Development of a MEMS Plate Based on Thin-Film Piezoelectric AlN Actuators for Biological Applications †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
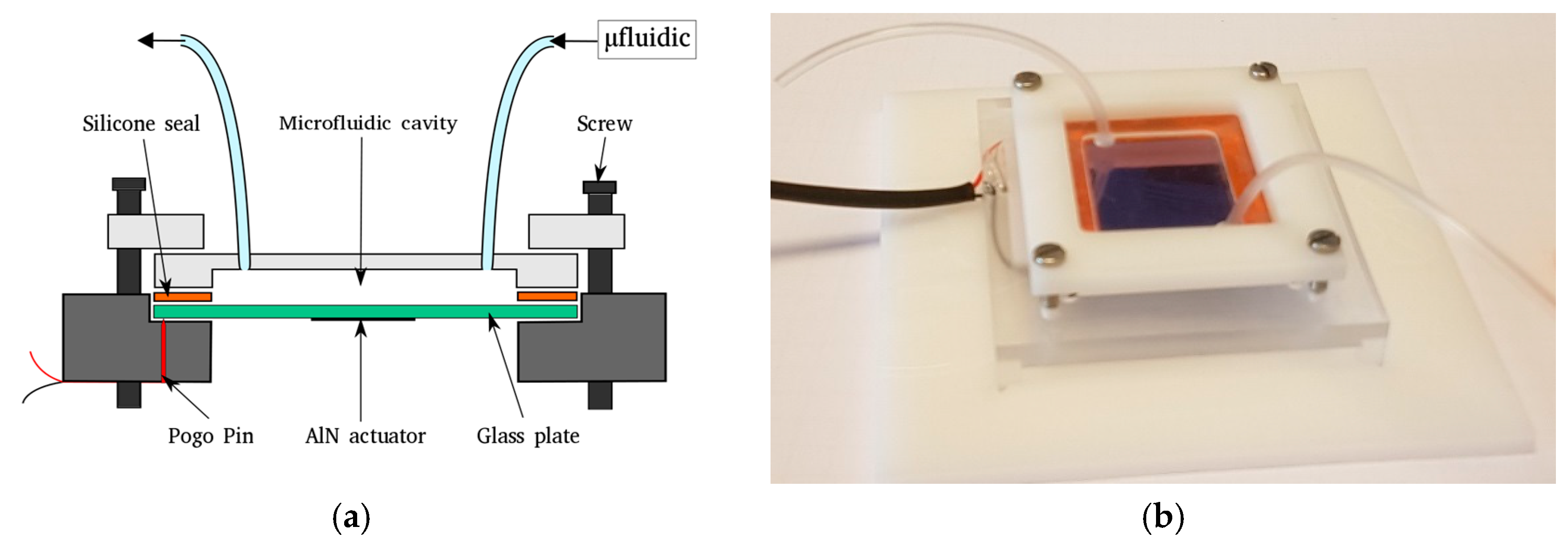
2.2. Microfluidic Packaging
2.3. Electromechanical Characterizations and FEM Simulations
3. Results
4. Conclusions
Conflicts of Interest
References
- Cermak, N.; Olcum, S.; Delgado, F.F.; Wasserman, S.C.; Payer, K.R.; Murakami, M.A.; Knudsen, S.M.; Kimmerling, R.J.; Stevens, M.M.; Kikuchi, Y.; et al. High-throughput measurement of single-cell growth rates using serial microfluidic mass sensor arrays. Nat. Biotechnol. 2016, 34, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Millet, L.J.; Kim, N.; Li, H.; Jin, X.; Popescu, G.; Aluru, N.R.; Hsia, K.J.; Bashir, R. Measurement of adherent cell mass and growth. Proc. Natl. Acad. Sci. USA 2010, 107, 20691–20696. [Google Scholar] [CrossRef] [PubMed]
- Fukui, W.; Kaneko, M.; Sakuma, S.; Kawahara, T.; Arai, F. μ-cell fatigue test. In Proceedings of the 2012 IEEE International Conference on Robotics and Automation (ICRA), Saint Paul, MN, USA, 14–18 May 2012; pp. 4600–4605. [Google Scholar]
- Wang, L.; Li, Y.-J.; Lin, A.; Choe, Y.; Gross, M.E.; Kim, E.S. A Self-Focusing Acoustic Transducer That Exploits Cytoskeletal Differences for Selective Cytolysis of Cancer Cells. J. Microelectromech. Syst. 2013, 22, 542–552. [Google Scholar] [CrossRef]
- Ruiz-Díez, V.; Hernando-García, J.; Ababneh, A.; Seidel, H.; Sánchez-Rojas, J.L. In-liquid characterization of in-plane and high order out-of-plane modes of AlN-based square microplates. Microsyst. Technol. 2016, 22, 1701–1708. [Google Scholar] [CrossRef]
- Ali, A.; Lee, J.E.-Y. Fully-differential AlN-on-Si wine glass mode resonator for enhanced characterization in water. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Debavelaere-Callens, D.; Peyre, L.; Campistron, P.; Hildebrand, H.F. On the use of ultrasounds to quantify the longitudinal threshold force to detach osteoblastic cells from a conditioned glass substrate. Biomol. Eng. 2007, 24, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Casset, F.; Danel, J.; Renaux, P.; Chappaz, C.; Bernard, F.; Sednaoui, T.; Basrour, S.; Desloges, B.; Fanget, S. 4-inch transparent plates based on thin-film AlN actuators for haptic applications. Mechatronics 2016, 40, 264–269. [Google Scholar] [CrossRef]


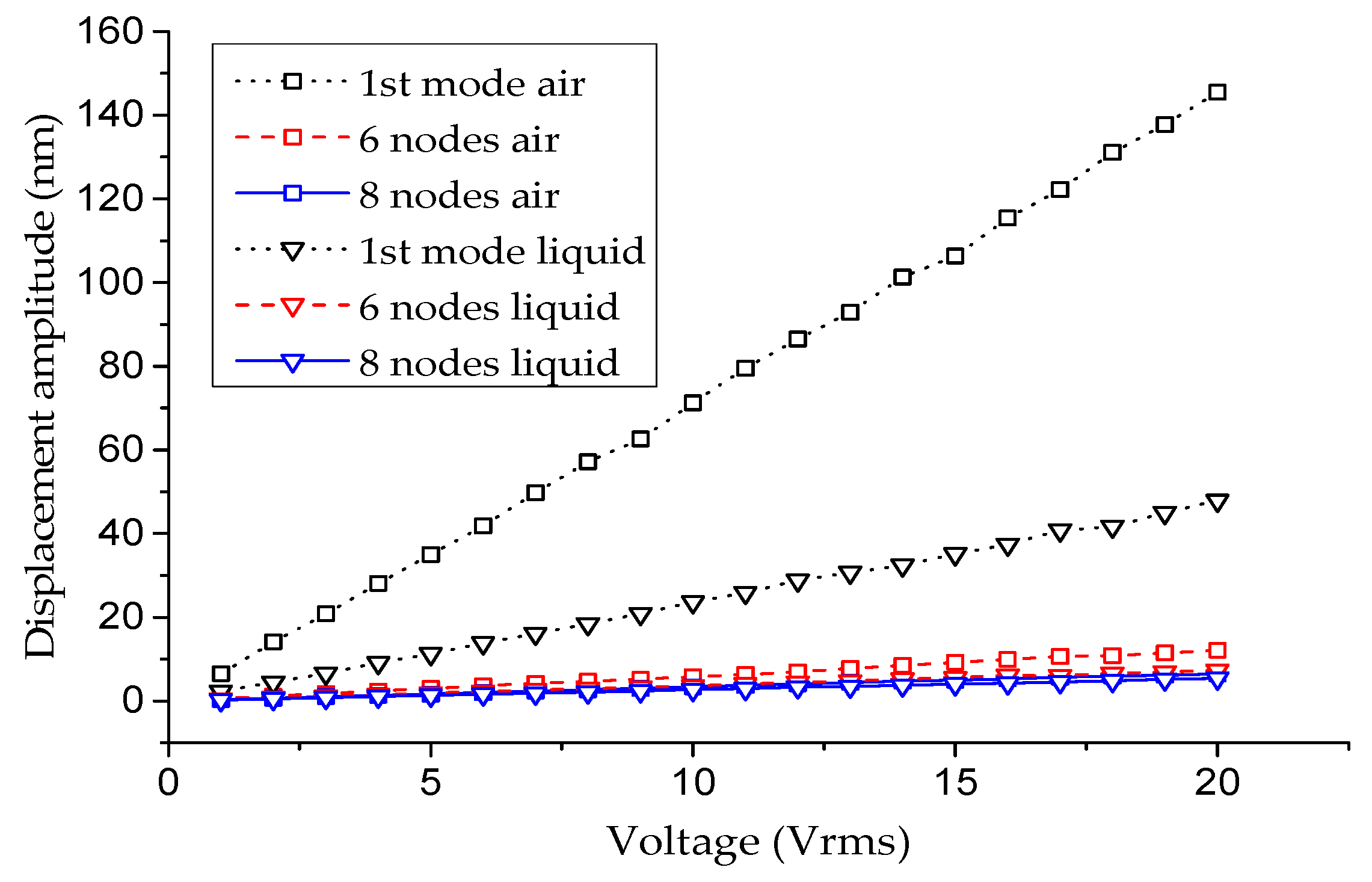
| Environment | Air | Liquid | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method | Displacement amplitudes @20 Vrms | Quality factor | Experimental | FEM | Relative error | Displacement amplitudes @20 Vrms | Quality factor | Experimental | FEM | Relative error |
| 1st mode | 146 nm | 22 | 5.47 kHz | 6.9 kHz | 20% | 48 nm | 13 | 3.75 kHz | 5.5 kHz | 31% |
| Lamb mode: 6 nodes | 12 nm | 20 | 98.92 kHz | 99.5 kHz | 0.6% | 7.3 nm | 18 | 76.50 kHz | 80 kHz | 4.4% |
| Lamb mode: 8 nodes | 6.4 nm | 30 | 144.7 kHz | 145.7 kHz | 0.7% | 5.4 nm | 33 | 121.4 kHz | 121 kHz | 0.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neff, B.; Casset, F.; Millet, A.; Agache, V.; Verplanck, N.; Boizot, F.; Fanget, S. Development of a MEMS Plate Based on Thin-Film Piezoelectric AlN Actuators for Biological Applications. Proceedings 2017, 1, 386. https://doi.org/10.3390/proceedings1040386
Neff B, Casset F, Millet A, Agache V, Verplanck N, Boizot F, Fanget S. Development of a MEMS Plate Based on Thin-Film Piezoelectric AlN Actuators for Biological Applications. Proceedings. 2017; 1(4):386. https://doi.org/10.3390/proceedings1040386
Chicago/Turabian StyleNeff, Baptiste, Fabrice Casset, Arnaud Millet, Vincent Agache, Nicolas Verplanck, François Boizot, and Stéphane Fanget. 2017. "Development of a MEMS Plate Based on Thin-Film Piezoelectric AlN Actuators for Biological Applications" Proceedings 1, no. 4: 386. https://doi.org/10.3390/proceedings1040386
APA StyleNeff, B., Casset, F., Millet, A., Agache, V., Verplanck, N., Boizot, F., & Fanget, S. (2017). Development of a MEMS Plate Based on Thin-Film Piezoelectric AlN Actuators for Biological Applications. Proceedings, 1(4), 386. https://doi.org/10.3390/proceedings1040386




