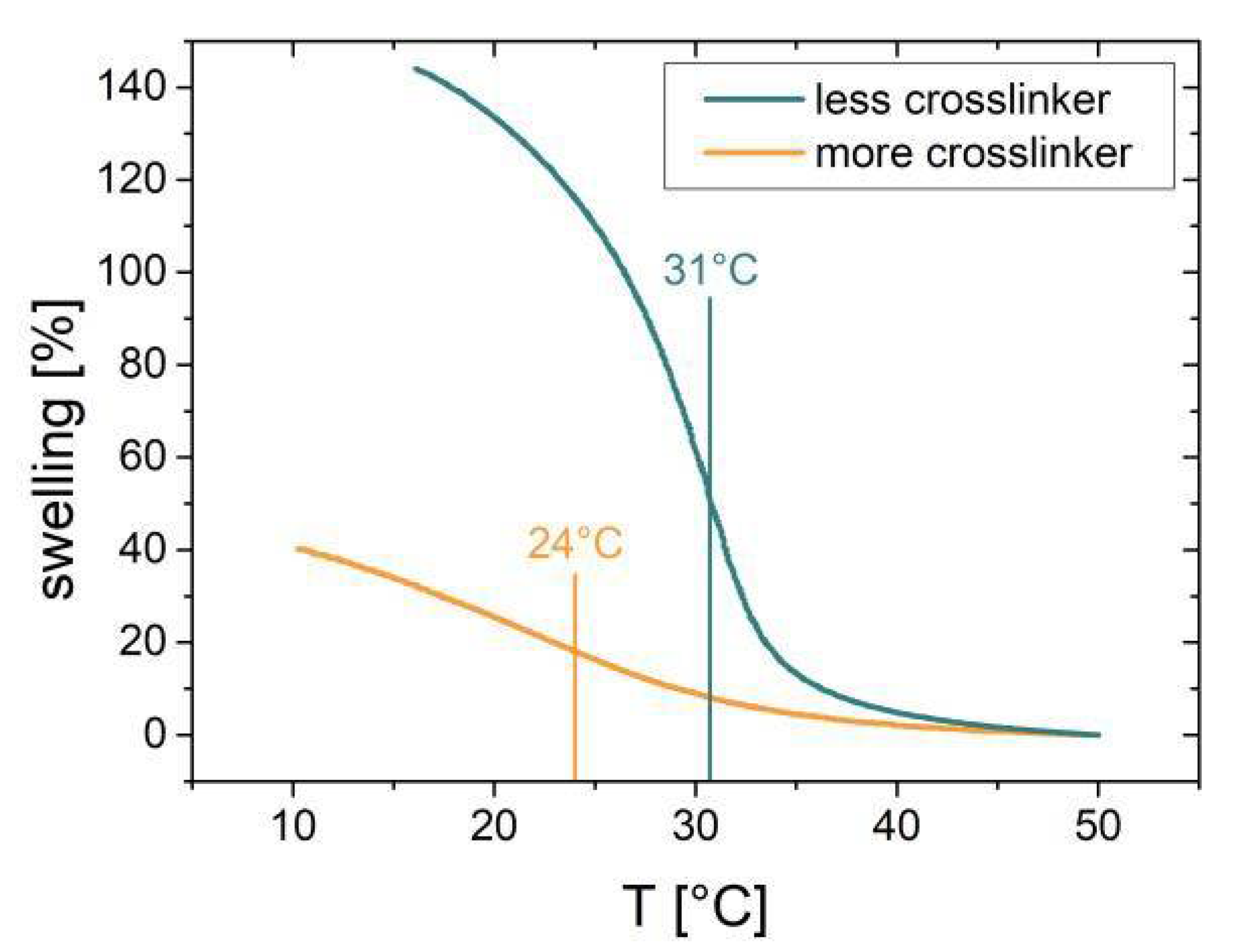
A series of samples with a nominal monomer-to-cross-linker ratio of 80:20 were prepared with film thicknesses in the range 30–150 nm. Furthermore, two nominally differently cross-linked (85:15 and 75:25) samples with a deposited film thickness of 100 nm were deposited. These samples, exhibiting different amounts of cross-linking and different film thicknesses, were investigated in terms of temperature dependent swelling behavior in water. Therefore, the film thickness (or swelling: film thickness normalized to the value at 50 °C) was plotted as a function of temperature. Two exemplary results for the differently cross-linked 100 nm samples are shown in
Figure 2.
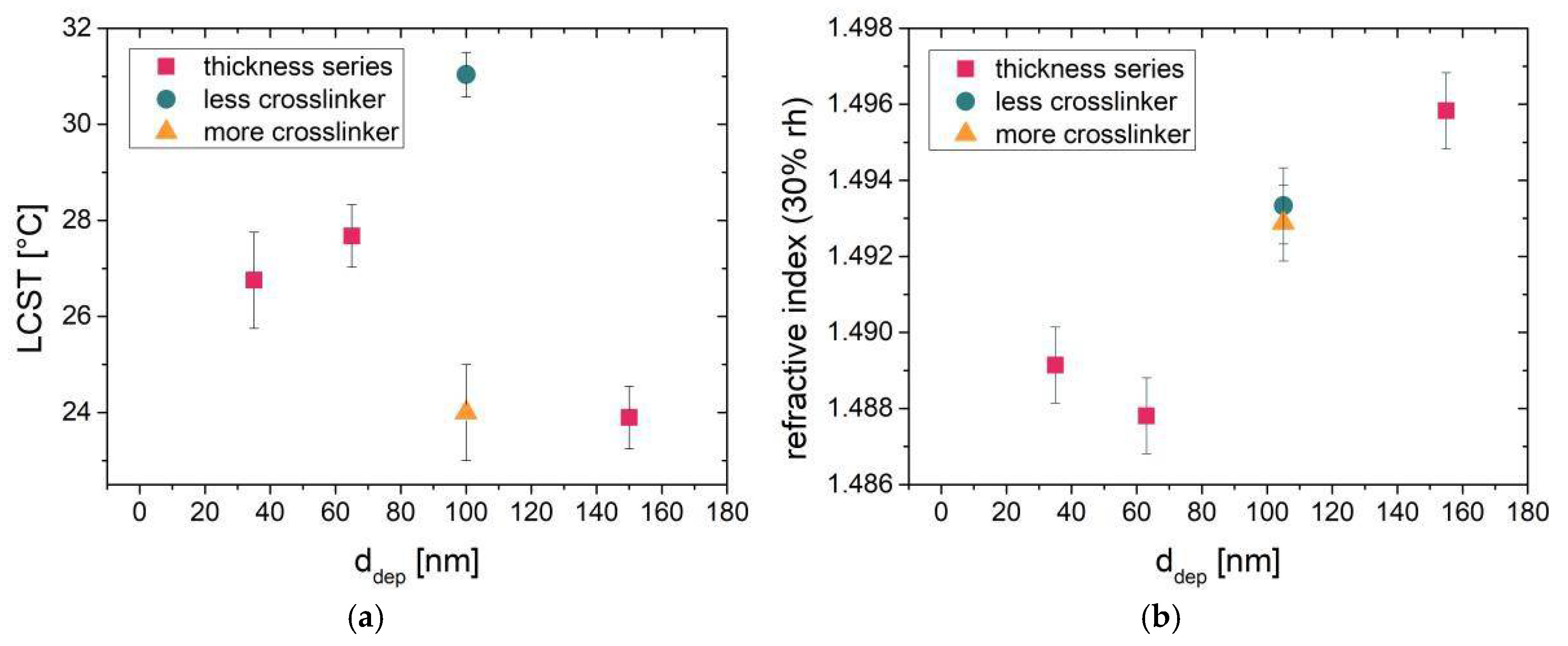
Hence, the LCSTs were evaluated as the points of inflection of the thickness vs. temperature curves for all of the samples (see
Figure 3a). The resulting LCSTs show that the transition temperature decreases with increasing amount of cross-linking and increasing film thickness.
Measuring the refractive index at a relative humidity of 30% gives information about the optical density of the films in this environment. The results of the optical density measurements in standard (moderately dry) environmental conditions (25 °C, rh 30%) are shown in
Figure 3b. The refractive index is found to increase with increasing the film thickness of the samples prepared with similar degrees of cross-linking. The 100 nm samples prepared with different amounts of cross-linking show similar refractive index values at 25 °C and in a relative humidity of 30%.
4. Discussion
It has been shown that the degree of cross-linking has an effect on the position of the LCST transition [
3]. The decrease in transition temperature with increasing amount of cross-linking can be attributed to two factors. First, more units of the hydrophobic cross-linker DEGDVE allow for repelling out water at lower temperatures. Second, polymer cross-linking reduces its mesh size limiting the amount of water diffusing into the polymer mesh [
4]. This can also be seen from the maximum swelling at low temperatures being lower for the more cross-linked film (~40% compared to ~150% for the less cross-linked sample; see
Figure 2). With this, a structural and a compositional component have been identified responsible for a change in swelling behavior. Furthermore, the film thickness also affects the LCST transition [
2]. As the thickest sample (150 nm) shows a significantly lower LCST, the refractive index of this sample is significantly higher than the ones of the thinner samples at 30% rh. The refractive index is connected to the (optical) density of the films. Thus, the “dry” density seems to be determining the position of the LCST transition; a higher refractive index gives a lower LCST. However, the refractive index values of the differently cross-linked 100 nm films are comparable, because the difference in chemical composition also affects the optical density. At 30% rh, the swelling occurring at these humidities additionally affects the measured refractive index, making it difficult to compare different chemical compositions in terms of optical density. On the other hand, a similarly cross-linked thickness series can be unambiguously compared in terms of density by spectroscopic ellipsometry measurements in relatively dry, comparable environmental conditions (25 °C, rh 30%). Therefore, the dry density could be successfully connected to the position of the LCST transition. A possible reason could be growth conditions changing as a function of deposition time, but further investigations need to be carried out to validate these assumptions. These more thorough, further investigations on the subject can be found in Muralter et al. [
5].