1. Introduction
Vitamin B
3 (nicotinic acid (NA) and nicotinamide (NAM), known as niacin) is present in various foods. NA and NAM play an important role in physiology. Niacin is a part of the cofactors nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate [
1,
2]. The recommended daily intake of niacin is about 15 mg/day [
3], but it is a semi-essential vitamin. It can be formed from the amino acid tryptophan in the human body, with approximately 60 mg of tryptophan being equivalent to 1 mg NA [
4]. During roasting of green coffee beans, NA is formed from the alkaloid trigonelline. Powdered coffee contains up to 300 mg/kg of NA depending on the degree of roasting. Nevertheless, in literature, information on the NA contents of commercially available coffee samples as well as the influence of brewing techniques on the NA content in prepared beverages is rather scarce. Therefore, we developed a fast and sensitive HPLC-MS/MS method to determine NA in coffee beverages. Additionally, the influence of brewing methods, degree of roasting, and brewing temperature was investigated.
2. Materials and Methods
2.1. Materials
Nicotinic acid (NA) and d4-nicotinic acid (d4-NA) were obtained from Toronto Research Chemicals (Toronto, Canada). Acetonitrile HPLC grade was acquired from VWR (Darmstadt, Germany). Formic acid p.a. was purchased from Sigma-Aldrich (Taufkirchen, Germany).
2.2. Preparation of the Coffee Beverages
Five commercially available coffee pad samples of ground coffee from the same manufacturer, containing approximately 7.4 g, (mild (taste intensity = 2), classic (taste intensity = 3), extra (taste intensity = 4), Espresso (taste intensity = 5), and decaffeinated (taste intensity = 4)) were purchased in a local supermarket in Germany. The “taste intensity”-value on a scale of 1 to 5 were provided on the packing box from the manufacturer to characterize the consumer expectation and is mainly influenced by the degree of roasting. All coffees were 100% arabica. After opening coffee powders were completely emptied in all cases, except in tests with the pad machine, and weighted. Coffee beverages were prepared in agreement with the described methods [
5] using the following machines: a standard “drip” coffee maker (Tchibo, Hamburg, Germany) fitted with filter paper, a Senseo coffee pad machine (Philips, Amsterdam, Netherlands) and an automatic coffee maker (Bosch, Gerlingen, Germany). Other samples were also prepared using: a manual plastic coffee filter with filter paper and boiling water; a pot for the “Turkish” coffee (powder and water heated together); a “French press” (Bodum AG, Triengen, Switzerland) and additionally, a so-called cold brew was prepared in the refrigerator for 24 h at +4 °C. For each method, triplicates were prepared and stored at −20 °C until the analysis. To investigate the influence of brewing temperatures, the ground coffee from the “classic” pads was emptied and brewed by hand filter at temperatures of 40, 60, 80, 90, and 100 °C.
2.3. Sample Preparation
Aliquots (n = 3) of the coffee beverages were diluted 1:100, in the case of the coffee brew prepared from “mild” or “decaffeinated” coffee, as well as prepared at a temperature of 40 °C, the samples were diluted 1:50. The diluted coffee samples were spiked with an isotopically labelled standard d4-NA to achieve a final concentration of 800 nM in each sample. After equilibration, the samples were passed through a syringe filter (Chromafil AO-45/25, Polyamid, 0.45 µm, Macherey-Nagel, Düren, Germany) and a 2 µL aliquotes were analyzed.
2.4. HPLC-ESI-MS/MS Analysis
HPLC-ESI-MS/MS system (1200 series, Agilent, Waldbronn, Germany and API 3200 triple quadrupole mass spectrometer from Applied Biosystems, Darmstadt, Germany) was used. NA was analyzed using a VDSpher PUR HILIC-Z column (100 × 2.0 mm, 100 Å, 5.0 µm, VDS optilab, Berlin, Germany) at 20 °C using 0.01% aqueous formic acid (eluent A) and acetonitrile (eluent B) at a flow rate of 1000 µL/min. The injection volume was 2 µl. Gradient started at 90% eluent B, isocratic for 0.5 min, then eluent B was reduced to 10% within 0.1 min. From minute 0.6 to 4.0, the system was kept isocratic at 10% eluent B. Afterwards the column was washed and equilibrated. The ESI-source was operated in positive ion mode (5.500 V), and nitrogen was used as nebulizer gas (55 psi), heater gas (65 psi, 350 °C), curtain gas (25 psi), and collision gas (2.0 psi). Nicotinic acid and d4-NA were detected in multiple reaction monitoring mode. MS transitions were: m/z 124→80 as qualifier and m/z 124→78 as quantifier and the isotopically labelled analogue m/z 128→84, respectively.
2.5. Quantitative Analysis and Method Validation
To quantitate NA mixtures at eight analyte/internal standard mole ratios in the range of concentrations from 80 to 10000 nM for each analyte and at 800 nM for the associated internal standard d
4-NA were analyzed by HPLC-MS/MS. The ratios of the peak areas of the ions selected for quantitation were plotted versus the weight ratio of analyte/internal standard. The linear regression coefficient (R
2) of the calibration curve was >0.99. Limits of detection and quantification, defined as signal to-noise ratios of 3:1 and 10:1 [
6], were 14.7 nM and 77.5 nM, respectively. Recovery rates were determined using 640, 800, and 960 nM of NA as well as the respective IS and were between 91 and 96%. The established HPLC-ESI-MS/MS method was fast and sensitive enough to determine nicotinic acid in coffee brews within 4 min.
Data were processed by Analyst 1.6 (AB Sciex, Darmstadt, Germany). Experimental results are reported as means of at least three independent preparations with ±SD as error bars.
3. Results
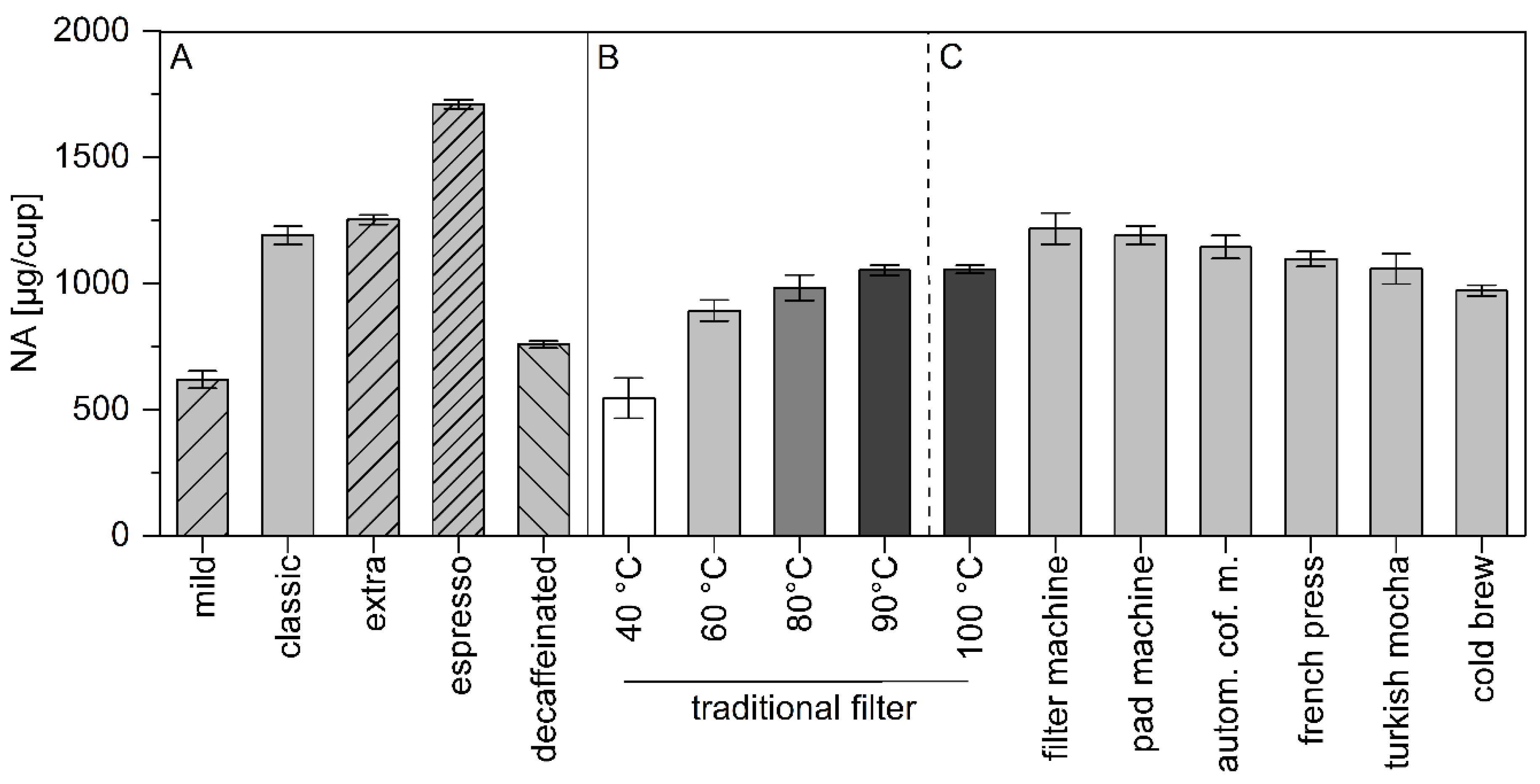
From the five commercially available coffee samples under study the respective brews were prepared by a pad machine and NA was quantitated. To observe the influence of the brewing method, a Senseo coffee pad machine using pads named “classic”, and the coffee powder from those pads was used to brew coffee by traditional filter, standard “drip” coffee maker, automatic coffee maker, manual plastic coffee filter with filter paper, “Turkish” coffee pot, “French press”, and so called cold brew method.
Figure 1 represents the calculated quantities of NA extracted from the powdered coffee [µg/cup of coffee].
It is demonstrated that the degree of roasting seems to influence the amount of NA: with higher roasting degree, the amount of NA is increased. Therefore, “espresso” showed the highest NA content with 1711 ± 18 µg/cup of coffee. The influence of the preparation seems to be minor. Here the automatic coffee maker and filter machine revealed highest amounts of NA (1144 ± 46 and 1217 ± 62 µg/cup), respectively. The lowest amount was determined in the cold brew with 972 ± 21 µg/cup NA.
One sample, namely the “classic” pad was used to observe the influence of brewing temperature on the NA transfer into the coffee brews. Water temperatures of 40, 60, 80, 90 and 100 °C were used to prepare traditional filtered coffee. Whereas at 40 °C the amount was only 545 ± 80 µg/cup, it increased with higher brewing temperatures: 892 ± 41 at 60 °C, 983 ± 41 at 80 °C, 1052 ± 20 at 90 °C and 1056 ± 17 µg/cup at 100 °C. At temperatures higher than 60 °C, the NA content of coffee beverages was almost independent of the brewing temperature reaching a plateau at 90 to 100 °C.
4. Discussion
We were able to show, that the NA amount in coffee is dependent on the degree of roasting. These results are in agreement with the study by Lang et al., who investigated the influence of different roasting conditions on NA formation. The authors found that hotter or longer roasted coffee beans contain more NA [
7]. Additionally, the brewing method seems to influence the amount of NA transferred into the respective brews. This can depend on the water temperature used to prepare the coffees. Here it was shown that the maximum amount of NA is liberated at water temperatures above 80 °C. In contrast, it was shown that the duration of extraction has an influence on the amount of extractable NA. With the cold brew method (4 °C for 24 h) it is possible to obtain about 972 ± 21 µg/cup NA in contrast to 545 ± 80 µg/cup NA with traditional filter at 40 °C. However, even the choice of brewing method itself can have an influence on the extractable amount of NA. Thus, mechanical methods that allow continuous extraction (e.g., pad, filter and automatic coffee maker) show a higher yield than discontinuous methods (e.g., French press and Turkish mocha).
The results are presented in µg NA extracted per cup of coffee, accounting for up to 11 mg NA per liter, present in the coffee brew prepared by the coffee pad machine from the “classic” coffee sample. This corresponds to 1.2 mg per 125 mL cup covering about 9% of the recommended daily intake (RDA) of 15 mg NA. [
3] It could be shown that coffee consumption contributes to the daily niacin (vitamin B
3) intake.
Author Contributions
Conceptualization, E.R.; investigation, L.B.; validation, L.B.; formal analyzes, L.B.; methodology, L.B., J.I.K. and S.S; project administration, E.R.; supervision, E.R.; writing—original draft preparation, E.R.; writing—review and editing, J.I.K., S.S. and E.R.
Funding
This article is based upon work from COST Action NutRedOx-CA16112 supported by COST (European Cooperation in Science and Technology).
Acknowledgments
We thank Tchibo GmbH, Hamburg, Germany for some financial support to buy the isotopically labelled standard.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Ann. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Sauve, A.A. NAD+ and vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Therap. 2008, 324, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12. [CrossRef]
- Horwitt, M.K.; Harper, A.E.; Henderson, L.M. Niacin-tryptophan relationships for evaluating niacin equivalents. Am. J. Clin. Nutr. 1981, 34, 423–427. [Google Scholar] [CrossRef]
- Deutscher Kaffeeverband e.V. Moderne Zubereitung von Kaffee in der Gastronomie, 2nd ed.; jungvornweg: Dresden, Germany, 2016. [Google Scholar]
- MacDougall, D.; Crummett, W.B.; Amore, F.J.; Cox, G.V.; Crosby, D.G.; Estes, F.L.; Lal, J.; Langner, R.R.; McClelland, N.I.; Phillips, W.F.; et al. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
- Lang, R.; Yagar, E.F.; Eggers, R.; Hofmann, T. Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J. Agric. Food Chem. 2008, 56, 11114–11121. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).